Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 7
AST-120 for the management of progression of chronic kidney disease
Authors Schulman G, Vanholder R, Niwa T
Received 8 December 2012
Accepted for publication 5 February 2013
Published 30 January 2014 Volume 2014:7 Pages 49—56
DOI https://doi.org/10.2147/IJNRD.S41339
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Gerald Schulman,1 Raymond Vanholder,2 Toshimitsu Niwa3
1Vanderbilt University School of Medicine, Nashville, TN, USA; 2University Hospital, Ghent, Belgium; 3Nagoya University Graduate School of Medicine, Nagoya, Japan
Abstract: Uremic toxins such as indoxyl sulfate contribute to the pathogenesis of chronic kidney disease (CKD) by promoting glomerulosclerosis and interstitial fibrosis with loss of nephrons and vascular damage. AST-120, an orally administered intestinal sorbent, adsorbs indole, a precursor of indoxyl sulfate, thereby reducing serum and urinary concentrations of indoxyl sulfate. AST-120 has been available in Japan since 1991, and subsequently Korea (2005), and the Philippines (2010) as an agent to prolong the time to initiation of hemodialysis and for improvement of uremic symptoms in patients with CKD. A Medline search was performed to identify data supporting clinical experience with AST-120 for managing CKD. Prospective open-label and double-blind trials as well as retrospective analyses were included. In prospective trials and retrospective analyses, AST-120 has been shown to prolong the time to initiation of hemodialysis, and slow decline in glomerular filtration rate and the increase serum creatinine. In an initial randomized, double-blind, placebo-controlled trial in the United States, AST-120 was associated with a significant dose-dependent reduction in serum indoxyl sulfate levels and a decrease in uremia-related malaise. The Evaluating Prevention of Progression in CKD (EPPIC) trials, two double-blind, placebo-controlled trials undertaken in North America/Latin America and Europe, are evaluating the efficacy of AST-120 for preventing the progression of CKD. The results of the EPPIC trials will further define the role of AST-120 in this debilitating condition.
Keywords: AST-120, chronic kidney disease, hemodialysis, indoxyl sulfate, uremic toxin
Introduction
For many patients, chronic kidney disease (CKD) is a progressive condition marked by deteriorating renal function ultimately leading to end-stage renal disease (ESRD). CKD is reported to affect 15.1% of the population and is associated with a substantial disease burden as evidenced by increased morbidity and mortality and reduced quality of life.1–4 Treatments to slow or prevent the progression of CKD have focused primarily on the management of the comorbidities leading to CKD, including hypertension and diabetes, as well as measures to reduce proteinuria and progressive glomerular and interstitial fibrosis with inhibitors of the renin–angiotensin–aldosterone system.5 However, other treatments (such as inhibitors of profibrotic cytokines and oxidative stress) that directly target the underlying pathophysiology that contributes to the progressive decline in renal function in patients with CKD are in development. In addition, preventing inadequate renal clearance subsequent to an initial insult and resulting nephron loss would avoid an overall increase of retained circulating uremic toxins.
The accumulation of uremic toxins, such as indoxyl sulfate and p-cresyl sulfate, is implicated in the progression of renal failure and cardiovascular disease.6,7 Data indicate that serum indoxyl sulfate levels are markedly increased in patients with renal disease and are related to disease severity.7,8 For example, a trial involving 139 patients at various stages of CKD found that indoxyl sulfate levels were inversely related to renal function (Figure 1)8 and directly related to aortic calcification and pulse wave velocity.8 Furthermore, elevated indoxyl sulfate levels were associated with increased mortality, even after adjustment for multiple variables (age, albumin and hemoglobin levels, aortic calcification, gender, diabetes status, phosphate levels, and pulse wave velocity).8
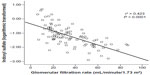  | Figure 1 Relationship between serum indoxyl sulfate levels and estimated glomerular filtration rate in 95 patients with chronic kidney disease stage 2–5.8 |
Elevated uremic toxins, such as indoxyl sulfate, promote the progression of CKD via a cycle of toxic accumulation and nephron loss.6 Organic anion transporters in the proximal tubule permit indoxyl sulfate uptake where it stimulates transforming growth factor beta 1, a profibrotic cytokine, in the renal parenchyma, ultimately leading to glomerulosclerosis and interstitial fibrosis.6 Indoxyl sulfate also induces oxidative stress via the promotion of free radical production, and the reduction of superoxide scavenging activity, resulting in tubular cell injury.6 Further pathways involved in tubular damage and deterioration of kidney function are: genetically mediated intracellular inflammation;9 epithelial-to-mesenchymal transition resulting in kidney fibrosis,10–13 which might be linked to stimulation of the renin–angiotensin–aldosterone system;10,14 activation of nuclear factor kappa-B and monocyte chemoattractant protein-1;15,16 and decreased Klotho expression.17,18 Given this role of uremic toxins in the pathophysiology of CKD progression, therapeutic approaches that decrease the level of uremic toxins are a rational method for inhibiting this progression. In this article, the clinical data supporting the role of AST-120 for slowing the progression of CKD will be reviewed.
Background on AST-120
AST-120 is an oral intestinal spherical carbon adsorbent consisting of porous carbon particles that are 0.2–0.4 mm in diameter and insoluble in water and common organic solvents.19 It adsorbs indole, a precursor of indoxyl sulfate derived from the metabolism of tryptophan by bacteria within the gastrointestinal tract, thereby attenuating indoxyl sulfate accumulation in patients with CKD.19 It differs from activated charcoal in its uniform composition, and it has a lower adsorption ability for amylase, pepsin, lipase, and chymotrypsin than charcoal.20
AST-120 decreases serum indoxyl sulfate levels in both dialyzed and undialyzed patients with CKD. In hemodialyzed patients with elevated levels of serum indoxyl sulfate (n = 26), administration of AST-120 (6 g/day) decreased serum indoxyl sulfate levels relative to untreated control patients at weeks 2, 4, 8, and 12 of the study (P < 0.05).19 In 22 undialyzed patients with chronic renal failure, 1 month of treatment with AST-120 (6 g/day) resulted in a reduction from baseline in both serum indoxyl sulfate (from 2.52 ± 0.43 mg/dL to 1.55 ± 0.33 mg/dL; P < 0.01 versus baseline) and urine indoxyl sulfate (from 75.3 ± 11.0 mg/day to 40.8 ± 7.2 mg/day; P < 0.01 versus baseline).21 In this study, there was no control group.21 In another trial, in 25 undialyzed patients with chronic renal failure, 6 months of AST-120 (6 g/day) resulted in a significant reduction from baseline of both serum and urine indoxyl sulfate (from 2.02 ± 0.28 mg/dL to 1.70 ± 0.35 mg/dL [P < 0.05 versus baseline] and from 66.8 ± 6.2 mg/day to 43.4 ± 9.2 mg/day [P < 0.05 versus baseline and control patients], respectively).22 This effect was evident within 1 month of treatment (P < 0.05).22 In contrast, serum and urine indoxyl sulfate levels were unchanged from baseline among control patients during the 6-month treatment period.22
AST-120 was approved in Japan in 1991 for prolonging the time to initiation of hemodialysis therapy and for improvement of uremic symptoms in patients with CKD. AST-120 was subsequently approved in Korea (2005) and the Philippines (2010) for prolonging the time to initiation of hemodialysis and for improvement of uremic symptoms in patients with CKD. Currently AST-120 is undergoing Phase III evaluation in North America/Latin America and Europe for the prevention of CKD progression.
Clinical data for AST-120 in patients with CKD
Prospective clinical trials
AST-120 has been evaluated in patients with CKD in a number of prospective clinical trials over the last 20 years (Table 1).23–28 In an open-label, randomized trial evaluating the effect of AST-120 (6 g/day) on glomerular filtration rate (GFR) directly measured by iothalamate clearance, patients with GFR of 20–70 mL/minute were enrolled in a 12-month observation period (n = 43) during which patients received dietary and blood pressure (BP) management.23 Patients whose GFR declined by ≥5 mL/minute during the observation period (n = 27) were eligible for inclusion in a 12-month treatment period. Thirteen patients continued receiving dietary and BP management alone (control group), while 14 patients added AST-120 to their regimen (AST-120 group). The mean change in GFR was not significantly different between the AST-120 and the control group following 12 months of treatment (primary outcome). However, when the mean change in GFR was compared between the treatment and observation periods, the rate of decline in GFR was significantly lowered in the AST-120 group (0.12 ± 0.15 mL/minute/month versus −1.11 ± 0.13 mL/minute/month in the treatment and observation periods, respectively; P < 0.001), but not in the control group (−0.34 ± 0.33 mL/minute/month versus −1.33 ± 0.22 mL/minute/month).23
Another trial evaluated the effect of treatment with AST-120 on progression of CKD as measured by changes in proteinuria, serum creatinine (sCr), and estimated GFR (eGFR) in 50 patients with chronic renal failure.24 Patients were assigned, without randomization, to either AST-120 (6 g/day) in addition to conventional therapy with antihypertensive, antihyperlipidemic, and antiplatelet agents or conventional therapy alone (control group). After 12 months, AST-120 treatment inhibited the increase in sCr and reduced proteinuria, urinary 8-hydroxydeoxyguanosine, urinary L-fatty acid-binding protein, and serum interleukin-6 levels compared with the control (P < 0.01 for all). However, the difference in sCr between AST-120-treated patients and control patients was only 0.4 mg/dL after 12 months of treatment. Furthermore, the addition of AST-120 did not significantly affect the decline in eGFR compared with control patients following 12 months of treatment.24
An open-label, randomized trial evaluated the efficacy of the addition of AST-120 (6 g/day) to conventional therapy in patients with early-stage diabetic nephropathy (n = 16).25 At baseline, the AST-120 and control groups were comparable with regard to clinical characteristics. After 12 months of treatment with AST-120, there was a significant reduction in sCr and urinary indoxyl sulfate levels in the AST-120 group compared with the control group (P < 0.001 and P < 0.01, respectively). These effects correlated to seven patients in the control group experiencing an increase in sCr > 2 mg/dL compared with only one patient in the AST-120 group. Furthermore, four patients in the control group initiated hemodialysis during this trial compared with only one patient in the AST-120 group. Overall, these results support the beneficial effects of AST-120 with regard to preserving renal function specifically in patients with early-stage diabetic nephropathy.25
The Carbonaceous Oral Adsorbent’s Effects on Progression of CKD (CAP-KD) study was a randomized trial conducted to evaluate the efficacy and safety of AST-120 in 460 patients with CKD (sCr < 5.0 mg/dL).26 Patients were randomized to AST-120 (6 g/day) in addition to conventional treatment (low-protein diet plus angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker) or conventional treatment alone.26 The primary composite endpoint was doubling of sCr, increase in sCr to ≥6.0 mg/dL, ESRD (need for hemodialysis or transplantation), or death. After 56 weeks of treatment, there was no difference between the AST-120 and control group with regard to the primary endpoint (42 patients in the AST-120 group versus 43 in the control group). However, the eGFR declined significantly less in the AST-120 group compared with the control group (P < 0.001; Figure 2). Further, the estimated creatinine clearance (CCr) decreased significantly less relative to baseline in the AST-120 group (−0.12 mL/minute/day) compared with the conventional treatment group (−0.15 mL/minute/day; P = 0.001).26
  | Figure 2 Estimated glomerular filtration rate in patients with chronic kidney disease receiving AST-120 6 g/day plus conventional treatment (n = 231) or conventional treatment alone (n = 229) for 56 weeks.26 |
In North America, a small double-blind, placebo-controlled, cross-over trial evaluated the effect of AST-120 on markers of renal function in patients with mild stable CKD (sCr 1.5–6.0 mg/dL).27 Patients (n = 20) received AST-120 (9 g/day) or placebo for 7 days followed by a 9-day washout period before switching to the alternative treatment. Throughout the study, patients followed a controlled diet. After 7 days of treatment, no significant difference in sCr, 24-hour urine creatinine, or CCr was noted between the AST-120 and placebo groups. These data suggest that AST-120 has no acute impact on creatinine balance, which supports the use of these parameters as acceptable measures of assessing renal function in patients receiving AST-120.27
A larger, double-blind, placebo-controlled trial evaluated the effect of various doses of AST-120 (2.7, 6.3, and 9.0 g/day) on serum indoxyl sulfate levels following 12 weeks of treatment in 157 patients with moderate to severe CKD.28 At baseline, patients had an sCr level of 3.0–6.0 mg/dL, a serum indoxyl sulfate level ≥ 0.50 mg/dL, and were following an adequate protein-intake diet. The primary endpoint was the change in serum indoxyl sulfate level from baseline with secondary endpoints including assessments of renal function (sCr levels, 1/sCr, proteinuria, creatinine excretion, and CCr) and symptoms of uremia (malaise, nausea, anorexia, pruritus, halitosis, or neuropathy). Indoxyl sulfate levels were reduced in a dose-dependent manner following 12 weeks of treatment with AST-120 (Figure 3). The change in indoxyl sulfate was significant versus baseline and placebo (P < 0.05) for the AST-120 6.3 and 9.0 g/day groups.28 During the 12-week trial, there were no significant changes in measures of renal function although patients in the AST-120 6.3 and 9.0 g/day groups experienced a dose-dependent decrease in malaise compared with placebo.28
  | Figure 3 Mean change from baseline in serum indoxyl sulfate level in patients (n = 154) receiving AST-120 dose of 2.7, 6.3, or 9.0 g/day, or placebo (P).28 |
Retrospective analyses
Several retrospective analyses have been performed, primarily in Japan, of patients with varying stages of CKD.29–33 One matched-control analysis followed 112 patients with CKD until initiation of hemodialysis.29 The effect of AST-120 treatment on the progression of CKD and initiation of hemodialysis was evaluated by retrospectively comparing the rate of eGFR reduction before and after initiation of AST-120 treatment (baseline). At baseline, proteinuria was higher and serum albumin was lower in the control group; however, an adjustment was made in the analysis for the degree of proteinuria. Prior to baseline, there was no significant difference between the AST-120 and control group in the rate of eGFR reduction. However, the rate of reduction in eGFR was significantly decreased in the AST-120 group (from −1.041 ± 1.177 mL/minute/month [before baseline] to −0.338 ± 0.317 mL/minute/month [after baseline]; P < 0.001).29 In contrast, there was no significant difference in the control group in the rate of eGFR before (−0.722 ± 0.885 mL/minute/month) and after baseline (−0.859 ± 0.978 mL/minute/month). After 24 months, the percentage of patients who had initiated hemodialysis was significantly lower in the AST-120 group compared with the control group (64.3% versus 94.5%; P < 0.001).29
Another retrospective pairwise-matching analysis evaluated the efficacy of AST-120 (6 g/day) plus conventional therapy for slowing the progression of CKD among patients admitted to hospital for CKD and later initiated on hemodialysis.30 Patients receiving AST-120 in combination with conventional therapy (n = 78) were compared with a control group (n = 78) who received conventional therapy alone. After 24 months, the hemodialysis-free rate was significantly higher in the AST-120 group relative to the control group (21.8% versus 1.3%, respectively; P < 0.001). The 50% hemodialysis-free period was also longer for the AST-120 group compared with the control group (9.0 months versus 4.1 months). Similar benefits of AST-120 with regard to hemodialysis-free rate and 50% hemodialysis-free period were observed in a subanalysis of diabetic versus nondiabetic patients. Furthermore, benefits were evident at early stages of renal disease: among patients with baseline sCr < 3 mg/dL, the 24-month hemodialysis-free period was significantly greater in the AST-120 group compared with the control group (57.1% versus 7.7%, respectively; P ≤ 0.001).30 More than 50% of the patients in the sCr < 3 mg/dL subgroup who were treated with AST-120 remained hemodialysis-free after 24 months. In contrast, 50% of the patients in the control group had initiated hemodialysis by 16.2 months.30 These results suggest that the addition of AST-120 to conventional therapy early in the treatment of CKD is beneficial for reducing disease progression. However, the criteria for initiating hemodialysis were not strictly defined and therefore may have been prone to bias. In addition, the proportion of patients with a baseline sCr level < 3 mg/dL ending on hemodialysis after 2 years was relatively high (close to 50%).30
Another analysis evaluated whether treatment with AST-120 prior to initiation of hemodialysis influences disease prognosis after initiation of hemodialysis.31 This analysis compared survival and cause of death between 101 patients who received AST-120 compared with 91 patients who did not. The 5-year survival rate in the AST-120 group was 72.6% compared with 52.6% in the control group (P = 0.018), corresponding to a 1.9-fold greater risk of death in the control group. Furthermore, the total number of deaths was significantly decreased in the AST-120 group compared with the control group (22 versus 32; P = 0.036).31
In a retrospective analysis of 100 patients with CKD who had not previously undergone hemodialysis, 12 months of treatment with AST-120 (6 g/day) improved the mean 1/sCr slope from −0.012 ± 0.013 before treatment to −0.006 ± 0.006 after treatment (P < 0.001).32 Benefit was observed regardless of the primary cause of renal dysfunction, age, or baseline sCr, and the greatest benefit was observed among patients who were treated with AST-120 for the longest duration.32 A potential bias of uncontrolled studies that are based on sCr or 1/sCr slope is that the seeming improvement in renal function during the course of the observation period may be due as much to loss of muscle mass as to preserved renal function.
Recently, a large, retrospective pair-matched analysis was conducted in 560 patients stratified according to whether or not they received AST-120 prior to initiation of hemodialysis.33 Overall, there was a significant difference in the 12-month and 24-month hemodialysis-free rate favoring the AST-120 group over the control group (25.0% versus 10.5%, respectively [12-month] and 13.7% versus 5.7%, respectively [24-month]; P < 0.001 for both). In addition, subgroup analyses showed that the 12-month and 24-month hemodialysis-free rate was higher in the AST-120 group irrespective of the presence of diabetic nephropathy or cardiovascular disease. However, comparison of 3-year, 5-year, and 10-year survival rates found no difference in overall survival between patients treated with AST-120 and those who were not treated with AST-120.33
Safety and tolerability of AST-120
Most adverse events associated with AST-120 treatment are, as expected, related to gastrointestinal function (eg, constipation, abdominal distension, nausea, flatulence, and diarrhea) and primarily mild to moderate in severity.26–28 Overall, the number and type of nongastrointestinal adverse events reported in placebo-controlled trials were similar between the AST-120 and control groups.26,28 One trial reported that AST-120 had no significant effect on the absorption of fat-soluble vitamins (ie, vitamins D and K).28 Discontinuation of treatment due to adverse events was reported in one trial (for both AST-120 and placebo patients),28 but other trials reported no discontinuations due to adverse events related to AST-120.23,27
Limitations and future directions
There are several limitations to the trials conducted to date. Open-label studies and retrospective analyses have the methodological deficiencies of non-blinding and/or lack of a placebo control. The primary limitation of the largest randomized, double-blind trial conducted in Japan (CAP-KD) was that only a small proportion of patients in either group reached the primary endpoint.26 Other limitations of CAP-KD included a short duration, a lower than expected percentage of patients with diabetic nephropathy, and a low percentage of patients with severe kidney disease.26 Furthermore, the initial randomized, double-blind trial conducted in the United States was not designed to assess clinical outcomes, as the primary endpoint was the change from baseline in serum indoxyl sulfate levels.28
A limitation of AST-120 treatment is the need for 30 pills daily. Unfortunately, compliance was not reported in most of the clinical trials that evaluated the efficacy and safety of AST-120. Therefore, it is difficult to ascertain the implications for treatment compliance resulting from the significant pill burden associated with AST-120. Patient education may be important to ensure compliance with AST-120 treatment in clinical practice.
Based on the promising results of initial trials and the need for more definitive clinical outcome data, large clinical trials have been initiated in North America/Latin America and Europe to evaluate the efficacy of AST-120 for the prevention of CKD progression. The Evaluating Prevention of Progression in CKD (EPPIC) trials are two double-blind, placebo-controlled trials to evaluate the efficacy of AST-120 for preventing progression of CKD in patients with moderate to severe disease who are not anticipated to require hemodialysis or renal transplant within 6 months of study entry. Patients were randomized to AST-120 (9 g/day), requiring ingestion of 30 capsules/day, or placebo. The primary endpoint of the EPPIC trials is the time to the triple composite endpoint of a doubling of sCr, initiation of hemodialysis, or kidney transplant. Over 2000 subjects have been enrolled and the data are currently being analyzed.
Conclusion
AST-120 has a long history in Japan and Asia for use in patients with CKD to prolong the time to initiation of hemodialysis and to improve uremic symptoms. The efficacy of the drug is supported by the results of clinical trials that demonstrate that AST-120 decreases serum indoxyl sulfate in a dose-dependent fashion, ameliorates the decline in renal function, and improves at least some symptoms of uremia. Data from ongoing clinical trials, particularly the EPPIC trials, are expected in late 2012 and will help clarify the role of AST-120 for slowing the progression of CKD.
Acknowledgments
Editorial assistance provided by ApotheCom (Yardley, PA, USA) was funded by Mitsubishi Tanabe Pharma Corporation.
Disclosure
Gerald Schulman has received research grants from Chromagen, Keryx, KUREHA Corporation, and Outsuka; has received consulting fees from KUREHA Corporation and Mitsubishi Tanabe Pharma Corporation; has received royalties from KUREHA Corporation; and is on the board of directors for Network 8 and the Tennessee Kidney Foundation. Raymond Vanholder has received research grants from Amgen, Baxter Health Care, Fresenius Medical Care, Fujisawa, Gambro, and Hoffman La Roche; has received consulting fees from Baxter Health Care, Bellco, Johnson and Johnson, and Mitsubishi Tanabe Pharma Corporation; and is the president of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA. Toshimitsu Niwa has no relationships to disclose. The authors report no other conflicts of interest in this work.
References
Mujais SK, Story K, Brouillette J, et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4(8):1293–1301. | |
US Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. | |
Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. | |
Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N; European Uremic Toxin Work Group. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20(6):1048–1056. | |
KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–S154. | |
Niwa T. Indoxyl sulfate is a nephro-vascular toxin. J Ren Nutr. 2010;20(Suppl 5):S2–S6. | |
Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med. 1994; 124(1):96–104. | |
Barreto FC, Barreto DV, Liabeuf S, et al; European Uremic Toxin Work Group (EUTox). Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(10):1551–1558. | |
Sun CY, Hsu HH, Wu MS. p-Cresol sulfate and indoxyl sulfate induce similar cellular inflammatory gene expressions in cultured proximal renal tubular cells. Nephrol Dial Transplant. 2013;28(1):70–78. | |
Sun CY, Chang SC, Wu MS. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS One. 2012;7(3):e34026. | |
Kim SH, Yu MA, Ryu ES, Jang YH, Kang DH. Indoxyl sulfate-induced epithelial-to-mesenchymal transition and apoptosis of renal tubular cells as novel mechanisms of progression of renal disease. Lab Invest. 2012;92(4):488–498. | |
Bolati D, Shimizu H, Higashiyama Y, Nishijima F, Niwa T. Indoxyl sulfate induces epithelial-to-mesenchymal transition in rat kidneys and human proximal tubular cells. Am J Nephrol. 2011;34(4):318–323. | |
Bolati D, Shimizu H, Niwa T. AST-120 ameliorates epithelial-to-mesenchymal transition and interstitial fibrosis in the kidneys of chronic kidney disease rats. J Ren Nutr. 2012;22(1):176–180. | |
Shimizu H, Hirose Y, Goto S, et al. Indoxyl sulfate enhances angiotensin II signaling through upregulation of epidermal growth factor receptor expression in vascular smooth muscle cells. Life Sci. 2012;91(5–6):172–177. | |
Shimizu H, Bolati D, Adijiang A, et al. NF-κB plays an important role in indoxyl sulfate-induced cellular senescence, fibrotic gene expression and inhibition of proliferation in proximal tubular cells. Am J Physiol Cell Physiol. 2011;301(5):C1201–C1212. | |
Masai N, Tatebe J, Yoshino G, Morita T. Indoxyl sulfate stimulates monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells by inducing oxidative stress through activation of the NADPH oxidase-nuclear factor-κB. Circ J. 2010;74(10):2216–2224. | |
Shimizu H, Bolati D, Adijiang A, et al. Indoxyl sulfate downregulates renal expression of Klotho through production of ROS and activation of nuclear factor-kB. Am J Nephrol. 2011;33(4):319–324. | |
Sun CY, Chang SC, Wu MS. Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int. 2012;81(7):640–650. | |
Niwa T, Emoto Y, Maeda K, Uehara Y, Yamada N, Shibata M. Oral sorbent suppresses accumulation of albumin-bound indoxyl sulphate in serum of haemodialysis patients. Nephrol Dial Transplant. 1991;6(2):105–109. | |
Kanai F, Takahama T, Yamazaki Z, Idezuki Y, Koide K. Effects of oral adsorbent on experimental uremic rats. Nihon Jinzo Gakkai Shi. 1986;28(9):1249–1259. | |
Niwa T, Tsukushi S, Ise M, et al. Indoxyl sulfate and progression of renal failure: effects of a low-protein diet and oral sorbent on indoxyl sulfate production in uremic rats and undialyzed uremic patients. Miner Electrolyte Metab. 1997;23(3–6):179–184. | |
Niwa T, Nomura T, Sugiyama S, Miyazaki T, Tsukushi S, Tsutsui S. The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int Suppl. 1997;62:S23–S28. | |
Shoji T, Wada A, Inoue K, et al. Prospective randomized study evaluating the efficacy of the spherical adsorptive carbon AST-120 in chronic kidney disease patients with moderate decrease in renal function. Nephron Clin Pract. 2007;105(3):c99–c107. | |
Nakamura T, Sato E, Fujiwara N, et al. Oral adsorbent AST-120 ameliorates tubular injury in chronic renal failure patients by reducing proteinuria and oxidative stress generation. Metabolism. 2011;60(2):260–264. | |
Konishi K, Nakano S, Tsuda S, Nakagawa A, Kigoshi T, Koya D. AST-120 (Kremezin) initiated in early stage chronic kidney disease stunts the progression of renal dysfunction in type 2 diabetic subjects. Diabetes Res Clin Pract. 2008;81(3):310–315. | |
Akizawa T, Asano Y, Morita S, et al; CAP-KD Study Group. Effect of a carbonaceous oral adsorbent on the progression of CKD: a multicenter, randomized, controlled trial. Am J Kidney Dis. 2009;54(3):459–467. | |
Marier JF, Lee J, Kambhampati SR, et al. Effect of repeated oral administrations of the oral adsorbent AST-120 on serum creatinine and other markers of renal function. A randomized controlled study in patients with chronic kidney disease. Am J Nephrol. 2006;26(2):136–141. | |
Schulman G, Agarwal R, Acharya M, Berl T, Blumenthal S, Kopyt N. A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am J Kidney Dis. 2006;47(4):565–577. | |
Maeda K, Hamada C, Hayashi T, et al. Efficacy of adsorbent in delaying dialysis initiation among chronic kidney disease patients. Dial Transplant. 2011;40(5):212–216. | |
Ueda H, Shibahara N, Takagi S, Inoue T, Katsuoka Y. AST-120, an oral adsorbent, delays the initiation of dialysis in patients with chronic kidney diseases. Ther Apher Dial. 2007;11(3):189–195. | |
Ueda H, Shibahara N, Takagi S, Inoue T, Katsuoka Y. AST-120 treatment in pre-dialysis period affects the prognosis in patients on hemodialysis. Ren Fail. 2008;30(9):856–860. | |
Maeda K, Hamada C, Hayashi T, et al. Long-term effects of the oral adsorbent, AST-120, in patients with chronic renal failure. J Int Med Res. 2009;37(1):205–213. | |
Hatakeyama S, Yamamoto H, Okamoto A, et al. Effect of an oral adsorbent, AST-120, on dialysis initiation and survival in patients with chronic kidney disease. Int J Nephrol. 2012;2012:376128. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

