Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Associations of TCF7L2 rs11196218 (A/G) and GLP-1R rs761386 (C/T) Gene Polymorphisms with Obesity in Chinese Population
Authors Xu T , Liu M, Liu Q, Wang B, Wang M , Qu M, Chen X, Wu J
Received 8 March 2021
Accepted for publication 16 May 2021
Published 1 June 2021 Volume 2021:14 Pages 2465—2472
DOI https://doi.org/10.2147/DMSO.S310069
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Ming-Hui Zou
Tiantian Xu,1 Mengmeng Liu,1 Qingjing Liu,1 Bian Wang,1 Min Wang,1,2 Minli Qu,1 Xin Chen,1 Jing Wu1,2
1Department of Endocrinology, Xiangya Hospital, Central South University, Changsha, People’s Republic of China; 2Hunan Engineering Research Center for Obesity and its Metabolic Complications, Xiangya Hospital, Central South University, Changsha, People’s Republic of China
Correspondence: Jing Wu
Department of Endocrinology, Xiangya Hospital, Central South University, Changsha, People’s Republic of China
Tel +86 13574120508
Email [email protected]
Introduction: This study aimed to investigate the genetic polymorphism associations with obesity of the transcription factor 7-like 2 (TCF7L2) gene rs11196218 (A/G) and glucagon-like peptide 1 receptor (GLP1-R) gene rs761386 (C/T) in the Chinese population.
Patients and Methods: This was a case–control pilot study involving 60 patients with obesity and 69 non-obesity Chinese adults, and the two groups were sex and age matched. Anthropometric indices of obesity, fasting blood glucose, blood pressure, and blood lipids were assessed. Both polymorphisms were genotyped using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF).
Results: There were significant differences in the allelic frequencies of the TCF7L2 rs11196218 and GLP1-R rs761386 between obesity and non-obesity groups (P = 0.003, OR = 2.32, 95% CI [1.31∼ 4.09]; P = 0.034, OR = 1.94, 95% CI [1.05∼ 3.60], respectively). In allele model, the genotypic frequencies of TCF7L2 rs11196218 and GLP1-R rs761386 also differed between obesity and non-obesity groups (P = 0.014 and 0.033, respectively). In dominant model, the TCF7L2 rs11196218 A-carrier (AA/AG) had a higher risk of obesity than GG genotype (P = 0.014, OR = 2.54, 95% CI [1.21∼ 5.35]). Comparison of clinical and biochemical parameters between genotypes showed no significant difference.
Conclusion: These findings suggest that the rs11196218 (A/G) polymorphism of the TCF7L2 gene and the rs761386 (C/T) polymorphism of the GLP1-R gene were associated with obesity in the Chinese population.
Keywords: obesity, genetic association, TCF7L2, GLP1-R, polymorphism, Chinese
Introduction
According to the World Health Organization (WHO), obesity is a medical condition where excess body fat has accumulated to an extent that may increase an individual’s health risks.1 The prevalence of obesity is high and ever-increasing worldwide – nearly 40% of adults were overweight and 10–15% were obese according to a report in 2016.2 It has become a public health problem, causing severe negative effects on personal health and social development.3–5 Obesity is a multifactor disease that is determined by both environmental and hereditary factors.6,7 Simulation studies have suggested that SNPs account for around 30% of variance in BMI, indicating that SNP polymorphism is one of the hereditary factors that needs further study.8 There is some evidence showing that the SNPs could have considerable biological effects. For instance, FTO SNPs variance could affect the gene expression by physically contacting the promoter or disrupting the binding sites of other genes, leading to the shifts of cell fates – from energy-burning beige adipocytes to energy-storing white adipocytes.9 However, for many obesity-related genes, the associations between SNPs polymorphism and obesity are still paradoxical and insufficient, which also hampered the functional characterization of genetic associations.10 This study mainly focuses on two genes: transcription factor 7-like 2 (TCF7L2) and glucagon-like peptide 1 receptor (GLP1-R).
TCF7L2 is a gene that encodes a high-mobility group (HMG) box-containing transcription factor that plays a key role in the Wnt signaling pathway. The protein has been implicated in blood glucose homeostasis. Several studies have found that genetic variants of this gene are associated with an increased risk of type 2 diabetes in Cameroonian and Danish individuals as well as gestational diabetes mellitus in Chinese individuals.11–14 In an Asian Indian population study, TCF7L2 polymorphism was also significantly associated with an increased risk of type 2 diabetes.15 Considering the strong connection between type 2 diabetes and obesity, the possible association between TCF7L2 and obesity has been explored.16,17 GLP1-R encodes a 7-transmembrane protein that functions as a receptor for glucagon-like peptide 1 (GLP-1) hormone, which stimulates glucose-induced insulin secretion. GLP1-R polymorphisms are associated with diabetes, while the associations between GLP1-R polymorphisms and obesity in different countries and ethnic groups vary a lot.18,19
Important advances have been made regarding the understanding of the genetic mechanisms underlying obesity in previous decades, but the research remains insufficient, especially in Chinese people, who comprise nearly 1/5 of the global population. To the best of our knowledge, there are no published data for the Chinese population on the roles of TCF7L2 or GLP1-R polymorphisms in obesity. This study aimed to investigate the associations of the TCF7L2 rs11196218 (A/G) and GLP-1R rs761386 (C/T) polymorphisms with obesity to improve our understanding of the effect of single-nucleotide polymorphisms (SNPs) on obesity in Chinese individuals.
Patients and Methods
Study Sample
We conducted a case–control study involving subjects of Chinese origin, aged 18 years old and above. The patients with obesity (body mass index [BMI] ≥28 kg/m2 and/or male WC≥90 cm, female WC≥85 cm) were recruited from the Outpatient Clinic and the non-obese controls (BMI 18.5–23.9 kg/m2) were recruited from the Physical Examination Center of Xiangya Hospital of Central South University. We only included patients who had a fasting blood glucose (FBG) level <5.6 mmol/L and without a history of diabetes. We finally invited 60 subjects with obesity and 69 normal-weight controls to participate in this study, all of whom agreed.
Clinical and Biochemical Data Collection and Genotyping
Clinical and biochemical data were collected. The data included sex, age, height, weight, BMI, waist-to-hip ratio (WHR), FBG, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), total triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). The TCF7L2 rs11196218 (A/G) and GLP-1R rs761386 (C/T) polymorphisms were genotyped using MassARRAY® MALDI-TOF System (Sequenom, Inc) after the PCR amplification.20 The primers used for rs11196218 are 5ʹ-ACGTTGGATGCTCTTAACCAACATGGCTTG-3ʹ and 5ʹ-ACGTTGGATGAATAAGTGTGCAAACGAGGG-3ʹ; the primers for rs761386 are 5ʹ-ACGTTGGATGGAGTGGCAGCTATGATAGGG-3ʹ and 5ʹ-ACGTTGGATGAGATGAGGAAGTTCACCTGC-3ʹ. The cycling parameters were 94 °C for 3 min; 40 cycles at 94 °C for 30 s, 56 °C for 25 s, 72 °C for 30 s; and a final extension step at 72 °C for 3 min. The detailed procedure is documented (https://www.gene-quantification.de/sequenom/).
Statistical Analysis
Statistical analysis was performed using SPSS 20.0, tests for deviation from the Hardy–Weinberg equilibrium as well as allelic and genotypic frequencies were performed with the online analysis tool SHEsis (http://analysis.bio-x.cn/myAnalysis.php).21 Continuous variables are expressed as mean ± standard deviation and categorical variables are expressed as frequencies and percentages. Differences in clinical and biological parameters were compared between groups using independent-sample t-tests (continuous variables) and chi-square tests (categorical variables). We adjusted confounding factors including sex and age in the regression analysis. The significance level was set at P-value < 0.05. Three different modes of inheritances (allele model, dominant model and recessive model) were analyzed. We performed the post hoc analysis of statistical power using the power calculator (https://clincalc.com/stats/Power.aspx).
Results
Clinical and Biochemical Characteristics of the Participants
We evaluated 129 Chinese participants, comprising 69 non-obese individuals and 60 patients with obesity (all of whom had a normal FBG level). Table 1 shows the clinical and biochemical characteristics of the subjects, comprising 78 women and 51 men. There were no significant differences between the two groups concerning sex composition, age and FBG (P> 0.05). BMI, WHR, SBP, DBP, TC, TG, and LDL-C were significantly higher in the obese group than in the non-obese group (P< 0.001). In contrast, HDL-C was significantly lower in the obese group compared to the non-obese group (P< 0.001).
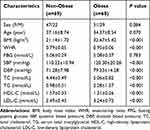 |
Table 1 Clinical and Biochemical Characteristics of the Studied Groups |
Allele and Genotype Distributions and Associations with Obesity
We performed the Hardy–Weinberg equilibrium test using the online tool SHEsis using the function “Single site analysis”. The allelic and genotypic frequencies of rs11196218 and rs761386 in the study sample, which are presented in Tables 2 and 3, were consistent with Hardy–Weinberg equilibrium (P>0.05), which means there were no other evolutionary forces (such as natural selection). Regarding the TCF7L2 rs11196218 polymorphism, the G allele was the major allele in the two groups (81.2% and 65.0%, respectively). There was a significantly higher proportion of A allele carriers in the obese group than the non-obese group (P=0.003), which indicates that the A allele of rs11196218 is probably a risk factor for obesity (OR=2.32, 95% CI [1.31~4.09]). Similarly, regarding the GLP1-R rs761386 polymorphism, the minor T allele was more frequent in the obese group compared with the non-obese group (P=0.034), which suggests that the T allele of rs761386 is a risk factor for obesity (OR=1.94, 95% CI [1.05~3.60]). Table 3 shows that the genotypic frequencies of rs11196218 and rs761386 between obesity and non-obesity group were also significantly different (χ2 = 8.558, P = 0.014 and χ2 = 6.795, P = 0.033, respectively). The frequency of TCF7L2 rs11196218 A-carrier AA and AG in the obesity group was higher than in the non-obesity group, while GG showed a lower frequency. Regarding the GLP1-R rs761386, the genotypic frequency of T-carrier, CT and TT, is higher in the obesity group than in the non-obesity group. These results are compatible with the allele difference showed in Table 2. To explore further, we studied the dominant and recessive model, separated the genotype into two groups for each SNP location, executed a binary logistic regression and took sex and age into count (Tables 4 and 5). For the dominant model, we compared TCF7L2 rs11196218 AA/AG with GG genotype, and GLP1R rs761386 CT/TT with TT genotype. In this regression, age, sex and genotype are independent variables, group (obesity or non-obesity) is the binary dependent variable. We found that in TCF7L2 rs11196218, genotype AA/AG had 2.54 times more risk of obesity (P = 0.014, OR = 2.54, 95% CI [1.21~5.35]) compared with GG. For GLP1-R rs761386, genotype CT/TT showed a marginal higher risk than CC group (P = 0.208, OR = 1.61, 95% CI [0.77~3.39]), but no statistic significance. For the recessive model, we compared TCF7L2 rs11196218 AA with AG/GG genotype, and GLP1-R rs761386 TT with CT/CC genotype. The results showed that for TCF7L2 rs11196218, the AA genotype had no significantly higher risk of obesity than the AG/GG group (P = 0.097, OR = 3.43, 95% CI [0.80~14.71]). Regarding the GLP1-R rs761386, there was no TT genotype in our study group; hence, it was unavailable to get meaningful results or make conclusions from the recessive model. Taken together, in the allele model, we found the difference of TCF7L2 rs11196218 and GLP1-R rs761386 polymorphisms between obesity and non-obesity groups; in the dominant model, TCF7L2 rs11196218 AA/AG showed a higher risk of obesity; there is no significant difference in the recessive model, potentially because of the limited sample size.
 |
Table 2 Hardy–Weinberg Equilibrium Test and Allelic Frequencies |
 |
Table 3 Genotypic Frequencies Between Non-Obese and Obese Group |
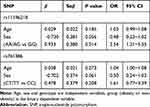 |
Table 4 Binary Logistic Regression Analysis of Obesity and Genotype – Dominant Model |
 |
Table 5 Binary Logistic Regression Analysis of Obesity and Genotype – Recessive Model |
Characteristics of Different Genotypes Associated with Obesity
Table 6 shows the comparisons of clinical and biochemical parameters between A allele carriers (AA/AG) and those with the GG genotype of TCF7L2 rs11196218, and between T allele carriers (CT/TT) and those with the CC genotype of GLP1-R rs761386. There was a significant difference in WHR between rs11196218 A allele carriers and those with the GG genotype. However, after the adjustment of sex and age, which were considered confounders, no significant difference was observed in these clinical characteristics (P>0.05) between the two groups.
 |
Table 6 Comparison of Clinical and Biological Parameters Between Genotypes |
Discussion
Although there are some studies on the effects of TCF7L2 and GLP1-R gene polymorphisms on obesity, the SNP loci that have been studied are still sparse, and there are no data about Chinese people. Moreover, the associations between these genetic polymorphisms and obesity vary among different research, the reason contributing to this may be the ethical groups, sex, age, and additional modulation by diabetes.22 Our study provides the first insight into the roles of the TCF7L2 rs11196218 and GLP1-R rs761386 polymorphisms in obesity in Chinese individuals, taking the confounders such as sex, age and blood glucose into consideration. We found that the TCF7L2 rs11196218 and GLP1-R rs761386 variants were associated with obesity.
In our study, we eliminated the interference of blood glucose in obese people on the results, the FBG level was normal in both obesity and non-obesity groups (Table 1). We also adjusted the effect of sex and age by logistic regression analysis. Thereafter, we found that there were significant differences in TCF7L2 and GLP1-R allele frequency and genotypic polymorphisms between obese and non-obese people, which can be seen in Tables 2–3. In allele model (Table 3), with a two-sided significance level of 0.05 and frequency of the TCF7L2 rs11196218 GG genotype of 66.7% in the non-obesity group and 41.7% in the obesity group, the power to detect the association of rs11196218 polymorphism and obesity reached 82.0%. For the GLP1-R rs761386, with the frequency of the rs761386 CC genotype of 69.6% in the non-obesity group and 56.7% in the obesity group, the power was 32.9%, which may lead to a false negative. In summary, the significant differences in the gene polymorphisms in our study are convincing, while the non-significant results could be false. The binary logic regression analysis indicated that in the recessive model, TCF7L2 rs11196218-A was associated with an increased risk of obesity in Chinese individuals. Furthermore, as shown in Table 6, we studied whether multiple clinical and biochemical characteristics were associated with the genotypes that predicted obesity, but no significant difference was observed.
TCF7L2 and GLP1-R encode proteins that are implicated in blood glucose homeostasis and glucose-induced insulin secretion. TCF7L2 and GLP1-R mechanistic studies suggested that TCF7L2 could impair β-cell function and down-regulate the expression of glucagon-like peptide 1 receptor (GLP-1R) and glucose-dependent insulinotropic polypeptide receptor (GIP-R), thus reducing insulin level.23 For the SNPs polymorphisms, recent studies have reported that variants in TCF7L2 (rs7903146) and WFS1 (rs10010131) could affect the response to exogenous GLP-1.24 One study showed that the presence of the T allele compared to the CC genotype in rs7903146 SNP of the TCF7L2 gene was associated with reduced fasting GV, suggesting that TCF7L2 is associated with altered gastric functions that may predispose to obesity.25 GLP-1R agonism enhances adjustable gastric banding in diet-induced obese rats and improves weight loss.26 However, there was no investigation of the associations between GLP1-R genetic polymorphisms and obesity or the mechanisms behind that. In our study, the significant SNPs (TCF7L2 rs11196218 and GLP1-R rs761386) were located within the intronic noncoding regions, and no previous studies have discovered any mechanisms of their actions, which awaits future investigation.
Obesity is affected by genetic profile and environmental risk factors, in which heritability is estimated to 40–70%.27 Previous studies of genetic associations with obesity suggested that many genes are related to obesity, including MC4R, BDNF, PCSK1, POMC, SH2B1, LEPR, NTRK2, FTO, IL-33, etc.9 Regarding TCF7L2 polymorphism, there was a study showing an absence of the association between rs12255372 variant and obesity in the Cameroonian population, as well as the European and American populations.16 Another study in a European population indicated that the TCF7L2 rs7903146 T allele was known to be a risk factor for type 2 diabetes, but not obesity.28 A meta-analysis about TCF7L2 rs11196218 suggested that there was an association between rs11196218 polymorphism and type 2 diabetes mellitus in the Asian population.23 Khan IA et al investigated six SNPs in six genes including TCF7L2, which was involved in β-cell dysfunction and insulin pathway, and they found that the TCF7L2 rs7903146 was associated with gestational diabetes in an Indian population.29 However, the detailed information on sex, age, anthropometric measurements and metabolic measurements lacked, so the interactive effects of these factors could not be adequately addressed.23 Another similar meta-analysis in the Chinese Han population did not show any association between the TCF7L2 gene rs11196218 A/G polymorphism and T2DM risk.30 As for GLP1-R gene polymorphism, a study in the European population revealed that rs2268641 in GLP1-R was significantly associated with BMI while rs9380825 is not.18,19 And there have not been studies about the GLP1-R rs761386 variant on obesity yet. Overall, the gene polymorphism findings are insufficient and controversial, which may be due to the ethical difference, study group and lack of consideration of confounding effects, such as obesity, sex and age. Therefore, to address the question of whether TCF7L2 rs11196218 is associated with obesity, it is important to have a well-matched obesity-specific gene polymorphism study and take confounders to count.
In summary, the study provides evidence and increases our understanding of the role of genetic polymorphisms in obesity. Our results suggest that the TCF7L2 rs11196218 and GLP1-R rs761386 gene polymorphisms are associated with obesity in the Chinese population.
This is the first study to evaluate the effect of TCF7L2 rs11196218 and GLP1-R rs761386 polymorphisms on obesity in the Chinese population. There are also several limitations. The number of SNP loci and the sample size may attenuate the power of our study. Since the genetic risk of obesity reflects the accumulation of multiple loci, each contributing a small portion of the total risk, large-scale screening of obesity-related gene candidates is required to better understand the genetic polymorphisms and the underlying mechanisms of genetic association in obesity. In addition, considering the connection between obesity and type 2 diabetes, longitudinal follow-up research would be helpful to understand the effect of these gene polymorphisms on the onset of type 2 diabetes and other metabolic diseases. In-depth studies with a larger scale and ethnic variation would help to elucidate the genetic associations with obesity in more detail.
Conclusion
Our study suggested that the allelic frequencies of TCF7L2 rs11196218 and GLP1-R rs761386 in allele model both differed in the obesity and non-obesity groups in the Chinese population. In the allele model and the dominant model, TCF7L2 rs11196218 A-carrier is a risk factor for obesity. In summary, the rs11196218 (A/G) polymorphism of the TCF7L2 gene and the rs761386 (C/T) polymorphism of the GLP1-R gene were associated with obesity in the Chinese population.
Statement of Ethics
This study was approved by the ethics committee of Xiangya Hospital of Central South University (No. 201601029) and was monitored by an independent Data and Safety Monitoring Board. All participants provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Funding
The study was financially supported by the Key Research & Development Plan, Hunan, China (2020SK2066) and the Project of Hunan Health Committee, Hunan, China (20201923).
Disclosure
The authors declare no conflicts of interest.
References
1. Schetz M, De Jong A, Deane AM, et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45(6):757–769. doi:10.1007/s00134-019-05594-1
2. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–1396. doi:10.1016/S0140-6736(16)30054-X
3. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi:10.1016/S0140-6736(14)60460-8
4. Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–567. doi:10.1016/S0140-6736(10)62037-5
5. Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Reprint of: healthy weight and obesity prevention: JACC health promotion series. J Am Coll Cardiol. 2018;72(23):3027–3052. doi:10.1016/j.jacc.2018.10.024
6. Reddon H, Gueant JL, Meyre D. The importance of gene-environment interactions in human obesity. Clin Sci (Lond). 2016;130:1571–1597.
7. Alharbi KK, Alshammary AF, Aljabri OS, Ali KI. Relationship between serum amyloid A1 (SAA1) gene polymorphisms studies with obesity in the Saudi Population. Diabetes Metab Syndr Obes. 2021;14:895–900. doi:10.2147/DMSO.S294948
8. Yang J, Bakshi A, Zhu Z, et al. Genetic variance estimation with imputed variants finds negligible missing heritability for human height and body mass index. Nat Genet. 2015;47(10):1114–1120. doi:10.1038/ng.3390
9. Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018;6(3):223–236. doi:10.1016/S2213-8587(17)30200-0
10. Yaghootkar H, Bancks MP, Jones SE, et al. Quantifying the extent to which index event biases influence large genetic association studies. Hum Mol Genet. 2017;26(5):1018–1030. doi:10.1093/hmg/ddw433
11. Guewo-Fokeng M, Sobngwi E, Atogho-Tiedeu B, et al. Contribution of the TCF7L2 rs7903146 (C/T) gene polymorphism to the susceptibility to type 2 diabetes mellitus in Cameroon. J Diabetes Metab Disord. 2015;14(1):26. doi:10.1186/s40200-015-0148-z
12. Wang K, Chen Q, Feng Y, et al. Single nucleotide polymorphisms in CDKAL1 gene are associated with risk of gestational diabetes mellitus in Chinese Population. J Diabetes Res. 2019;2019:3618103. doi:10.1155/2019/3618103
13. Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38(3):320–323. doi:10.1038/ng1732
14. Ye D, Fei Y, Ling Q, et al. Polymorphisms in TCF7L2 gene are associated with gestational diabetes mellitus in Chinese Han population. Sci Rep. 2016;6(1):30686. doi:10.1038/srep30686
15. Khan IA, Poornima S, Jahan P, Rao P, Hasan Q. Type 2 diabetes mellitus and the association of candidate genes in Asian Indian Population from Hyderabad, India. J Clin Diagn Res. 2015;9:C1–C5.
16. Ngwa EN, Sobngwi E, Atogho-Tiedeu B, et al. Association between the rs12255372 variant of the TCF7L2 gene and obesity in a Cameroonian population. BMC Res Notes. 2015;8(1):717. doi:10.1186/s13104-015-1661-3
17. Nguimmo-Metsadjio A, Atogho-Tiedeu B, Noubiap JJ, et al. Investigation of the association between the TCF7L2 rs7903146 (C/T) gene polymorphism and obesity in a Cameroonian population: a pilot study. J Health Popul Nutr. 2017;36(1):12. doi:10.1186/s41043-017-0087-z
18. Chung WK, Patki A, Matsuoka N, et al. Analysis of 30 genes (355 SNPS) related to energy homeostasis for association with adiposity in European-American and Yup’ik Eskimo populations. Hum Hered. 2009;67(3):193–205. doi:10.1159/000181158
19. Li P, Tiwari HK, Lin WY, et al. Genetic association analysis of 30 genes related to obesity in a European American population. Int J Obes (Lond). 2014;38(5):724–729. doi:10.1038/ijo.2013.140
20. Feucherolles M, Poppert S, Utzinger J, Becker SL. MALDI-TOF mass spectrometry as a diagnostic tool in human and veterinary helminthology: a systematic review. Parasit Vectors. 2019;12(1):245. doi:10.1186/s13071-019-3493-9
21. Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15(2):97–98. doi:10.1038/sj.cr.7290272
22. Corella D, Coltell O, Sorli JV, et al. Polymorphism of the transcription factor 7-Like 2 gene (TCF7L2) interacts with obesity on Type-2 diabetes in the PREDIMED Study emphasizing the heterogeneity of genetic variants in Type-2 diabetes risk prediction: time for obesity-specific genetic risk scores. Nutrients. 2016;8.
23. Zhai Y, Zhao J, You H, et al. Association of the rs11196218 polymorphism in TCF7L2 with type 2 diabetes mellitus in Asian population. Meta Gene. 2014;2:332–341. doi:10.1016/j.mgene.2014.04.006
24. Lin CH, Lee YS, Huang YY, Hsieh SH, Chen ZS, Tsai CN. Polymorphisms of GLP-1 receptor gene and response to GLP-1 analogue in patients with poorly controlled type 2 diabetes. J Diabetes Res. 2015;2015:176949. doi:10.1155/2015/176949
25. Vazquez-Roque MI, Camilleri M, Vella A, Carlson P, Laugen J, Zinsmeister AR. Association of TCF7L2 allelic variations with gastric function, satiation, and GLP-1 levels. Clin Transl Sci. 2011;4(3):183–187. doi:10.1111/j.1752-8062.2011.00284.x
26. Habegger KM, Kirchner H, Yi CX, et al. GLP-1R agonism enhances adjustable gastric banding in diet-induced obese rats. Diabetes. 2013;62(9):3261–3267. doi:10.2337/db13-0117
27. Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27(4):325–351. doi:10.1023/A:1025635913927
28. Cauchi S, Choquet H, Gutierrez-Aguilar R, et al. Effects of TCF7L2 Polymorphisms on Obesity in European Populations. Obesity (Silver Spring). 2008;16(2):476–482. doi:10.1038/oby.2007.77
29. Khan IA, Jahan P, Hasan Q, Rao P. Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. Diabetes Metab Syndr. 2019;13(1):688–694. doi:10.1016/j.dsx.2018.11.035
30. Ma E, Wang H, Guo J, Tian R, Wei L. The association between the rs11196218A/G polymorphism of the TCF7L2 gene and type 2 diabetes in the Chinese Han population: a meta-analysis. Clinics (Sao Paulo). 2015;70(8):593–599. doi:10.6061/clinics/2015(08)10
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
