Back to Journals » Nature and Science of Sleep » Volume 13
Associations of Sleep Insufficiency and Chronotype with Inflammatory Cytokines in College Students
Authors Zhai S, Tao S, Wu X, Zou L, Yang Y, Xie Y, Li T, Zhang D, Qu Y, Tao F
Received 17 July 2021
Accepted for publication 15 September 2021
Published 27 September 2021 Volume 2021:13 Pages 1675—1685
DOI https://doi.org/10.2147/NSS.S329894
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sarah L Appleton
Shuang Zhai,1 Shuman Tao,2,3 Xiaoyan Wu,1,3 Liwei Zou,1 Yajuan Yang,4 Yang Xie,1 Tingting Li,1 Dan Zhang,1 Yang Qu,1 Fangbiao Tao1,3
1Department of Maternal, Child & Adolescent Health, School of Public Health, Anhui Medical University, Hefei, People’s Republic of China; 2Department of Nephrology, The Second Hospital of Anhui Medical University, Hefei, People’s Republic of China; 3MOE Key Laboratory of Population Health Across Life Cycle/NHC Key Laboratory of Study on Abnormal Gametes and Reproductive Tract, Hefei, People’s Republic of China; 4School of Nursing, Anhui Medical University, Hefei, People’s Republic of China
Correspondence: Shuman Tao
Department of Nephrology, The Second Hospital of Anhui Medical University, Hefei, 230601, People’s Republic of China
Tel +86 15077927106
Email [email protected]
Purpose: Sleep insufficiency and circadian rhythm disturbances in college students have become prominent. Current findings show that sleep insufficiency is closely related to inflammation. Studies on the correlation between chronotype and inflammatory factors are still lacking. Therefore, this research intended to examine the relationships between sleep duration, chronotype and inflammatory cytokines in young adults, and to estimate the correlation between chronotype and inflammatory cytokines stratified by sleep duration.
Patients and Methods: We conducted a cross-sectional study in April and May 2019. Participants were recruited from two colleges located in central China. The Pittsburgh Sleep Quality Index (PSQI) and the Morning and Evening Questionnaire-5 (MEQ-5) were administered to assess sleep duration and chronotype. Sleep duration less than 7 hours was defined as insufficient sleep. Fasting venous blood was collected to measure plasma levels of inflammatory markers including IL-1β, IL-6, TNF-α and IL-10.
Results: A total of 723 participants were included in this study, with a mean age of 18.68 years (standard deviation=0.99). After adjusting for confounding factors, the results of generalized linear model showed that sleep insufficiency was positively correlated with IL-1β, TNF-α and IL-10; and evening-types (E-types) were positively associated with the levels of IL-1β and IL-6 (p< 0.05). Compared to the control group (sleep sufficiency and M-types), there were positive interaction effects of sleep insufficiency and neutral-types (N-types) on the levels of IL-1β, IL-6, TNF-α and IL-10 (p< 0.05). The hierarchical regression model showed that N-types and E-types were positively correlated to the levels of IL-1β, IL-6, TNF-α and IL-10 among college students with sleep insufficiency (p< 0.05).
Conclusion: The levels of inflammatory markers were higher among college students with sleep insufficiency and E-types. N-types and E-types were positively correlated with IL-1β, IL-6, TNF-α and IL-10 among college students with sleep insufficiency.
Keywords: sleep duration, circadian rhythm, chronic inflammation, young adults
Introduction
College life represents an important transition period for young adults characterized by increased social involvement. During this critical period, lifestyles acquired will affect the future life of young college students.1 Insufficient sleep is a serious public health problem in college students.2 It is generally considered that chronotype is one of an expression of circadian rhythm.3 The most common methods of classification used in chronotype is morning-types (M-types), neutral-types (N-types) and evening-types (E-types).4 M-types prefer to sleep and get up early, and it is most efficient to perform physical and intellectual activities in the morning; E-types prefer to sleep and get up late, and feel and perform better in the afternoon or evening; N-types display between these two types.5 Changes in chronotype are primarily characterized by delayed sleep phase syndrome in late adolescence.6 Several studies have demonstrated that circadian rhythm disorder also exists at surprising levels in the college students.7,8 Circadian rhythm regulation or adequate sleep is evidently essential for normal metabolic health.9 It has been found that unhealthy sleep habits adversely affect physical, cognitive, emotional and academic well-being.10 Specifically, sleep problems affect circadian hormonal profiles and inflammatory markers, and may damage executive function, attention and decision-making capacity.11
Like many biological processes, sleep is associated with immunity and inflammation and changes in circulating immune cells.11 Inflammation is a process by which the body responds to injury or infection.12 Cytokines are proved to be biomarkers of chronic low-grade inflammation which transmitting information from the immune system, regulating the inflammatory processes and mediating local and systemic reactions.13 Interleukin-10 (IL-10),14 interleukin-1β (IL-1β),15 interleukin-6 (IL-6),16 and tumor necrosis factor-α (TNF-α)17 are important cytokines involved in chronic inflammation. IL-10 is generally considered to be an anti-inflammatory cytokine, which can inhibit the production of pro-inflammatory markers.18 Sleep duration has been suggested to be related to an increased risk of cardiovascular morbidity, neurocognitive impairment, diabetes and mortality. Inflammation is considered to be one of the main associations between sleep duration or sleep disorders and these illnesses.10,19 Unfortunately, there are currently rare research on the relationship between chronotype and inflammatory factors.
Rarely, investigation on the interaction between sleep duration and chronotype, and limited substantive evidence on the interaction effects of them on inflammation in college students. Research reported that daytime sleepiness and E-types were related to self-regulation.20 Investigation has revealed that in adolescents with insomnia, shorter sleep duration was associated with increased levels of inflammation.21 What is more, a survey in the United States showed that the combined effects of adolescents’ sleep duration and quality were predictors of adolescents’ social mood adjustment.22 On the contrary, a Swedish study showed that there was no interaction between adolescents’ sleep duration and sleep problems on health.23
To be sure, it is imperative to examine the interaction between sleep duration and chronotype, which can promote the health and function of the entire life process. Therefore, a cross-sectional study was conducted to make inquiries about the relationships between sleep duration, chronotype and its interaction effects on inflammatory cytokines among college students in China.
Materials and Methods
Participants and Procedure
Participants were enrolled in the College Student Behavior and Health Cohort Study, which is an ongoing cohort designed to track behaviors, physical and mental health in college students from the first year to the third year in China. Baseline data was analyzed in the present study. Participants were selected from a medical university located in Hefei, Anhui Province and a distinguished federated university situated in Shangrao, Jiangxi Province between April and May 2019. Cluster random sampling was used, with the faculty as the primary sampling unit. The baseline survey consisted of electronic questionnaires scanned with smartphones and physical examination. We received a total of 1135 valid questionnaires that collected baseline information on sociodemographic and health-related factors, and the response rate was 98.6%. Blood samples were collected in 771 students. Finally, a total of 723 valid respondents both have survey data and blood samples were analyzed. The study protocol was approved by the Ethics Committee of Anhui Medical University (No. 20170291). Written informed consents were obtained from all participants before completing the survey, which was conducted in accordance with the principles of the Declaration of Helsinki.
Measures
Demographic Factors
Sociodemographic data were collected by questionnaire, including gender, age, height, weight, residential area (rural or urban), any siblings (yes or no), self-reported family economy (low, medium or high), self-rated health (high, medium or low), parental education level (primary school and below, middle school or senior high school and above).
Sleep Duration and Chronotype
Sleep duration during the past month was assessed by a question in the Pittsburgh Sleep Quality Index (PSQI): “During the past month, how many hours of actual sleep did you get at night?”.24 According to sleep time duration recommendations proposed by National Sleep Foundation of America, the appropriate sleep duration for young adults (18–25 years) is between 7 and 9 hours. Therefore, responses were categorized into two groups: less than 7 hours (sleep insufficiency) and 7 hours or more (sleep sufficiency).25 The Morning and Evening Questionnaire-5 (MEQ-5) was well validated and used as a self-reported measurement of chronotype.26 The MEQ-5 questionnaire comprised five items, which evaluated the habit of sleep among participants. In accordance with total scores (4 to 25 points), chronotype could be parted into three groups including evening-types (4 to 11 points), neutral-types (12 to 17 points), and morning-types (18 to 25 points).
Inflammatory Cytokines
Laboratory analyses were performed on blood samples to evaluate IL-1β, IL-6, TNF-α and IL-10, as systemic inflammatory markers. During the physical examination, 5 mL of fasting venous blood samples were collected on 6:00 to 8:00 in the morning using vacuum blood collection tube with anticoagulant (EDTA). The blood samples were centrifuged for 10 min at a set speed of 3000 revolutions per minute within 2 hours. The upper plasma samples were stored at −80°C. Liquid-phase protein suspension chip (Luminex) was used in the detection process. Plasma inflammatory markers were detected by multi-bead enzyme-free analyzer MILLIPLEX ® MAP instrument (Merck Millipore). Within the analysis of variation, the intra-assay coefficient of variation of IL-1β, IL-6, TNF-α and IL-10 were all <5%, and the inter-assay coefficient of variation were <15%, <20%, <15%, <20%, respectively. The assay had a lower detection limit of IL-1β, IL-6, TNF-α and IL-10 of 0.14 pg/mL, 0.11 pg/mL, 0.16 pg/mL, 0.56 pg/mL, respectively. All inflammatory markers were log transformed to settle skewness.
Covariates
Health behaviors included cigarette use, alcohol use, physical activity, afternoon napping and symptoms of depression. Two questions from the Young Risk Behavior Surveillance System questionnaire were modified to measure current cigarette and alcohol use.27 Cigarette use was measured by “How many days did you smoke during the past month?” Alcohol use was measured by “How many days did you have at least one drink during the past month?” The answers are recoded into “yes” or “no”. Physical activity was assessed using International Physical Activity Questionnaire Short Form (IPAQ-SF) was used to measure physical activity.28 Physical activity level was divided into 3 categories: low, moderate and high. Afternoon napping was measured by “Are you in the habit of taking afternoon napping?” and answers are recoded into “yes” or “no”.24 Symptoms of depression were measured by the Patient Health Questionnaire 9 (PHQ-9).29 PHQ-9 was used to screen for depressive symptoms during the last 2 weeks, which contains 9 items. The total score (0 to 27 points) was divided into a dichotomous variable with a critical value of 4 points.
Statistical Analyses
All statistical analyzes were conducted using SPSS version 23.0 (SPSS, Chicago, IL, USA). Continuous variables of the normal distribution in present study were represented as mean and standard deviation (SD), while categorical variables were summarized using frequencies (n) and percentages (%). Two independent sample t-test, and Chi-square tests were used to assess the demographic characteristics stratified by sleep duration. ANOVA was performed to assess the differences in levels of inflammatory factors between chronotype. The generalized linear models we performed were to investigate the relationships between sleep duration, chronotype and inflammatory factors. We also introduced the interactive terms of sleep duration * chronotype. Regression coefficients were measured for the explanatory factors, adjusting for age, self-rated health, physical activity, afternoon napping and depressive symptoms. Existing findings showed that E-types were characterized by late bedtimes and greater physical and mental activity in the evening and cause more sleep problems, worse sleep quality, and more behavioral problems compared to M-types. Therefore, the healthier group, M-types were recognized as the control group.30,31 All statistical tests were two-sided and significance was set at p<0.05.
Results
Participant Characteristics
Table 1 manifests the demographic descriptive information of 723 participants (32.9% males and 67.1% females) aged 16–26 years old (M=18.68, SD=0.99). Of the 723 participants, the proportion of sleep insufficiency were 56.2%. The proportions of M-types, N-types and E-types were 14.8%, 74.8% and 10.4% among college students. Nevertheless, no sex-based difference was found in sleep duration (p=0.955). There were no significant differences on BMI (p=0.080), residential area (p=0.202), any siblings (p=0.717), self-reported family economy (p=0.361), parental educational level (p=0.944; p=0.464), cigarette use (p=0.185) and alcohol use (p=0.942) between different groups of sleep duration. College students with higher age, lower self-rated health status, having a nap habit, lower level of physical activity or suffering from depressive symptoms were more likely to experience sleep insufficiency (p<0.05). The mean level of IL-1β, IL-6, TNF-α and IL-10 were 0.32±0.26 pg/mL, 0.72±0.32 pg/mL, 0.77±0.21 pg/mL and 1.92±0.31 pg/mL, respectively. There were statistical differences in the levels of all inflammatory cytokines in this study among sleep duration.
 |
Table 1 Characteristics of the Study Sample |
The Comparison of Inflammatory Cytokines Among Different Groups of Sleep Duration or Chronotype
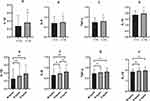
Figure 1 shows the comparison of inflammatory cytokines among different groups of sleep duration or chronotype. There were statistical differences in the levels of IL-1β, IL-6, TNF-α and IL-10 in this study among different groups of sleep duration or chronotype (p<0.05). Further, the levels of IL-1β, IL-6 and TNF-α and IL-10 were significant higher in sleep insufficiency and E-types group.
Generalized Linear Model Analysis of Sleep Duration, Chronotype and Inflammatory Cytokines
Table 2 describes the results of sleep duration, chronotype and inflammatory cytokines analysis using the generalized linear model. The results indicated that inflammatory cytokines were statistically positively correlated with sleep duration or chronotype. After controlling for age, self-rated health, physical activity, afternoon napping, and depressive symptoms, the level of inflammatory cytokines were higher in sleep insufficiency group (IL-1β: B=0.064, p=0.001; TNF-α: B=0.042, p=0.008; IL-10: B=0.089, p<0.001). The levels of inflammatory cytokines were higher in E-types (IL-1β: B=0.096, p=0.013; IL-6: B=0.125, p=0.009).
 |
Table 2 Generalized Linear Models of Sleep Duration, Chronotype and Inflammatory Cytokines |
The interaction effects of sleep duration and chronotype on inflammatory cytokines are shown in Table 2. Crude and adjusted models showed that among college students sleep duration and chronotype had a positive multiplicative interaction effect on inflammatory cytokines. Compared to the reference group (sleep sufficiency and M-types), students with sleep insufficiency and N-types were more likely to be with higher levels of inflammatory markers including IL-1β (B=0.144, p=0.008), IL-6 (B=0.197, p=0.003), TNF-α (B=0.140, p=0.001) and IL-10 (B=0.190, p=0.004).
Generalized Linear Models of the Associations Between Chronotype and Inflammatory Cytokines Stratified by Sleep Duration
Figure 2 shows the results of the generalized linear models of the connections between chronotype and inflammatory cytokines stratified by sleep duration. E-types were statistically positively correlated with the levels of inflammatory markers in students with sleep insufficiency. After controlling for age, self-rated health, physical activity, afternoon napping, and depressive symptoms, N-types (IL-1β: B=0.117, p=0.004; IL-6: B=0.161, p=0.001; TNF-α: B=0.107, p=0.001; IL-10: B=0.135, p=0.003) and E-types (IL-1β: B=0.139, p=0.006; IL-6: B=0.201, p=0.001; TNF-α: B=0.119, p=0.003; IL-10: B=0.146, p=0.012) were statistically positively correlated with the level of inflammatory markers in college students reported sleep insufficiency.
Discussion
As far as we know, this is the first study to investigate the independent effects of sleep duration, chronotype and combination of them in relation to inflammation in college students’ crowd. The findings suggested that sleep insufficiency and E-types were positively related to the level of inflammatory markers. Furthermore, we confirmed the interaction effects of sleep insufficiency and E-types on the level of inflammatory markers among Chinese college students. The paradox is that sleep duration and chronotype were positively associated with IL-10, which is considered an anti-inflammatory cytokine under most circumstances.
We observed that 56.2% of participants reported sleep insufficiency, which was lower than in previous studies.32 Furthermore, the proportion of M-types, N-types and E-types among college students was 14.8%, 74.8% and 10.4%, respectively. Similarly, in another investigation of 1st-year college students, the distribution of chronotype also got consistent results.33 Moreover, the percentage of sleep insufficiency in E-types students was 72.0%, which was higher than that of M-types (39.7%) and N-types (57.3%). The current results were compatible with formerly covered in Korean adolescents as well.34
College students should represent a specially and huge group in society. The lifestyles developed during college period could have a broad and profound impact on health such as immune system.1 Inflammation underlies widely various physiological and pathological processes.35 Inflammation mechanism is an important defense mechanism for the body to restore homeostasis and tissue function and exists in most non-communicable chronic diseases.36 The present study indicated that sleep insufficiency was related to higher levels of inflammatory factors including IL-1β, TNF-α and IL-10, which is consistent with other studies.37,38 In addition, we also found that E-types were statistically related to higher levels of IL-1β and IL-6. Yet little research has examined the effect of chronotype on inflammation in college students. Recently, there was a pilot study on the role of inflammatory factors in sleep, circadian preference, and health found that in adolescents with higher CRP, shorter sleep duration was associated with greater emotional health risks; however, among adolescents with higher IL-6, small effect size simple slopes were observed between evening preference and behavior.39
A major finding of this present research was that N-types and E-types were only associated with elevated levels of inflammatory factors in college students with insufficient sleep. Short sleep duration has been proved to be with systemic Inflammation in young adults.40,41 Sleep duration and chronotype are interconnected. A large school-based study showed that none of the chronotype met the minimum recommended amount of sleep duration on school nights; and E-types had shorter school day sleep duration than M-types, with N-types displaying a moderate sleep duration between these two extremes.42 Perhaps this is why compare to M-types, N-types and E-types were only associated with elevated levels of inflammatory factors, and the correlation sizes of N-types on inflammatory factors were lower than those of E-types. Insufficient sleep is strongly associated with sympathetic activation, and elevated levels of epinephrine and norepinephrine could be served as its evidence.43,44 The changes in the levels of these two nerve mediators may be caused in part by stimulation of leukocyte adrenergic receptors and activation of nuclear factor (NF)-κB-mediated NLRP3 inflammasome inflammatory signaling pathways.45,46 Likewise, it has been posited that circadian rhythm disorder triggers an immune cascade that eventually activates inflammatory factors, which are general markers of systemic inflammation.47 For this reason, the results of this study further support the proposal that sleep is related to inflammation. Future research should explore the associations of sleep insufficiency and chronotype with activation of the sympathetic nervous system.
In the present investigation, it was extraordinary that insufficient sleep or E-types was associated with higher levels of IL10, and IL-10 was considered to be an anti-inflammatory cytokine in most instances. Nevertheless, some studies have also found contradictory results for IL-10.48 However, similar results were obtained when the circadian rhythm was disturbed. IL-10 was decreased due to circadian misalignment in a randomized controlled trial of adults in the United States.49 One potential explanation is that IL-10 may require cofactors to function as an anti-inflammatory mediator.50 Moreover, IL-10 has different signaling mechanism pathways for each subset of immune cells.51 Recently, the possibility of pro-inflammation of IL-10 has been reported. For example, when used during cancer treatment, it may be pro-inflammatory.52 Thus, additional rigorous scientific research is needed to better clarify the potential and vital role of IL-10 in the relationship between sleep and immune system.
One of the advantages of the present study was a multitude of sample of Chinese college students as participants, which may cause our findings more compelling. Moreover, we explored the interactions between sleep duration and chronotype on the level of inflammation, and further stratified by sleep duration, which more accurately estimate the relationship of them. Last but not least, we analyzed the relationships between a variety of inflammatory biomarkers, not just one, sleep duration and chronotype that make the results more convincing. Nonetheless, the following restrictions were recognized in this research. Firstly, our research was not a longitudinal study that has limitation in determining the causal relationship of sleep duration, chronotype and inflammation among college students. Our research is still ongoing and further longitudinal analysis will be performed in the future. Secondly, data of sleep pattern were based on subjective responses of college students, which were not able to avoid recall bias. Further studies should be undertaken, including more objective measures of sleep patterns such as polysomnography and actigraphy.
Conclusion
Our findings suggested that sleep insufficiency, N-types and E-types were positively associated with inflammation. In addition, N-types and E-types were particularly related to inflammation factors among Chinese college students with sleep insufficiency. Inflammation may be an intermediate mechanism between sleep and health in college students. The present findings should be replicated in longitudinal studies to determine the direction of this association. Our study provides evidence that maintaining a healthy sleep pattern, such as getting enough sleep duration and maintaining a healthy routine, are advantageous to healthy development in college students.
Abbreviations
BMI, body mass index; B, regression coefficient; CI, confidence interval; IL-10, interleukin-10; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; M-types, Morning-types; N-types, Neutral-types; E-types, Evening-types.
Acknowledgments
The authors sincerely thank the consent of data access from College Student Behavior and Health Cohort Study, carried out by the Institute of Anhui Medical University, and all participants involved in this study for their full support.
Funding
This study was supported by National Natural Science Foundation of China (81803257, 81773455), Scientific Research of BSKY from Anhui Medical University (XJ201824), and Open Project Program of MOE Key Laboratory of Population Health Across Life Cycle (JK20205).
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Ge Y, Xin S, Luan D, et al. Association of physical activity, sedentary time, and sleep duration on the health-related quality of life of college students in Northeast China. Health Qual Life Outcomes. 2019;17(1):124. doi:10.1186/s12955-019-1194-x
2. Glavin EE, Matthew J, Spaeth AM. Gender differences in the relationship between exercise, sleep, and mood in young adults. Health Educ Behav. 2021;12:1090198120986782. doi:10.1177/1090198120986782
3. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110.
4. Vitale JA, Roveda E, Montaruli A, et al. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405–415. doi:10.3109/07420528.2014.986273
5. Haraden DA, Mullin BC, Hankin BL. The relationship between depression and chronotype: a longitudinal assessment during childhood and adolescence. Depress Anxiety. 2017;34(10):967–976. doi:10.1002/da.22682
6. Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16(3):258–262. doi:10.1093/sleep/16.3.258
7. Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 2010;46(2):124–132. doi:10.1016/j.jadohealth.2009.06.016
8. Owens H, Christian B, Polivka B. Sleep behaviors in traditional-age college students: a state of the science review with implications for practice. J Am Assoc Nurse Pract. 2017;29(11):695–703. doi:10.1002/2327-6924.12520
9. Depner CM, Stothard ER, Wright KP. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14(7):507. doi:10.1007/s11892-014-0507-z
10. Humphries RK, Bath DM, Burton NW. Dysfunctional beliefs, sleep hygiene and sleep quality in university students. Health Promot J Austr. 2021. doi:10.1002/hpja.471
11. Choshen-Hillel S, Ishqer A, Mahameed F, et al. Acute and chronic sleep deprivation in residents: cognition and stress biomarkers. Med Educ. 2021;55(2):174–184. doi:10.1111/medu.14296
12. Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi:10.1161/CIRCRESAHA.118.313591
13. Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. doi:10.1152/physrev.00010.2018
14. Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi:10.1111/j.1749-6632.2009.05300.x
15. Rethorst CD, Greer TL, Toups MS, Bernstein I, Carmody TJ, Trivedi MH. IL-1β and BDNF are associated with improvement in hypersomnia but not insomnia following exercise in major depressive disorder. Transl Psychiatry. 2015;5(8):e611. doi:10.1038/tp.2015.104
16. Rohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci. 2012;1261:88–96. doi:10.1111/j.1749-6632.2012.06634.x
17. Rockstrom MD, Chen L, Taishi P, et al. Tumor necrosis factor alpha in sleep regulation. Sleep Med Rev. 2018;40:69–78. doi:10.1016/j.smrv.2017.10.005
18. Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50(4):871–891. doi:10.1016/j.immuni.2019.03.020
19. Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. doi:10.1146/annurev-psych-010213-115205
20. Owens JA, Dearth-Wesley T, Lewin D, Gioia G, Whitaker RC. Self-regulation and sleep duration, sleepiness, and chronotype in adolescents. Pediatrics. 2016;138(6):e20161406. doi:10.1542/peds.2016-1406
21. Fernandez-Mendoza J, Baker JH, Vgontzas AN, Gaines J, Liao D, Bixler EO. Insomnia symptoms with objective short sleep duration are associated with systemic inflammation in adolescents. Brain Behav Immun. 2017;61:110–116. doi:10.1016/j.bbi.2016.12.026
22. El-Sheikh M, Saini EK, Gillis BT, Kelly RJ. Interactions between sleep duration and quality as predictors of adolescents’ adjustment. Sleep Health. 2019;5(2):180–186. doi:10.1016/j.sleh.2018.11.004
23. Norell-Clarke A, Hagquist C. Child and adolescent sleep duration recommendations in relation to psychological and somatic complaints based on data between 1985 and 2013 from 11 to 15 year-olds. J Adolesc. 2018;68:12–21. doi:10.1016/j.adolescence.2018.07.006
24. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
25. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–43. doi:10.1016/j.sleh.2014.12.010
26. Kanagarajan K, Gou K, Antinora C, et al. Morningness-Eveningness questionnaire in bipolar disorder. Psychiatry Res. 2018;262:102–107. doi:10.1016/j.psychres.2018.02.004
27. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance - United States, 2015. MMWR Surveill Summ. 2016;65(6):1–174. doi:10.15585/mmwr.ss6506a1
28. Nolan RC, Raynor AJ, Berry NM, May EJ. Self-reported physical activity using the international physical activity questionnaire (IPAQ) in Australian adults with type 2 diabetes, with and without peripheral neuropathy. Can J Diabetes. 2016;40(6):576–579. doi:10.1016/j.jcjd.2016.05.013
29. Kroenke K, Spitzer RL, Williams JB, et al. The PHQ-9 validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
30. Wang Y, Chen Z, Guo F, et al. Sleep patterns and their association with depression and behavior problems among Chinese adolescents in different grades. Psych J. 2017;6(4):253–262. doi:10.1002/pchj.189
31. Li T, Xie Y, Tao S, et al. Chronotype, sleep, and depressive symptoms among Chinese college students: a cross-sectional study. Front Neurol. 2020;11:592825. doi:10.3389/fneur.2020.592825
32. Li L, Wang YY, Wang SB, et al. Sleep duration and sleep patterns in Chinese university students: a comprehensive meta-analysis. J Clin Sleep Med. 2017;13(10):1153–1162. doi:10.5664/jcsm.6760
33. Zhou J, Hsiao FC, Shi X, et al. Chronotype and depressive symptoms: a moderated mediation model of sleep quality and resilience in the 1st-year college students. J Clin Psychol. 2021;77(1):340–355. doi:10.1002/jclp.23037
34. Yim SH, Yang KI, Kim JH, Hwangbo Y, Kim D, Hong SB. Association between eveningness preference, socio-behavioral factors, and insomnia symptoms in Korean adolescents. Sleep Med. 2021;82:144–150. doi:10.1016/j.sleep.2021.03.016
35. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi:10.1038/nature07201
36. Arulselvan P, Fard MT, Tan WS, et al. Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev. 2016;2016:5276130. doi:10.1155/2016/5276130
37. Clinton JM, Davis CJ, Zielinski MR, Jewett KA, Krueger JM. Biochemical regulation of sleep and sleep biomarkers. J Clin Sleep Med. 2011;7(5 Suppl):S38–S42. doi:10.5664/JCSM.1360
38. Taraz M, Khatami MR, Hajiseyedjavadi M, et al. Association between antiinflammatory cytokine, IL-10, and sleep quality in patients on maintenance hemodialysis. Hemodial Int. 2013;17(3):382–390. doi:10.1111/hdi.12035
39. Dolsen MR, Harvey AG. IL-6, sTNF-R2, and CRP in the context of sleep, circadian preference, and health in adolescents with eveningness chronotype: cross-sectional and longitudinal treatment effects. Psychoneuroendocrinology. 2021;129:105241. doi:10.1016/j.psyneuen.2021.105241
40. Bakour C, Schwartz S, O’Rourke K, et al. Sleep duration trajectories and systemic inflammation in young adults: results from the national longitudinal study of adolescent to adult health (add health). Sleep. 2017;40(11):zsx156. doi:10.1093/sleep/zsx156
41. Park H, Chiang JJ, Bower JE, et al. Sleep and inflammation during adolescents’ transition to young adulthood. J Adolesc Health. 2020;67(6):821–828. doi:10.1016/j.jadohealth.2020.04.015
42. Saxvig IW, Evanger LN, Pallesen S, et al. Circadian typology and implications for adolescent sleep health. Results from a large, cross-sectional, school-based study. Sleep Med. 2021;83:63–70. doi:10.1016/j.sleep.2021.04.020
43. Floam S, Simpson N, Nemeth E, Scott-Sutherland J, Gautam S, Haack M. Sleep characteristics as predictor variables of stress systems markers in insomnia disorder. J Sleep Res. 2015;24(3):296–304. doi:10.1111/jsr.12259
44. Fernandez-Mendoza J, Vgontzas AN, Calhoun SL, et al. Insomnia symptoms, objective sleep duration and hypothalamic-pituitary-adrenal activity in children. Eur J Clin Invest. 2014;44(5):493–500. doi:10.1111/eci.12263
45. Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64(6):538–540. doi:10.1016/j.biopsych.2008.05.004
46. Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. doi:10.3390/ijms20133328
47. Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13(3):190–198. doi:10.1038/nri3386
48. Figueiredo-Braga M, Cornaby C, Cortez A, et al. Influence of biological therapeutics, cytokines, and disease activity on depression in rheumatoid arthritis. J Immunol Res. 2018;2018:5954897. doi:10.1155/2018/5954897
49. Wright KP, Drake AL, Frey DJ, et al. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015;47:24–34. doi:10.1016/j.bbi.2015.01.004
50. Lee SW, Hong YS, Chun CM, et al. Anti-inflammatory effects of IL-4 and IL-10 on human polymorphonuclear leukocytes. J Korean Med Sci. 2002;17(1):7–14. doi:10.3346/jkms.2002.17.1.7
51. Sanjabi S, Zenewicz LA, Kamanaka M, Flavell R. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9(4):447–453. doi:10.1016/j.coph.2009.04.008
52. Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM. The dual role of IL-10. Trends Immunol. 2003;24(1):36–43. doi:10.1016/s1471-4906(02)00009-1
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.