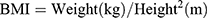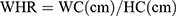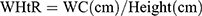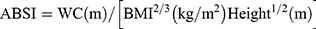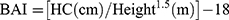Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Associations of Gain in Weight-Related Anthropometric Indices with a Marker of Lipid Peroxidation: A Cohort Study Among Urban Adults in China
Authors Xu T, Wang B, Cao L, Qiu W , Zhang Z, Chen A, Chen W
Received 21 April 2020
Accepted for publication 8 July 2020
Published 18 August 2020 Volume 2020:13 Pages 2877—2887
DOI https://doi.org/10.2147/DMSO.S259194
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Tao Xu,1,2 Bin Wang,1,2 Limin Cao,1,2 Weihong Qiu,1,2 Zhuang Zhang,1,2 Ailian Chen,1,2 Weihong Chen1,2
1Department of Occupational and Environmental Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, People’s Republic of China; 2Key Laboratory of Environment and Health, Ministry of Education and Ministry of Environmental Protection, State Key Laboratory of Environmental Health (Incubating), School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, People’s Republic of China
Correspondence: Weihong Chen
Department of Occupational and Environmental Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, People’s Republic of China
Tel +86 27 83691677
Email [email protected]
Background: Obesity is reported to be associated with oxidative stress which can cause lipid peroxidation. However, the effects of gain in various weight-related anthropometric indices on lipid peroxidation remain unclear. We aimed to examine the cross-sectional and longitudinal associations between altered weight-related anthropometric indices and a marker of lipid peroxidation among urban adults in China.
Methods: A total of 3762 participants from the Wuhan-Zhuhai cohort were included in the present study, with a follow-up of 3 years. Six weight-related anthropometric indicators were measured and calculated, including waist circumference (WC), body mass index (BMI), waist-to-hip ratio (WHR), waist-to-height ratio (WHtR), a body shape index (ABSI), and body adiposity index (BAI). Individual urinary 8-iso-prostaglandin-F2α (8-iso-PGF2α) was determined via enzyme-linked immunosorbent assay to evaluate lipid peroxidation. We used generalized linear models to analyze the cross-sectional and longitudinal associations of weight-related anthropometric indices with a marker of lipid peroxidation and stratified analyses to estimate effect modification.
Results: We found significant relationships between WHR, WHtR, ABSI, and urinary 8-iso-PGF2α at baseline. Each 1% increase in WHR, WHtR, and ABSI was significantly associated with a 0.007, 0.004, and 0.104 increase in log-transformed 8-iso-PGF2α concentration, respectively (P< 0.05). In longitudinal analysis, positive dose–response relationships were observed between gains in BMI, BAI, and increased 8-iso-PGF2α after adjusting for potential confounders (Ptrend< 0.05). We also found that gender and smoking status modified the association of BMI gain and 8-iso-PGF2α increment, and such an association was more obvious in female and non-smokers.
Conclusion: Our research implied that gain in anthropometric indices may result in a higher level of lipid peroxidation.
Keywords: weight-related anthropometric indices, lipid peroxidation, obesity, cohort study
Introduction
Obesity, a widespread pandemic leading to poor health nowadays, has attracted worldwide public attention. According to the recent World Health Organization report, more than one third of the adult population were categorized as overweight or obese globally, as were over 340 million children and adolescents.1 Several weight-related indices have been used to assess adiposity, among which body mass index (BMI) is the most widely used.2 Compared with BMI, waist circumference (WC) and waist-to-hip ratio (WHR) have advantages in reflecting body fat distribution and measuring central obesity that is linked to greater health risks.3,4 Additionally, other weight-related anthropometric indices were proposed to assess the accumulation of adipose, including waist-to-height ratio (WHtR), a body shape index (ABSI), and body adiposity index (BAI). A review from Italian scholars reported that obesity may activate the innate immune system and promote oxidative stress.5
Oxidative stress arises from a systemic disequilibrium of oxidation–antioxidation system due to overproductions of reactive oxygen species (ROS) or reactive nitrogen species (RNS), which could contribute to the initiation and progression of obesity-related diseases.6–8 Cell membranes and macromolecules may be damaged once this imbalance appears. Lipids, especially polyunsaturated fatty acids, are susceptible biomolecules that could be attacked by ROS, and generate peroxides ultimately. F2-isoprostanes (F2-isoP) are regarded as reliable markers among numerous lipid peroxides.9 The 8-iso-prostaglandin-F2α (8-iso-PGF2α), one well-known part of F2-isoP class, is usually detected in urine to reflect the level of lipid peroxidation.10 Some published literatures have suggested that elevated 8-iso-PGF2α was associated with obesity and fat accumulation.11,12
To better achieve the early prevention of obesity-related diseases, further understanding on the relationship between adiposity and lipid peroxidation is necessary. Ohmori et al13 found that the levels of plasma 8-iso-PGF2α were significantly higher in the high BMI group. In the Framingham Heart Study, positive associations of BMI and WHR with urinary 8-iso-PGF2α were also documented.14 However, a cross-sectional study of 677 Japanese people showed that BMI has no significant relevance to urinary 8-iso-PGF2α,15 which was inconsistent with the abovementioned findings. In addition to such controversial associations, independent effects of some new weight-related anthropometric parameters, such as ABSI and BAI, on urinary 8-iso-PGF2α remain largely unknown. And the study regarding the longitudinal associations of gain in weight-related anthropometric indices with urinary 8-iso-PGF2α change is limited. Accordingly, we aimed to investigate the effects of different weight-related anthropometric indices on urinary 8-iso-PGF2α levels, and further examine whether there are longitudinal associations between gain in weight-related anthropometric indices and altered urinary 8-iso-PGF2α levels among urban adults in China.
Methods
Study Design and Population
Data used in this study were originated from the Wuhan-Zhuhai cohort, which is a community-based prospective cohort established between 2011 and 2012 as described previously.16 A total of 4812 participants aged 18–80 years who lived in the communities of Wuhan or Zhuhai city for more than 5 years were recruited at baseline survey. Participants were followed up every 3 years. In the current cross-sectional analysis, 3762 subjects were included after excluding those with a history of nephritis (n=43), missing information on 8-iso-PGF2α (n=926), and weight-related anthropometric indices (n=81) at baseline. The first follow-up survey was finished after 3 years, and a total of 2777 participants were recruited. We excluded participants who had missing data on weight-related anthropometric indices (n=1069) or failed to provide sufficient urine samples (n=322). Finally, 1386 participants were included in longitudinal analysis. Participants included and excluded showed no significant difference in basic characteristics including age and gender. The study has approval from the Ethics and Human Subject Committee of Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was gained from residents before participation.
Covariate Data Collection
Covariates, including basic demographic information, lifestyle (smoking status, alcohol drinking, regular physical activity, cooking status), and history of disease, were collected through face-to-face interview by professionals. Smoking status covered ever smoking (current and former smoking) and never smoking. Drinking status covered ever drinking (current and former drinking) and never drinking. Physical activity was defined as exercising regularly at least two times per week and for more than 20 minutes each time within the past 6 months. Diet information (intake frequencies of grains, fruit, vegetables, meat, and smoked each day or week) were also collected.
Weight-Related Anthropometric Indices
Every participant underwent a physical examination that included anthropometric measurements, like weight, height, WC, and hip circumference (HC). All of them were accomplished through standardized protocol and technique. In brief, height and weight were measured via weighing scale and a ruler (RGZ-120 Jiangsu Suhong Medical Device Co., Ltd, Changzhou, China). During the measurement, participants stood upright and kept the heels, sacrum, and scapula in line. WC was done at the midpoint between the iliac crest and the lower costal margin, and HC at the maximum circumference of the buttocks. Other weight-related anthropometric indices, including BMI, WHR, WHtR, ABSI, and BAI, were calculated as follows.17–19
The gain in weight-related anthropometric indices were derived through the values of corresponding indicators in first follow-up minus the values at baseline. Overweight (≥24 kg/m2) is defined based on the BMI cut-off values presented by the Working Group on Obesity in China.20
Urinary 8-Iso-PGF2α Determination
We collected spot morning urine to detect the levels of urinary 8-iso-PGF2α that have been widely accepted in large epidemiological investigations previously. The levels of urinary 8-iso-PGF2α were determined using a commercially available ELISA kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instruction. We controlled the intra- and inter-assay coefficients of variation below 5% and 10%, respectively. Urinary 8-iso-PGF2α concentration was calibrated by urinary creatinine (Cr) and finally expressed as ng/mmol Cr. Increased urinary 8-iso-PGF2α for each participant is the result of 8-iso-PGF2α in first follow-up minus the result at baseline.
Statistical Analysis
Urinary 8-iso-PGF2α concentration was log-transformed because of its right skewed distribution. Demographic characteristics across urinary 8-iso-PGF2α quartiles were compared using one-way ANOVA for continuous variables and Chi-square test for categorical variables.
The associations of weight-related and gain in weight-related anthropometric indices with urinary 8-iso-PGF2α and altered urinary 8-iso-PGF2α levels were evaluated using generalized linear models with adjustment for potential confounders, including age, gender, city, education, smoking status, alcohol drinking, physical activity, cooking meals at home, intake frequency of each kind of food (fruit, meat, smoked), history of hypertension, diabetes, and coronary heart disease.
Continuous models were used to calculate the regression coefficient and 95% confidence intervals of altered urinary 8-iso-PGF2α by each 1-unit increase in weight-related anthropometric indices, and categorical models to estimate regression coefficient of urinary 8-iso-PGF2α change across quartiles of different anthropometric indices gain. Furthermore, we assessed the relationships between altered urinary 8-iso-PGF2α and transition of overweight status during a 3-year period in two models. One was adjusted for single study factor, and another for all potential confounders. Statistical modification was performed by inputting an interaction term of interested weight-related anthropometric indices multiplied by the stratification covariates (age, gender, smoking status, alcohol consumption, and physical activity) in the generalized linear models, so as to clarify the modification effect of baseline demographic characteristics. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline Characteristics of Participants
The 8-iso-PGF2α concentrations of all participants in urine samples ranged from 38.01–108.39 ng/mmol Cr, with a median value of 61.75 ng/mmol Cr. Baseline characteristics of the study population by quartiles of urinary 8-iso-PGF2α concentration are shown in Table 1. The mean age of 3762 subjects (1156 male, 30.73%) was 52.07±13.56 years old. Male individuals, cigarette smokers, alcohol drinkers, and those who ate vegetables, fruits, and smoked food frequently were more likely to have a higher urinary 8-iso-PGF2α level, whereas the results were the opposite among individuals who exercise regularly, eat more meat, and usually cook meals at home (P<0.05). As for weight-related anthropometric parameters, no differences were found across urinary 8-iso-PGF2α quartiles except for WHR and ABSI.
 |
Table 1 Baseline Characteristics of Study Population by Quartiles of Urinary 8-Iso-PGF2α Concentration (N=3762) |
Cross-Sectional Associations of Weight-Related Anthropometric Indices with Urinary 8-Iso-PGF2α
The cross-sectional relationships between various weight-related anthropometric indices and urinary 8-iso-PGF2α in all participants and subgroups of non-overweight and overweight are shown in Table 2. With adjustment for potential covariates, WHR, WHtR, and ABSI rather than BMI, WC, or BAI were significantly associated with 8-iso-PGF2α in all participants (Table 2 and Figure 1). Each 1% increase in WHR, WHtR, and ABSI was significantly associated with a 0.007, 0.004, and 0.104 increase in log-transformed 8-iso-PGF2α concentration, respectively (P<0.05). Additional subgroup analysis showed similar unanimous associations, and we found that the association of WHtR with 8-iso-PGF2α is significant in the subgroup of non-overweight instead of overweight. The simple scatter plots with crude data between those relationships are shown in Supplementary Figure 1.
 |
Table 2 Associations Between Weight-Related Anthropometric Indices and Urinary 8-Iso-PGF2α Levels at Baseline (N=3762) |
Longitudinal Associations of Gain in Weight-Related Anthropometric Indices with Altered Urinary 8-Iso-PGF2α
As shown in Figure 2, the results of generalized linear models indicated positive dose–response relationships between increased 8-iso-PGF2α and gain in BMI and BAI, after adjusting for potential confounders (all Ptrend<0.05). Consistent with categorical analyses, continuous analyses exhibited that average increase in 8-iso-PGF2α was 2.65% (95% CI=0.32–4.98%) with per 1-unit gain in BAI (P<0.05). Change in other weight-related anthropometric indices (BMI, WC, WHR, WHtR, and ABSI) were positively associated with an increase in 8-iso-PGF2α without statistical significance (Supplementary Table 1). When contrasting with those overweight at baseline but non-overweight at follow-up, females who were non-overweight at baseline but overweight at follow-up showed a 42.15% (95% CI=8.62–75.68%) increase in urinary 8-iso-PGF2α levels (Table 3).
 |
Table 3 Regression Coefficients of Change in Urinary 8-Iso-PGF2α by Overweight at Baseline and 3-Year Follow-Up |
Stratification analyses of selected characteristics are presented in Table 4. Gender and smoking status were potential effect modifiers of the association between BMI gain and altered urinary 8-iso-PGF2α levels (P for interaction<0.05), and no statistical significance was noted in other interaction terms. The relationships between BMI, as well as BAI gain and increased urinary 8-iso-PGF2α, were more remarkable among females and nonsmokers. Thus, we then examined such associations in subgroups of different genders and smoking status. Among females or nonsmokers, but not males or smokers, significant dose–response relationships between anthropometric indices (BMI and BAI) gain and increased urinary 8-iso-PGF2α still existed. In addition, individuals who cooked at home exhibited more pronounced associations compared with those did not cook (Supplementary Tables 2 and 3).
 |
Table 4 Associations Between Gain in Weight-Related Anthropometric Indices and Change in Urinary 8-Iso-PGF2α Levels During 3-Year Follow-Up, Stratified by Selected Characteristics |
Discussion
In this study, we explored the cross-sectional and longitudinal associations of various weight-related anthropometric indices with urinary 8-iso-PGF2α, a biomarker of lipid peroxidation. We observed that urinary 8-iso-PGF2α concentrations were increased along with elevated WHR, WHtR, and ABSI (an indicator reflecting body fat distributions) among all participants in the cross-sectional analysis. After follow-up of 3 years, we found significant dose–response relationships between gain in BMI and BAI and increased urinary 8-iso-PGF2α in longitudinal analysis. And such a relationship was more obvious in females and non-smokers than in males and smokers.
Data from a Framingham Heart Study involving 1250 adults showed that subcutaneous adipose tissue and visceral adipose tissue were related to higher urinary 8-iso-PGF2α, which indicated a direct cross-sectional association of visceral adiposity and oxidative stress.21 In line with that, subsequent studies implied both general adiposity (accessed by BMI) and central adiposity (measured by WC and WHtR) were positively and similarly associated with lipid peroxidation.22–25 Besides, evidence from Japanese scholars found that the relationship between urinary F2-isoprostane (a lipid peroxidation marker) excretion and BMI showed a U-shaped curve with the lowest F2-isoprostane level at BMI 23 among 15 subjects.26 Indeed, our research also showed positive relationships between weight-related anthropometric indices (WHR, WHtR, ABSI) and urinary 8-iso-PGF2α concentrations (a stable biomarker of lipid peroxidation), which indicated central obesity may contribute to lipid peroxidation by examining a large population in China. However, with respect to BMI, we did not find its effect on urinary 8-iso-PGF2α at baseline, though this result was in accordance with a Japanese study conducted on 677 healthy adults.15 The possible explanations for the discrepancy between our findings and previously published data might be the differences in biological specimens and detection method of lipid peroxides. Blood specimens and mass spectrometric methods were applied in some prior research. While considering the short half-life of plasma F2-isoP and large sample size,15,27 immunoassays in urine may be a more suitable method.
To our knowledge, our present results provided vital insight into altered urinary 8-iso-PGF2α (a biomarker used to reflect the degree of lipid peroxidation) influenced by gain in weight-related anthropometric indices, which were largely unknown before. In the present study, as BMI or BAI levels keep increasing after 3 years, urinary 8-iso-PGF2α concentrations were gradually increased. Furthermore, compared with the female who lost weight during 3-year follow-up, those with gained weight had increased urinary 8-iso-PGF2α, suggesting that the levels of lipid peroxidation may strengthen with growing fat accumulation over time, especially in women. A study by Chae et al28 found that 122 overweight/obese participants exhibited decreased urinary 8-iso-PGF2α after 3-year-long intervention involving daily 100-kcal calorie deficits. Il’yasova et al29 discovered that 2-year calorie restriction in healthy volunteers caused a statistical reduction in F2-isoP at both time points. Nevertheless, in the Insulin Resistance Atherosclerosis Study (IRAs) Cohort followed by 5 years, increased urinary F2-isoP levels predicted lower weight gain using the data from 299 individuals,30 which showed incongruity with our results. The heterogeneity may be owing to the discrepancy of subject characteristics and sample size.
The explicit mechanism underlying the associations between weight-related anthropometric indices gain and higher level of lipid peroxidation over time remain not elucidated. The following pathways were likely to be involved. Enlarged white adipose tissue lead to the synthesis of various adipokines, which facilitate the generation of lipid peroxides in turn.31 Among adipokines, leptin serves as a crucial regulator for energy expenditure and food intake, and plays an important role in the formation of lipid peroxides; so as inflammatory cytokines from macrophages and monocytes, like TNF-α, IL-1, and IL-6.23,32 Experimental models have observed that the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase pathway (NOX) is another major source of ROS.33 NOX, particularly NOX4, shares the capacity to transfer electrons and convey various cellular signals.34 Marchesi et al35 found that vascular superoxide and NADPH oxidase activity was aroused in perivascular adipose tissue of New Zealand obese mice. In addition, susceptibility to lipid peroxidative injury is aggravating in obese individuals because of reduced antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX).31 Compared to those with a BMI of more than 40 kg/m2, subjects with healthy BMI had significant higher SOD and GPX.36 And it has been proved that supplementation with antioxidants decreased systemic oxidative stress and plasma 8-iso-PGF2α concentrations among those of overweight or obese.37 Finally, visceral fat accumulation triggers increased lipid peroxidation through excess free fatty acids.5 As Randle et al38 identified that mitochondrial NADH/NAD+ ratio rose with high levels of fatty acid oxidation.
Interestingly, the females were more likely to have stronger associations between anthropometric indices (BMI and BAI) gain and 8-iso-PGF2α increment, supporting the hypothesis that significant sex interactions were found between plasma 8-iso-PGF2α levels and most measures of adiposity.23 The prior literatures have indicated that a higher adipose tissue volume in women than men may be responsible for the gender differences.23 Another possible interpretation was menopausal status. Most researches have shown increased lipid peroxidation levels and decreased antioxidant status were discovered in postmenopausal women,39,40 and women over 50 years old took up a large percentage in our study. The concrete reasons for the gender difference in the relationships need to be investigated in the future.
There are several strengths in our study. First and foremost, we clarified the relationships of weight-related anthropometric indices and urinary 8-iso-PGF2α via both cross-sectional and longitudinal analyses in a relatively large population. Besides, several weight-related anthropometric indices which could evaluate the fat accumulation from different dimensions were applied to offer a better understanding of the associations. Third, we adjusted for potential confounders such as cigarette smoking and drinking. As for our limitations, participants involved in this study were followed with a short interval, as such, the indices of anthropometry and lipid peroxidation may not reflect a long-term change. Further studies are needed to explore their associations for a long period in a larger population. Another limitation is that information about dietary intake habits, smoking, and drinking status were obtained through questionnaires, and possible bias in recall may exist. In addition, although all persons participating in physical examination were trained by professionals on the basis of the same standard, there is also the chance for bias in anthropometric measurement. Further studies are needed to confirm our results.
Conclusions
In summary, we found that WHR, WHtR, and ABSI are powerful predictors of lipid peroxidation that was reflected by urinary 8-iso-PGF2α levels and associated closely with obesity-related health risks. Among females, pronounced dose-dependent relationships were reported between gain in BMI, BAI, and increased urinary 8-iso-PGF2α. The study highlights that BAI and BMI may also be suitable indicators of dynamic response to lipid peroxidation.
Abbreviations
WC, waist circumference; BMI, body mass index; WHR, waist-to-hip ratio; WHtR, waist-to-height ratio; ABSI, a body shape index; BAI, body adiposity index; HC, hip circumference; 8-iso-PGF2α, 8-iso-prostaglandin-F2α; Cr, creatinine; ROS, reactive oxygen species; RNS, reactive nitrogen species; F2-isoP, F2-isoprostanes; NADPH, nicotinamide adenine dinucleotide phosphate; NOX, nicotinamide adenine dinucleotide phosphate oxidase pathway; SOD, superoxide dismutase; CAT, catalase; GPX, glutathione peroxidase; SD, standard deviation.
Data Sharing Statement
The datasets used and analyzed in this study are available from the corresponding author with reasonable request.
Ethics Approval and Informed Consent
The study was approval by the Ethics and Human Subject Committee of Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was gained from residents before participation.
Acknowledgments
We sincerely appreciated all participants recruited in the study and the support from the study team.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. WHO. Fact sheet: obesity and overweight. Available from: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
2. Reilly JJ, El-Hamdouchi A, Diouf A, Monyeki A, Somda SA. Determining the worldwide prevalence of obesity. Lancet. 2018;391(10132):1773–1774. doi:10.1016/S0140-6736(18)30794-3
3. Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105–2120. doi:10.1056/NEJMoa0801891
4. Boggs DA, Rosenberg L, Cozier YC, et al. General and abdominal obesity and risk of death among black women. N Engl J Med. 2011;365(10):901–908. doi:10.1056/NEJMoa1104119
5. Marseglia L, Manti S, D’Angelo G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014;16(1):378–400. doi:10.3390/ijms16010378
6. Higashi Y, Maruhashi T, Noma K, Kihara Y. Oxidative stress and endothelial dysfunction: clinical evidence and therapeutic implications. Trends Cardiovasc Med. 2014;24(4):165–169. doi:10.1016/j.tcm.2013.12.001
7. Brunelli E, La Russa D, Pellegrino D. Impaired oxidative status is strongly associated with cardiovascular risk factors. Oxid Med Cell Longev. 2017;2017:6480145. doi:10.1155/2017/6480145
8. Bigagli E, Lodovici M. Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxid Med Cell Longev. 2019;2019:5953685. doi:10.1155/2019/5953685
9. Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ
10. Schwedhelm E, Bartling A, Lenzen H, et al. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109(7):843–848. doi:10.1161/01.CIR.0000116761.93647.30
11. Urakawa H, Katsuki A, Sumida Y, et al. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88(10):4673–4676. doi:10.1210/jc.2003-030202
12. Costabile G, Della Pepa G, Bozzetto L, et al. Urine 8-isoprostane in relation to adiposity and insulin resistance in individuals at high cardiometabolic risk. Metab Syndr Relat Disord. 2015;13(4):187–191. doi:10.1089/met.2014.0119
13. Ohmori K, Ebihara S, Kuriyama S, et al. The relationship between body mass index and a plasma lipid peroxidation biomarker in an older, healthy Asian community. Ann Epidemiol. 2005;15(1):80–84. doi:10.1016/j.annepidem.2004.04.001
14. Keaney JF
15. Sakano N, Wang DH, Takahashi N, et al. Oxidative stress biomarkers and lifestyles in Japanese healthy people. J Clin Biochem Nutr. 2009;44(2):185–195. doi:10.3164/jcbn.08-252
16. Song Y, Hou J, Huang X, et al. The Wuhan-Zhuhai (WHZH) cohort study of environmental air particulate matter and the pathogenesis of cardiopulmonary diseases: study design, methods and baseline characteristics of the cohort. BMC Public Health. 2014;14:994. doi:10.1186/1471-2458-14-994
17. Krakauer NY, Krakauer JC, Li S. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7(7):e39504. doi:10.1371/journal.pone.0039504
18. Wang H, Liu A, Zhao T, et al. Comparison of anthropometric indices for predicting the risk of metabolic syndrome and its components in Chinese adults: a prospective, longitudinal study. BMJ Open. 2017;7(9):e016062. doi:10.1136/bmjopen-2017-016062
19. Tripolino C, Irace C, Carallo C, Scavelli FB, Gnasso A. Body fat and blood rheology: evaluation of the association between different adiposity indices and blood viscosity. Clin Hemorheol Microcirc. 2017;65(3):241–248. doi:10.3233/CH-16172
20. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
21. Pou KM, Massaro JM, Udo H, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress. Circulation. 2007;116(11):1234–1241. doi:10.1161/CIRCULATIONAHA.107.710509
22. Wu SH, Shu XO, Chow WH, et al. Adiposity and fat distribution in relation to inflammation and oxidative stress in a relatively lean population of Chinese women. Dis Markers. 2013;34(4):279–293. doi:10.1155/2013/437076
23. Kanaya AM, Wassel CL, Stoddard PJ, et al. F 2 -Isoprostanes and adiposity in older adults. Obesity. 2011;19(4):861–867. doi:10.1038/oby.2010.243
24. An H, Du X, Huang X, et al. Obesity, altered oxidative stress, and clinical correlates in chronic schizophrenia patients. Transl Psychiatry. 2018;8(1):258. doi:10.1038/s41398-018-0303-7
25. Mure K, Yoshimura N, Hashimoto M, et al. Urinary 8-iso-prostaglandin F2α as a marker of metabolic risks in the general Japanese population: the ROAD study. Obesity. 2015;23(7):1517–1524. doi:10.1002/oby.21130
26. Narukawa T, Anzai Y, Murakami T, Isogai R, Nakagawa S, Yamada H. Effects of Body Mass Index (BMI), dietary intake and serum antioxidant vitamin concentration on urinary 8-hydroxydeoxyguanosine and F2-isoprostane excretions. Aging Mech Dis. 2011;8(1):1–6. doi:10.3793/jaam.8.1
27. Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28(4):505–513. doi:10.1016/S0891-5849(99)00264-6
28. Chae JS, Paik JK, Kang R, et al. Mild weight loss reduces inflammatory cytokines, leukocyte count, and oxidative stress in overweight and moderately obese participants treated for 3 years with dietary modification. Nutr Res. 2013;33(3):195–203. doi:10.1016/j.nutres.2013.01.005
29. Il’yasova D, Fontana L, Bhapkar M, et al. Effects of 2 years of caloric restriction on oxidative status assessed by urinary F2-isoprostanes: the CALERIE 2 randomized clinical trial. Aging Cell. 2018;17(2):e12719.
30. Il’yasova D, Wang F, Spasojevic I, Base K, D’Agostino RB
31. Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12(5):3117–3132. doi:10.3390/ijms12053117
32. Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem. 2012;68(4):701–711. doi:10.1007/s13105-012-0154-2
33. Lopez-Acosta O, de Los Angeles Fortis-barrera M, Barrios-Maya MA, Ramirez AR, Aguilar FJA, El-Hafidi M. Reactive oxygen species from NADPH oxidase and mitochondria participate in the proliferation of aortic smooth muscle cells from a model of metabolic syndrome. Oxid Med Cell Longev. 2018;2018:5835072. doi:10.1155/2018/5835072
34. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi:10.1152/physrev.00044.2005
35. Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54(6):1384–1392. doi:10.1161/HYPERTENSIONAHA.109.138305
36. Olusi SO. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int J Obes Relat Metab Disord. 2002;26(9):1159–1164. doi:10.1038/sj.ijo.0802066
37. Sutherland WH, Manning PJ, Walker RJ, de Jong SA, Ryalls AR, Berry EA. Vitamin E supplementation and plasma 8-isoprostane and adiponectin in overweight subjects. Obesity. 2007;15(2):386–391. doi:10.1038/oby.2007.546
38. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785–789. doi:10.1016/S0140-6736(63)91500-9
39. Pansini F, Cervellati C, Guariento A, et al. Oxidative stress, body fat composition, and endocrine status in pre- and postmenopausal women. Menopause. 2008;15(1):112–118. doi:10.1097/gme.0b013e318068b285
40. Sánchez-Rodríguez MA, Zacarías-Flores M, Arronte-Rosales A, Correa-Muñoz E, Mendoza-Núñez VM. Menopause as risk factor for oxidative stress. Menopause. 2012;19(3):361–367. doi:10.1097/gme.0b013e318229977d
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.