Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Associations of Clinical Characteristics and Intestinal Flora Imbalance in Stable Chronic Obstructive Pulmonary Disease (COPD) Patients and the Construction of an Early Warning Model
Authors Zeng X, Yang H, Yang Y, Gu X, Ma X, Zhu T
Received 24 July 2021
Accepted for publication 29 November 2021
Published 18 December 2021 Volume 2021:16 Pages 3417—3428
DOI https://doi.org/10.2147/COPD.S330976
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Xuetao Zeng,1,* Hongfeng Yang,2,* Yan Yang,1 Xinnan Gu,1 Xiuqin Ma,1 Taofeng Zhu1
1Department of Respiratory and Critical Medicine, The Affiliated Yixing Hospital of Jiangsu University, Yixing, Jiangsu, People’s Republic of China; 2Department of Critical Medicine,The Affiliated People’s Hospital of Jiangsu University, Zhenjiang, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiuqin Ma; Taofeng Zhu
The Affiliated Yixing Hospital of Jiangsu University, No. 75 Tongzhenguan Road, Yixing, Jiangsu, 214200, People’s Republic of China
Tel +86-510-879210911
; +86-510-87920912
Fax +86-510-87921110
Email [email protected]; [email protected]
Objective: Establish a simple predictive model and scoring rule that is suitable for clinical medical staff in respiratory departments to assess intestinal flora imbalance occurrence in stable chronic obstructive pulmonary disease (COPD) patients.
Methods: From January 1, 2019, to December 31, 2020, COPD patients (195 cases) – who attended the Outpatient Department, Respiratory and Critical Care, Yixing Hospital, Jiangsu University – were enrolled in a cross-sectional study. Based on stool examination results, patients were divided into experimental (41 cases) and control (154 cases) groups. Single-factor and logistic regression analyses were performed with the baseline data of the two groups to obtain a new predictive model, which was further simplified.
Results: Five predictive factors composed the model: body mass index (BMI), serum albumin (ALB), Charlson’s Comorbidity Index (CCI), gastrointestinal symptom score (GSRs), and Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification. The model to predict intestinal flora imbalance in stable COPD patients had an area under the ROC curve (AUC) of 0.953 [95% CI (0.924, 0.982)]. After simplifying the scoring rules, the AUC was 0.767 [95% CI (0.676, 0.858)].
Conclusion: In the current study, we obtained a model that could effectively predict intestinal flora imbalance risk in stable COPD patients, being suitable for implementation in early treatments to improve the prognosis. Moreover, all indicators can be easily and simply obtained.
Keywords: lung disease, chronic obstruction, early warning score, gastrointestinal tract, flora imbalance, predictive model
Background
Chronic Obstructive Pulmonary Disease (COPD) is common in middle-aged and elderly people, with a prevalence superior to 27% in people over 60 years.1 The COPD characteristics include small airway injury remodeling, incomplete reversible and gradually aggravating dyspnea, and a high disability rate. Long-term chronic inflammation also has a strong destructive effect on the airway tissue and structure. The morbidity and mortality of COPD have been increasing recently, affecting over 250 million people worldwide, and might be the third leading death cause in the world by the end of 2021.2 Stable COPD patients account for about 210 million people worldwide and have a considerable economical burden.3 For example, in the United States and Asia, COPD hospitalization cost accounts for nearly half of the total medical expenses.4 Due to the development and improvement of lung-intestine axis studies, increasing attention has been paid to the role of the intestinal flora in COPD. Intestinal flora imbalance can lead to an increase in potentially pathogenic bacteria, which can also greatly increase bacterial translocations. Further, the gram-negative bacilli increase can aggravate intestinal endotoxin release, which can cause indirect lung damage and accelerate COPD progression.5 Additionally, the intestinal flora can metabolize choline and phosphatidylcholine to produce trimethylamine (TMA), then oxidized into TMAO by the liver (after being absorbed into the blood). Previously, TMAO was associated with long-term all-cause mortality in COPD patients.6 On the other hand, the increased metabolic demand of COPD patients can lead to intestinal mucosal ischemia and hypoxia, intestinal tract integrity damage, finally causing intestinal flora imbalance.7 Therefore, even stable COPD patients should be alerted to the occurrence of intestinal flora imbalance, and vulnerable patients should be identified. Therefore, in the present study, our main goal was to establish an early warning model and specific scoring rules for intestinal flora disorders in stable COPD patients. Thus, clinicians can more intuitively and conveniently identify early patients, then carry out interventions to improve prognosis. Our secondary objective was to construct a model that can be used as a risk stratification basis for intestinal flora disorders in stable COPD patients.
Methods
Study Design
This cross-sectional and observational study was conducted at a medical Center, Yixing People’s Hospital, affiliated with Jiangsu University, from January 1, 2019, to December 31, 2020. Subjects were invited to participate at the Respiratory and Critical Care Clinic, where their demographic information, pulmonary artery systolic pressure (PASP),8 laboratory tests, Modified British Medical Research Council Questionnaire (mMRC), Chronic Obstructive Pulmonary Disease assessment test (CAT) scores,9 Charlson’s Comorbidity Index Questionnaire (CCI),10 Gastrointestinal Symptom Rating scale (GSRs), and lung function test was obtained. The study was approved by the Ethical Committee of the Affiliated Yixing Hospital of Jiangsu University (Batch No.2019-002). All participants signed an informed consent form. The study was conducted following the Declaration of Helsinki.
Study Subjects
We selected COPD patients who attended the Respiratory and Critical Care Clinic, Yixing People’s Hospital, affiliated to the Jiangsu University, from January 1, 2019, to December 31, 2020, as the research subjects.
The inclusion criteria were:
- (1) met the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) diagnostic criteria (revised in 2019):11 lung function test (after inhaling bronchodilators): [first second forced expiratory volume (FEV1)/forced vital capacity (FVC)] < 0.7; respiratory physicians helped to confirm that it was stable: no acute exacerbation in the past 8 weeks; no clear infection history; signed the relevant informed consent form of this study.
- (2) intestinal flora disorders diagnostic criteria followed previous recommendations for diagnosis and treatment.12,13 Intestinal flora disorders were diagnosed if laboratory tests presented any of the following results: Fecal laboratory examination: ① enterococci and bacilli number were observed and counted under oil microscope, and the cocci proportion was > 40%; (2)(Bifidobacterium/Enterobacter) < 1; ③ Gram-negative bacilli abnormal type ≥ 50%. Gram-positive bacilli abnormal type < 68%.
- (3) presence of complete clinical data, including demographic characteristics, fecal flora smear or culture results, oxygen partial pressure, and lung function.
The exclusion criteria were:
- (1) primary intestinal diseases led to intestinal flora imbalance.
- (2) presented diseases such as pulmonary interstitial fibrosis and active tuberculosis.
- (3) used antibiotics, systemic intravenous hormones, and/or probiotics 8 weeks before admission.
- (4) Incomplete data or dropped out.
First, 213 stable COPD patients were enrolled. Eighteen patients were excluded due to primary intestinal diseases, pulmonary interstitial fibrosis, antibiotics or probiotics use in the past 8 weeks, incomplete data, and midway withdrawal. Finally, 195 patients were divided into experimental (41 cases) and control (154 cases) groups, according to whether they were diagnosed with intestinal flora imbalance or not (Figure 1).
 |
Figure 1 Patient flow diagram. |
Research Tools
Data Collection
We collected the general clinical data of all subjects, including sex, age, smoking status, drinking status, body mass index (BMI), disease course, COPD hospital admission in the past year, and inhaled corticosteroids and drugs as required (leakage rate < 20%). Pulmonary function indicators were also collected, including forced expiratory volume ratio in 1 second to predicted value (FEV1%pred), forced expiratory volume ratio to forced vital capacity (FEV1/FVC), and GOLD classification (divided into 4 groups according to the GOLD rating: GOLD I - FEV1%pred ≥ 80%; GOLD II - 50% ≤ FEV1%pred < 80%; GOLD III - 30% ≤ FEV1%pred < 50%; GOLD IV - FEV1%pred < 30%). Additionally, laboratory data were collected: oxygen partial pressure (PaO2), serum albumin (ALB), triglyceride (TG), total cholesterol (TC), serum creatinine(Scr), B-type natriuretic peptide (BNP), hemoglobin (Hb), white blood cell count (WBC), eosinophil counts, lymphocyte counts, fasting blood glucose, PASP, mMRC and CAT scores, and CCI Questionnaire.10
Gastrointestinal Symptom Rating Scale (GSRs)
Gastrointestinal Symptom Rating scale (GSRs) scores14 included abdominal pains (abdominal pain, nausea, vomiting), reflux (heartburn, belching, acid reflux), diarrhea (fecal diarrhea, incontinence, and urgency), dyspepsia (abdominal ringing and distension, increased exhaustion), and constipation (infrequent defecation, stool tumor, incomplete defecation). Each symptom score ranged from 0 to 3. The scores of asymptomatic, mild, moderate, and severe symptoms were 0, 1, 2, and 3, respectively. The total symptoms score was between 0–15.
Blood Sample Collection and Testing
We collected 10 mL of fasting cubital venous blood from all subjects. Then, blood samples were placed in a 10 mL anticoagulation tube and sent for examination within 30 min. The blood collection tube was turned upside down several times to fully mix it. After 1 h at room temperature, the plasma was separated by centrifugation at 3500 rpm/10 min. Then, the supernatant was aspirated and placed in an EP tube and stored at −80 °C. After samples were collected, we tested different indicators. WBC, lymphocytes, and eosinophils counts were detected by an automatic five-segment hematology analyzer (Japanese Sysmex company). An automatic biochemical analyzer (Siemens ADVIA 1200) was used to detect serum albumin, creatinine, blood glucose, and blood lipids (TG, TC). We used an arterial blood gas analyzer (Siemens RAPIDLAB1265, Germany) to measure PaO2, and an automatic immune analyzer (Roche Cobas e602, Germany) to measure BNP. The PASP was determined by continuous-wave Doppler echocardiography (PHILIPS iE-Elite). The maximum tricuspid regurgitation velocity was measured by professional physicians in the Department of Cardiac Ultrasound, and the PASP was finally estimated. All operations were strictly carried out according to the instructions.
Intestinal Bacteria Microscopy and Culture
We used a dedicated clean toilet to collect feces samples (2.5 g). Only middle part feces samples were collected (the surface part was removed). During sampling, we avoid touching the inner surface of the container and ensured that there was no pollution (eg urine). We used 0.5 g of fresh feces to perform routine microscopic examinations within 10 min and smeared 2 sheets (the thickness was suitable to see the paper writing through the smear). We waited for the smear specimen to dry and fix, then perform Gram staining and oiling. Then, bacilli and cocci numbers were counted under the microscope and the ratio was calculated. When the cocci proportion was > 40%, the intestinal flora imbalance was diagnosed.
We used another 0.5 g of the fecal specimen and placed it into a test tube containing diluent (9 mL) and glass beads, marked it, mixed it thoroughly on a mixer, and serially diluted it (10−1–10−8). MRS agar (Qingdao Haibo Biological Company, China) was dissolved in boiling water and placed in a 48 °C constant temperature water bath for standby. Then, 0.1 mL of the suspension was inoculated in MRS agar and cultured for 48–72 h. The China blue agar (Zhengzhou Antu Bioengineering Co., Ltd) was rewarmed to 25 °C. Next, 0.1 mL of the suspension was inoculated in the agar medium and cultured at 36 °C for 24–48 h. An appropriate colony was selected to calculate the B/E value. A B/E > 1 indicated normal intestinal flora, and a B/E < 1 indicated intestinal flora disorder.
Lung Function Test
Subjects rested for 10 min before lung function test examination. They inhaled a uniform albuterol dose (400 μg) and were tested for FEV1, FEV1%pred, FEV1/FVC. The Japan Jest pulmonary function instrument (CHESTAC-8800) was used.
Statistical Analyses
According to stool microscopy and culture results, two stable COPD groups were divided (with and without intestinal flora imbalance) to analyze the characteristics of the general clinical data. All data were analyzed using SPSS 22.0 (IBM, NY, USA). Normally distributed data are expressed as means±standard 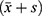 and comparisons between two groups were performed by the t-test. Non-normally distributed data are expressed by medians [1st quartile; 3rd quartile, M (P25, P75)]. In that case, comparisons between groups were performed by the Mann–Whitney U rank-sum test.
and comparisons between two groups were performed by the t-test. Non-normally distributed data are expressed by medians [1st quartile; 3rd quartile, M (P25, P75)]. In that case, comparisons between groups were performed by the Mann–Whitney U rank-sum test.
Count data are expressed as frequencies (composition ratios). Comparisons between the two groups were performed by the χ2 test, and statistically significant variables were included. The ROC curve was drawn to obtain the best cut-off value and Youden index of the above variables. The continuous variables among them were transformed into binary variables according to the cut-off value. Then, the variables with statistical differences between the two groups in the general data and laboratory comparison were screened out and entered the univariate logistic regression analysis with a p-value < 0.05 as the standard. Next, the variables with statistical differences (p-value < 0.05) in the univariate logistic regression analysis were included in a multivariate logistic regression by entry method. A preliminary predictive model was established for the selected candidate variables. The model fit was tested by Hosmer & Lemeshow. The corresponding β-value was given as an integer to assign a score. Then, a simplified early warning model scoring rule was established. Finally, the ROC curve was used to verify the effectiveness of the early warning scoring model for COPD’s intestinal flora imbalance. All curves were drawn with GraphPad Prism software. A p-value < 0.05 was considered statistically significant.
Results
COPD Patients’ Clinical Features
The clinical features of COPD patients are shown in Table 1. We collected information of 195 subjects: 41 (21.0%) had intestinal flora imbalance (case group) and 154 (79.0%) did not (control group).
 |
Table 1 Comparison of General Data Between the Two Groups |
Comparative Analysis of the Intestinal Flora Imbalance Base Data of COPD Patients
Compared with the control, the case group had a lower BMI, worse pulmonary function, lower arterial oxygen partial pressure, and albumin concentration (p < 0.05). The median CAT scores of the case and control groups were 14 (9, 19) and 9 (7, 14), respectively. The median mMRC score was 2 (1, 3) for the control and 1 (0, 2) for the case group. The CCI score was 3 (2, 4) for control and 1 (0.75,2) for the case. The GSRs scores of the control and case groups were 2 (1, 4) and 1 (0,1), respectively. Therefore, the case group had higher CAT, mMRC, CCI, and GSRs scores compared to the control (p < 0. 05). The other factors did not significantly differ between the two groups (p > 0.05) (Table 1).
Establishment and Simplification of a Predictive Model for Intestinal Flora Disorders in Stable COPD Patients
Preliminarily Screen of Variables for Logistic Regression Analysis
Based on the results presented in Table 1, the BMI, FEV1%pred, PaO2, ALB, GOLD classification, CAT, mMRC, CCI, and GSRs scores were selected as candidate predictors. The SPSS 22.0 software was used to obtain the best cut-off value and Youden index of those continuous variables (Table 2). According to the best cut-off values, 9 continuous variables were transformed into binary ones (Table 3): BMI ≤ 23.25 kg/m2 = 0, BMI>23.25 kg/m2 = 1; FEV1%pred ≤ 49.75% = 0, FEV1%pred>49.75% = 1; PaO2 ≤ 97 mmHg = 0, PaO2 > 97 mmHg = 1; ALB ≤ 32.5 g/L = 0, ALB > 32.5 g/L = 1;CCI scores ≤ 2 = 0, CCI scores>2 = 1; mMRC scores ≤ 2 = 0, mMRC scores > 2 = 1; CAT scores ≤ 9 = 0, CAT scores > 9 = 1; GSRs scores ≤ 2 = 0, GSRs scores > 2 = 1; GOLD classification: I–II = 0, III–IV = 1. Then, these categorical variables were incorporated into the univariate logistic regression analysis using the entry method and the intestinal flora imbalance occurrence was the returned result. Parameters estimation and test results are summarized in Table 4.
 |
Table 2 The Best Cut-Off Value of Predictor Variables and Youden Index |
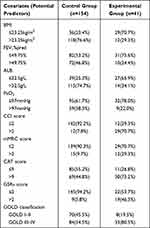 |
Table 3 Comparison of Categorical Predictors Between the Two Groups |
 |
Table 4 Univariate and Multivariate Logistic Regression Analysis of Intestinal Flora Imbalance in Patients with Stable COPD |
Establishment of a Predictive Model for Intestinal Flora Imbalance in COPD Patients by Logistic Regression Analysis
Meaningful indicators (p < 0.05) selected in the univariate logistic regression analysis were included in the multivariate logistic regression. Parameters estimation and testing results are also shown in Table 4. Five statistically significant (p < 0.05) and independent variables (BMI, ALB, CCI score, GSRs score, GOLD classification) were finally included in the model. The model sensitivity was 90.2% and the specificity 87.7%. Additionally, the model fit was tested by Hosmer & Lemeshow: X2 = 9.683, P = 0.288. The logistic regression equation is:
Logit(p) = −4.332 + (−2.406 BMI) + (−1.280 ALB) + (3.244 CCI score) + (2.840 GSRs score) + (3.237 GOLD classification);
The equation was transformed as follows:
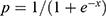 ; e: natural logarithm (~2.718).
; e: natural logarithm (~2.718).
x= −4.332 + (−2.406 BMI) + (−1.280 ALB) + (3.244 CCI score) + (2.840 GSRs score) + (3.237 GOLD classification).
ROC Curves of the Predictive Model and Its Variables for Intestinal Flora Imbalance Occurrence in COPD Patients
Next, we used the area under the ROC curves (AUC) of the five variables (BMI, ALB, CCI scores, GSRs scores, and GOLD classification) included in the model and the combined diagnosis predictive model (PRE_1) to test their predictive value regarding intestinal flora imbalance in COPD patients. The AUC were: 0.782 [95% CI (0.709, 0.856)], 0.740 [95% CI (0.660, 0.820)], 0.898 [95% CI (0.845, 0.951)], 0.831 [95% CI (0.764, 0.897)], 0.657 [95% CI (0.567, 0.747)], 0.953 [95% CI (0.924, 0.982)]. The combined diagnosis model was superior to a single variable test power (Figures 2 and 3).
 |
Figure 2 ROC curve: 5 predictors predict the risk of intestinal flora imbalance in COPD. |
 |
Figure 3 ROC curve: ROC of PRE_1. |
Model Simplification
After retrieving the statistically significant regression coefficients (β-value) of BMI, ALB, CCI score, GSRs score, and GOLD classification in the model as integers (2, 1, 3, 3, 3; Table 4), a simplified scoring model (PRE_2) was established: BMI ≤ 23.25kg/m2 2 points, > 23.25kg/m2 0 points; ALB ≤ 32.5g/L 1 point, > 32.5g/L 0 points; CCI scores ≤ 2 points were assigned 0 points, > 2 points were assigned 3 points; GSRs scores ≤ 2 points are assigned 0 points, > 2 points are assigned 3 points; GOLD classification: I–II 0 points, III–IV 3 points. The simplified model scores were between 0–12 points (Table 5) and its AUC value was 0.767 [95% CI (0.676, 0.858)]. This model (PRE_2) had a moderate predictive power for the intestinal flora imbalance risk in COPD patients (Figure 4). The model best critical value score was > 7 (the optimal cut-off value of the PRE _ 2 model was obtained using the ROC curve) and was used as the basis for the stratification of intestinal flora imbalance risk in stable COPD patients. Finally, patients could be divided into a low-risk group (≤ 7 points) and a high-risk group (> 7 points).
 |
Table 5 Risk Score Sheet for Intestinal Flora Imbalance in Patients with Stable COPD |
 |
Figure 4 ROC curve: ROC of PRE_2. |
Discussion
We found that BMI, ALB, CCI score, GSRs score, and GOLD classification were intestinal flora imbalance risk factors in COPD patients and presented a moderate predictive value for COPD patients’ prognosis. Among them, CCI scores > 2 and GSRs scores > 2 increased the risk of intestinal flora disorders in COPD patients by 25.633 and 17.113 times, respectively. BMI > 23.25 kg/m2 and ALB > 32.5 g/L might be protective factors, since they decreased the risk of intestinal flora disorders in stable COPD patients by 0.090 and 0.278 times, respectively.
BMI is often regarded as a nutritional status indicator of the body. Also, previous studies have demonstrated associations between low BMI and COPD patients’ exacerbation.15 A 3-year South Korean follow-up BMI study aiming to predict COPD prognosis found that the BMI decrease was independently related to COPD deterioration and mortality increases (HR = 3.167).16 COPD patients often present energy expenditure increase. When malnutrition occurs, the body’s defense ability decreases, resulting in intestinal flora imbalance with COPD deterioration. Serum albumin is another nutritional status indicator. It can be used to predict the in-hospital mortality (ALB level of 30.5 g/L) and readmission rate within 30 days (ALB level of 30.1g/L) of COPD patients.17 When hypoalbuminemia occurs, it leads to malnutrition and low immunity in COPD patients, colloidal osmotic pressure decreases, intestinal wall edemas, and intestinal mucosa structural disorders, finally affecting the intestinal flora ecological stability and resulting in flora imbalance.18,19 Furthermore, the albumin/globulin ratio has a more predictive value than the serum albumin single index, and it can be used as a reference index to evaluate the condition of elderly COPD patients.20 Although the albumin/globulin ratio can determine whether COPD patients are complicated with infections and predict prognosis, it was not used in the current study. An Italian study showed that the CCI score was an independent acute exacerbation and death risk factor in COPD patients. In this study, the COPD patients’ death rate increased by 4.8% when the CCI score was > 1.21 Both COPD and its complications can aggravate the weakness and disability progress of COPD patients,22 finally leading to intestinal flora imbalance. Their interaction accelerates the disease process and the COPD acute exacerbation number. Additionally, some studies have shown that the gastrointestinal symptoms scores in COPD patients are higher than healthy individuals (2.12/1.96) and a negative correlation between gastrointestinal symptoms and mental health impairment (r = −0.49, p < 0.001).23 COPD patients have more chances to develop psychological disorders, such as anxiety and depression, which can lead to the disappearance of the dominant intestinal flora, intestinal wall barrier function loss, and intestinal endotoxin release, thereby promoting intestinal flora imbalance and gastrointestinal symptoms.24 Previously, it has been reported that the gut microbiome can promote the development of mental disorders such as depression and anxiety.25 COPD is also associated with a higher prevalence of anxiety and depression than the general population, 49 with reported rates ranging from 7% to 50% and from 10% to 57%, respectively.26 Therefore, the intestinal flora imbalance may also be one of the reasons for anxiety and depression in COPD patients, and accelerate COPD progress. Compared with non-COPD and COPD GOLD I patients, GOLD II–IV patients have significantly higher symptoms (CAT scores) and lower lung function indexes (including FEV1/FVC, FVC). Especially, GOLD III–IV COPD patients are considered to have good clinical significance for judging COPD prognosis.27 Patients with moderate or severe pulmonary dysfunction often have more clinical symptoms and higher acute exacerbation risk. According to the Global Initiative for Chronic Obstructive Lung Disease, these patients are usually placed into the GOLD D group. Compared with A group patients, D group patients have a higher (25 times) death risk.28 This indicates that worse lung function can easily lead to COPD acute exacerbation, thus increasing the hospitalization number, and further aggravating infection risk, and causing intestinal flora disorder.
Age is also regarded as a risk factor for intestinal flora disorder in COPD patients. Older patients often have more serious organ and gastrointestinal function declines. Therefore, elderly patients are more prone to present declined gastrointestinal function, facilitating enterotoxin accumulation, then leading to dysbacteriosis and COPD development acceleration.29 However, age was not one of the risk factors in our present study (p>0.05), even with a majority of elderly patients.
We found that any single indicator, including BMI, ALB, CCI score, GSRs score, and GOLD classification, was not effective to predict intestinal flora imbalance occurrence in COPD patients, with AUCs of 0.782, 0.740, 0.898, 0.831, and 0.657, respectively. Therefore, we combined these 5 risk factors to construct a new prediction model. The model predictive value for intestinal flora imbalance in stable COPD patients was better than any single variable. The AUC was 0.953, the sensitivity was 90.2%, and the specificity was 87.7%, which indicated that the model had great clinical predictive value. Therefore, the model provided a basis for early screening and identification of COPD patients that are susceptible to intestinal flora imbalance. However, the prediction model calculation process was relatively cumbersome, and it was not suitable for rapid clinical judgment and early identification of intestinal flora imbalance in COPD patients. Thus, we simplified the model by assigning the corresponding β-values of the 5 predictors to integer scores, obtaining a stable period. The predictive scoring rules for intestinal flora imbalance in COPD patients were: BMI ≤ 23.25 kg/m2 (2 points), ALB ≤ 32.5 g/L (1 point), CCI score > 2 (3 points), GSRs score > 2 (3 points), GOLD classification III–IV (3 points). The fast screening of high-risk patients, who were susceptible to intestinal flora imbalance, by the scores presented a good predictive performance (AUC of 0.767). The simplified scoring model might allow early interventions in this group of patients to extend the COPD stable period and improve prognosis. Although PRE_2 also showed a moderate predictive value for intestinal flora imbalance in COPD patients, it was not as good as PRE_1. This can be related to the fact that some data was lost while pursuing simplification and convenience, resulting in the decline of the accuracy in the simplified predictive model (PRE_2).
Overall, PRE_1 presented a high diagnostic value for intestinal flora imbalance in stable COPD patients. However, its calculation process was relatively cumbersome. We suggest that clinicians use this model when they want to pursue an accurate prediction rate, which can be achieved using a calculator or incorporating the formula into Excel. At the same time, when clinicians need to quickly obtain the prediction rate without paying special attention to the accuracy, it is recommended to use CCI or GSRs alone.
Study Limitations
Here, we conducted a single-center retrospective study with a small number of observed samples. Besides, other factors that were not included in the analysis - such as antibiotics use and glucocorticoids total dose – might also impact the authenticity of our conclusions. Therefore, sample size expansion, follow-up time prolongation, and a multicenter prospective study can be further performed to access these issues. In the present study, only ROC curves were used to evaluate the simplified scoring model prediction value for the occurrence of intestinal dysbiosis in stable COPD patients. In the future, we intend to include stable COPD patients as a validation cohort to verify the clinical efficacy of our current model and optimize it for clinical application.
Conclusion
A simplified scoring model (PRE_2) was established with five indicators (BMI, ALB, CCI score, GSRs score, and GOLD classification) and showed a moderate predictive value for intestinal dysbiosis in stable COPD patients. Besides, all indicators can be easily detected and obtained. The scoring model could identify high-risk groups, who are susceptible to intestinal flora imbalance, allowing the implementation of interventional measures, such as oral probiotics, as soon as possible. Finally, by balancing the intestinal flora and microecological internal environment, a reduction in the number of acute attacks in COPD patients and the improvement of their prognosis can be achieved.
Abbreviations
COPD, Chronic Obstructive Pulmonary Disease; BMI, Body mass index; ALB, serum albumin; CCI, Charlson comorbidity index; GSRs, gastrointestinal symptom score; CAT, Chronic Obstructive Pulmonary Disease assessment test score; mMRC, Modified British Medical Research Council questionnaire; GOLD, Global Initiative for Chronic Obstructive Lung Disease; TMA, trimethylamine; FEV1, first second forced expiratory volume; FVC, forced vital capacity; FEV1%pred, ratio of forced expiratory volume in 1 second to predicted value; PaO2, arterial oxygen partial pressure; TG, triglyceride; TC, total cholesterol; Scr, endogenous creatinine; BNP, B-type natriuretic peptide; PASP, pulmonary artery systolic pressure; Hb, hemoglobin; WBC, white blood cell count; ROC curve, receiver operating characteristic curve; AUC, area under the curve.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy or ethical restrictions.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the People’s Hospital of Yixing City, Jiangsu Province (batch number: 2019-002), and all the subjects signed the informed consent form.
Author Contributions
All authors contributed to data analysis and drafting or revising the article, have agreed on submitting the article to this journal, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported in part by the National Natural Science Foundation of China (81802102), the Science Foundation of Jiangsu Health Commission (No. Z2018011) and the Top Talent Support Program for young and middle-aged people of Wuxi (Yixing) Health Committee (grant no. BJ2020107).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Riley CM, Sciurba FC. Diagnosis and outpatient management of chronic obstructive pulmonary disease: a review. JAMA. 2019;321(8):786–797. doi:10.1001/jama.2019.0131
2. Iheanacho I, Zhang S, King D, et al. Economic burden of chronic obstructive pulmonary disease (COPD): a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:439–460. doi:10.2147/COPD.S234942
3. Wu YK, Lan CC, Tzeng IS, et al. The COPD-readmission (CORE) score: a novel prediction model for one-year chronic obstructive pulmonary disease readmissions. J Formos Med Assoc. 2021;120(3):1005–1013. doi:10.1016/j.jfma.2020.08.043
4. Anees UR, Ahmad Hassali MA, Muhammad SA, et al. The economic burden of chronic obstructive pulmonary disease (COPD) in the USA, Europe, and Asia: results from a systematic review of the literature. Expert Rev Pharmacoecon Outcomes Res. 2020;20(6):661–672. doi:10.1080/14737167.2020.1678385
5. Mayhew D, Devos N, Lambert C, et al. Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax. 2018;73(5):422–430. doi:10.1136/thoraxjnl-2017-210408
6. Ottiger M, Nickler M, Steuer C, et al. Gut, microbiota-dependent trimethylamine-N-oxide is associated with long-term all-cause mortality in patients with exacerbated chronic obstructive pulmonary disease. Nutrition. 2018;45:135–141 e131. doi:10.1016/j.nut.2017.07.001
7. Sun Z, Zhu QL, Shen Y, et al. Dynamic changes of gut and lung microorganisms during chronic obstructive pulmonary disease exacerbations. Kaohsiung J Med Sci. 2020;36(2):107–113. doi:10.1002/kjm2.12147
8. Chen Y, Liu C, Lu W, et al. Clinical characteristics and risk factors of pulmonary hypertension associated with chronic respiratory diseases: a retrospective study. J Thorac Dis. 2016;8(3):350–358. doi:10.21037/jtd.2016.02.58
9. Cheng SL, Lin CH, Wang CC, et al. Comparison between COPD Assessment Test (CAT) and modified Medical Research Council (mMRC) dyspnea scores for evaluation of clinical symptoms, comorbidities and medical resources utilization in COPD patients. J Formos Med Assoc. 2019;118(1 Pt 3):429–435. doi:10.1016/j.jfma.2018.06.018
10. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
11. Miravitlles M, Vogelmeier C, Roche N, et al. A review of national guidelines for management of COPD in Europe. Eur Respir J. 2016;47(2):625–637. doi:10.1183/13993003.01170-2015
12. Tuddenham S, Sears CL. The intestinal microbiome and health. Curr Opin Infect Dis. 2015;28(5):464–470. doi:10.1097/QCO.0000000000000196
13. Kostidis S, Kokova D, Dementeva N, et al. 1 H-NMRanalysis of feces: new possibilities in the helminthes infections research. BMC Infect Dis. 2017;17(1):275. doi:10.1186/s12879-017-2351-7
14. Zaika S, Paliy I, Chernobrovyi V, et al. The study and comparative analysis of GerdQ and GSRS Questionnaires on gastroesophageal reflux disease diagnostics. Prz Gastroenterol. 2020;15(4):323–329. doi:10.5114/pg.2020.101561
15. Yamaya M, Usami O, Nakayama S, et al. Malnutrition, airflow limitation and severe emphysema are risks for exacerbation of chronic obstructive pulmonary disease in Japanese subjects: a retrospective single-center study. Int J Chron Obstruct Pulmon Dis. 2020;15:857–868. doi:10.2147/COPD.S238457
16. Kim EK, Singh D, Park JH, et al. Impact of body mass index change on the prognosis of chronic obstructive pulmonary disease. Respiration. 2020;99(11):943–953. doi:10.1159/000511022
17. Chen R, Xing L, You C, et al. Prediction of prognosis in chronic obstructive pulmonary disease patients with respiratory failure: a comparison of three nutritional assessment methods. Eur J Intern Med. 2018;57:70–75. doi:10.1016/j.ejim.2018.06.006
18. Zinellu E, Fois AG, Sotgiu E, et al. Serum albumin concentrations in stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Clin Med. 2021;10:2. doi:10.3390/jcm10020269
19. Sprooten RTM, Lenaerts K, Braeken DCW, et al. Increased small intestinal permeability during severe acute exacerbations of COPD. Respiration. 2018;95(5):334–342. doi:10.1159/000485935
20. Qin J, Qin Y, Wu Y, et al. Application of albumin/globulin ratio in elderly patients with acute exacerbation of chronic obstructive pulmonary disease. J Thorac Dis. 2018;10(8):4923–4930. doi:10.21037/jtd.2018.07.47
21. Montagnani A, Mathieu G, Pomero F, et al. Hospitalization and mortality for acute exacerbation of chronic obstructive pulmonary disease (COPD): an Italian population-based study. Eur Rev Med Pharmacol Sci. 2020;24(12):6899–6907. doi:10.26355/eurrev_202006_21681
22. Spece LJ, Epler EM, Donovan LM, et al. Role of comorbidities in treatment and outcomes after chronic obstructive pulmonary disease exacerbations. Ann Am Thorac Soc. 2018;15(9):1033–1038. doi:10.1513/AnnalsATS.201804-255OC
23. Niklasson A, Strid H, Simren M, et al. Prevalence of gastrointestinal symptoms in patients with chronic obstructive pulmonary disease. Eur J Gastroenterol Hepatol. 2008;20(4):335–341. doi:10.1097/MEG.0b013e3282f2d0ec
24. Rutten EPA, Lenaerts K, Buurman WA, et al. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest. 2014;145(2):245–252. doi:10.1378/chest.13-0584
25. Zareifopoulos N, Bellou A, Spiropoulou A, et al. Prevalence, contribution to disease burden and management of comorbid depression and anxiety in chronic obstructive pulmonary disease: a narrative review. COPD. 2019;16(5–6):406–417. doi:10.1080/15412555.2019.1679102
26. Zheng S, Zhu Y, Wu W, et al. A correlation study of intestinal microflora and first-episode depression in Chinese patients and healthy volunteers. Brain Behav. 2021;11(8):e02036. doi:10.1002/brb3.2036
27. Su KC, Ko HK, Chou KT, et al. An accurate prediction model to identify undiagnosed at-risk patients with COPD: a cross-sectional case-finding study. NPJ Prim Care Respir Med. 2019;29(1):22. doi:10.1038/s41533-019-0135-9
28. Lee SJ, Yun SS, Ju S, et al. Validity of the GOLD 2017 classification in the prediction of mortality and respiratory hospitalization in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:911–919. doi:10.2147/COPD.S191362
29. Kim J, Lee CH, Hwang SS, et al. The ability of different scoring systems to predict mortality in chronic obstructive pulmonary disease patients: a prospective cohort study. Respiration. 2019;98(6):495–502. doi:10.1159/000502826
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
