Back to Journals » Journal of Inflammation Research » Volume 14
Associations Between Tenascin-C and Testosterone Deficiency in Men with Major Depressive Disorder: A Cross-Sectional Retrospective Study
Received 20 December 2020
Accepted for publication 3 March 2021
Published 16 March 2021 Volume 2021:14 Pages 897—905
DOI https://doi.org/10.2147/JIR.S298270
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Rui Peng, Yan Li
Department of Clinical Laboratory, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, 430060, People’s Republic of China
Correspondence: Yan Li
Department of Clinical Laboratory, Renmin Hospital of Wuhan University, 238 Jiefang Road, Wuhan, People’s Republic of China
Tel +86 2788071553
Email [email protected]
Background: Elevated levels of tenascin-C are linked to increased risk and severity of major depressive disorder (MDD), while testosterone shows a protective effect. The present study explored associations between serum levels of tenascin-C and testosterone in Chinese men with MDD.
Methods: Testosterone and tenascin-C levels were measured in sera of 412 men with MDD and 237 age- and sex-matched controls. Serum levels of thyroid hormone, lipids, and high-sensitivity C-reactive protein (hs-CRP) were also quantified. Potential associations were examined using covariance, subgroup analysis, and multivariate linear regression analyses.
Results: Significantly higher concentrations of tenascin-C were detected in sera of subjects with MDD than in controls. Among subjects with MDD, testosterone concentrations inversely correlated with tenascin-C levels. This relationship was observed when patients were stratified by age at onset; duration or severity of depression; or concentration of thyroid hormones, low- or high-density lipoprotein, or hs-CRP. The negative association remained even when the statistical model was adjusted for age, smoking status, alcohol use, and body mass index. Linear regression with bootstrap resampling confirmed that high tenascin-C levels inversely correlated with testosterone levels.
Conclusion: In men with MDD, high tenascin-C concentrations correlate with testosterone deficiency. The combination of elevated tenascin-C and testosterone deficiency may be associated with MDD progression.
Keywords: tenascin-C, testosterone, major depressive disorder
Introduction
Major depressive disorder (MDD) is considered a major public health challenge and is predicted to become a significant cause of disability worldwide in 2020.1 This disorder is associated with high treatment costs, and it places a heavy burden on patients and their families.2 MDD has been linked to dysregulation of neurotransmitters, dysfunction of the hypothalamic-pituitary-thyroid (HPT) axis, and inflammatory responses.3,4 The causes and mechanisms underlying depression remain poorly understood, preventing the identification of biomarkers that might guide diagnosis or treatment. Thus, identifying new biological markers may bring deeper insights and improved diagnosis for MDD.
Tenascin-C (TNC) participates in cell adhesion, inflammatory response, and tissue remodeling in many tissues, including brain. It is well known that TNC may contribute to the development of hippocampal cells via regulating the neuron–glia interactions.4 A study in serum proteomic profiling of MDD has found that TNC is a serum-based biomarker of depression, suggesting that this molecular alteration is associated with acute depressed symptomatology.5 Therefore, elevated TNC levels in serum may indicate depressive pathophysiology. TNC is perhaps a promising marker for assessing MDD risk and treatment efficacy.
In men, low testosterone is strongly linked to elevated risk of depression.6 However, some researchers reviewed that low levels of testosterone are not associated with the increased rates of MDD.7 The limited studies or small samples may lead to these inconsistent conclusions. In addition, administering testosterone to gonadectomized male animals reduces anxiety and depression-like symptoms.8 These effects are eliminated when the body aromatizes testosterone into estrogens; hence, excess aromatase activity can cause testosterone deficiency in men, which increases their risk of depression.9 Our previous study also demonstrated that testosterone could suppress the neuronal apoptosis induced by corticosterone through Traf6/TAK1 pathway.10 Furthermore, testosterone feedback regulates thyroid hormone levels, lipid metabolism, and inflammatory response.11–13 It has suggested an inverse link between testosterone and TNC; the hormone may regulate TNC expression in certain cancers.14 Additionally, we previously demonstrated that high TNC levels in depressed patients were associated with suicide attempts and severity of depression, which may be a risk factor for suicide attempts of MDD.15 Thus, it is supposed that testosterone deficiency in depressed patients may contribute to the induction of TNC levels, and further promote the neuroinflammatory response and stimulate the development of MDD.
Therefore, we hypothesized that lower circulating concentrations of testosterone in men with depression may be associated with the increases in the levels of TNC, which in turn may increase the risk of MDD onset or progression. We tested this idea in the present study of Chinese men with MDD.
Methods
Study Population
In this retrospective study, a consecutive sample of 412 men diagnosed with MDD and 237 sex- and age-matched healthy controls without MDD were recruited from the Department of Psychiatry at Renmin Hospital of Wuhan University. These participants were selected by a qualified psychiatrist before enrollment in our study. The selection was performed according to the previous report.16 Briefly, the process includes five steps: (1) a standard psychiatric interview; (2) depressive symptoms were assessed based on a Structured Clinical Interview Diagnosis (SCID) on the first day of admission, which refers to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5); (3) severity of depressive symptoms was assessed using the Patient Health Questionnaire-9 (PHQ-9) and the Beck’s Suicidal Ideation Scale (SSI); (4) a detailed evaluation of early psychiatric disease; (5) a sociodemographic condition and clinical data were recorded using predesigned questionnaires. In the present study, a PHQ-9 score ≥10 indicates the presence of depressive symptoms. The severity of depressed symptoms was divided into not depressed (0–4 scores), mild (5–9 scores), moderate (10–19 scores), and severe (20–27 scores), according to the previous observation.17
Data were collected on sociodemographic characteristics (age and years of education), lifestyle and health indicators (smoking status, alcohol use, and body mass index (BMI)), and clinical characteristics (symptom duration and age at onset). Smoking and alcohol use were evaluated by asking the subjects whether they smoked or drank, and if they did, they were asked to show the numbers of cigarettes smoked or drinking in present. BMI was evaluated by measuring the weight and height of both individuals on the same scale and calculated by dividing weight (in kilograms) by the square of height (in meters); a BMI of below 18.5 represents underweight, 18.5–24.9 represents normal weight, 25.0–29.9 represents overweight, and 30 or above indicates obesity (WHO, 2016). Patients with MDD suffering from depressed symptoms for at least 4 weeks or longer and hospitalized individuals were enrolled in our research. Exclusion criteria were the use of illegal agents, substance abuse within the past 3 months, history of heart disease, kidney or inflammatory diseases, acute or chronic infection, tumors and major somatic disorders. Additionally, the use of antidepressant or antipsychotic agents can contribute to the alterations of serum cytokine contents, thus, both individuals were free of medications for at least 2 weeks before sampling.
The Medical Ethics Review Committee of Renmin Hospital of Wuhan University approved this study protocol. Clinical trial registration number is No. 2020783. And all subjects provided written informed consent prior to study enrollment. We declare that our study was conducted in accordance with the Declaration of Helsinki.
Sample Collection
Blood specimens (5 mL) were drawn from the antecubital vein of all participants after an overnight fast. Serum samples were centrifuged at 1000× g for 15 min. The supernatant was recovered in 2-mL cryogenic vials and frozen at −80 °C until the time of assay.
Biomarkers of Depression Risk
Serum TNC levels were measured using a commercially available indirect sandwich enzyme-linked immunosorbent assay involving capture antibodies and biotinylated detection antibodies (CUSABIO, Wuhan, China). The kit has a manufacturer-specified detection limit of 7.8 pg/mL. All samples were measured in duplicate. The following lipid-related biomarkers were assayed as described18 using an Advia 2400 automatic analyzer (Siemens, Erlangen, Germany): total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and high-sensitivity C-reactive protein (hs-CRP). The following thyroid-related biomarkers were determined using an Advia Centaur CP system (Siemens): free thyroxine, free triiodothyronine, thyroid-stimulating hormone, and testosterone.
Statistical Analysis
Data were analyzed using SPSS 19.0 (IBM, NY, USA), and figures were generated using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). Continuous data were reported as mean ± SD if normally distributed, or as median if data were skewed. Categorical data were reported as percentages. Intergroup differences in normally distributed continuous data were assessed for significance using Student’s t test, while differences in skewed continuous data were assessed using the Mann–Whitney test. Differences in categorical data were assessed using the χ2-test. Cohen’s d effect sizes (d) were calculated for serum concentrations of testosterone and TNC in MDD patients and healthy controls.
For analyses of groups stratified by clinicodemographic factors as well as for analysis of covariance ANCOVA, we transformed TNC levels to a log10 scale and adjusted for age, smoking status, alcohol use, and body mass index. Multivariate linear regression with bootstrap resampling was used to assess the correlation between TNC and testosterone, after adjusting for age, duration and severity of disease, and levels of lipid- and thyroid-related biomarkers. P<0.05 was regarded as statistically significant.
Results
Study Population
The mean age of all 649 subjects was 35.89 ± 13.57 yr (Table 1). Men with MDD were more likely to be older, less educated, and to have worse lifestyle and health indicators. The average age at MDD diagnosis was 29.34 ± 13.52 yr, and the mean duration of disease was 36.47 ± 31.72 months. The severity of depression was “severe” in 26.73% of patients.
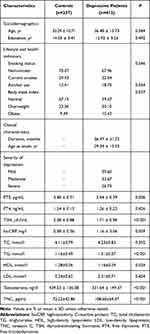 |
Table 1 Characteristics of the Study Population |
MDD patients showed significantly lower serum levels of free triiodothyronine, thyroid-stimulating hormone, and high-density lipoprotein cholesterol. Conversely, patients showed significantly higher serum levels of hs-CRP and triglyceride. The two groups showed similar levels of free thyroxine, total cholesterol, and low-density lipoprotein cholesterol. Additionally, our study demonstrated that serum contents of testosterone were dramatically lower in MDD subjects in comparison to healthy controls (P<0.001) with Cohen’s d effect size difference of −0.69. Furthermore, the serum concentrations of TNC in MDD patients were significantly increased compared with healthy controls (P<0.001) with Cohen’s d effect size difference of −0.62.
Characteristics of MDD Patients After Stratification by Testosterone Levels
The 412 patients with MDD were stratified into tertiles based on testosterone levels: <237.64 ng/dl was the low tertile (n=136), ≥237.64 ng/dl to ≤346.20 ng/dl was the middle tertile (n=137), and >346.20 ng/dl was the high tertile (n=139). The three groups did not differ significantly in age, smoking status, alcohol use, body mass index, or age at onset (Figure 1A-C). Patients in the lowest tertile had had more severe MDD for significantly longer than those in the middle and highest tertiles (Figure 1D-E). Patients in the lowest tertile also had significantly higher concentrations of free triiodothyronine and high-density lipoprotein cholesterol (Figure 1F). However, all groups showed similar levels of the remaining biomarkers for depression.
Association Between Serum TNC and Testosterone Levels in Men with MDD
TNC levels were compared among men with MDD stratified by testosterone level. When covariance analyses adjusted for age, smoking status, alcohol use, body mass index, age at onset, and severity of depression, the concentration of TNC fell dramatically (346.20 pg/mL to 126.55±15.58 pg/mL) with increasing testosterone levels (Figure 2).
Relationship Between Testosterone and TNC Levels in Men with MDD After Stratification by Clinicodemographic Variables
Patients were stratified according to age, smoking status, alcohol use, and body mass index. Then, a linear regression was performed to relate log10-transformed TNC levels in patients with their respective testosterone levels (Table 2). In nearly all cases, serum levels of TNC and testosterone negatively correlated with each other, and testosterone was particularly low in older patients who had suffered from more severe depression for longer periods of time. These patients also had lower concentrations of free triiodothyronine and free thyroxine, but higher levels of thyroid-stimulating hormone, low- and high-density lipoprotein cholesterol and hs-CRP.
 |
Table 2 Adjusted Relationships Between Serum Levels of Testosterone and TNC Across All Patients Stratified by Clinical Factors |
Clinicodemographic Variables Affecting the Relationship Between Levels of Testosterone and TNC in Men with MDD
Linear regression with bootstrap resampling (n=368) also showed a significant negative association between testosterone and TNC after adjusting for age, duration and severity of depression, and depression biomarkers (β-coefficient = −0.037, P=0.004; Table 3).
 |
Table 3 Multivariate Linear Regression to Identify Factors Affecting the Relationship Between Levels of Testosterone and Tenascin-C in Serum Across All Patients |
Discussion
To our knowledge, this cross-sectional study is the first report analyzing correlations between serum levels of TNC and testosterone in men with MDD. We found that MDD was associated with significantly higher TNC and significantly lower testosterone levels after adjusting for age, smoking status, alcohol use, body mass index, age at onset, and severity of depression. The two biomarker levels correlated negatively with each other after patients were stratified by almost any clinicodemographic variable considered in our study. This negative correlation was confirmed in adjusted multivariate linear regression with bootstrap resampling.
TNC is an extracellular matrix glycoprotein expressed not only during the normal development of multiple organisms but also in the progression of pathological processes, including inflammation and tissue impairment as well as psychiatric disorders.19–21 It acts as a growth factor and immunomodulator and has been implicated in cognitive decline.22 TNC deficiency improves dopaminergic neuron survival and regulates microglial functions in vitro23,24 suggesting that TNC may help drive neurodegeneration in the central nervous system. In contrast, under physiological conditions, TNC helps maintain neurons and promotes regeneration of the peripheral nervous system.25 These findings suggest that under pathological conditions, TNC may contribute to the onset and progression of mental illness, which may include MDD.
Due to the role of TNC, it may serve as a viable biomarker for nervous system diseases, particularly those derived from inflammatory disorders, such as psychiatric disease. In vitro studies have described a positive association between levels of TNC and C-reactive protein in endothelial cells.26 In patients with Parkinson’s disease, higher TNC levels correlate with age at disease onset and with the presence of dementia.19 Conversely, TNC deficiency in mice improves locomotion and exploration as well as reduces anxiety-related behaviors.27 More work should be performed to test the reliability of TNC as a biomarker of neuronal diseases and determine cut-off levels predictive of pathology.
Our results suggest that low circulating testosterone levels are associated with longer duration and higher severity of MDD. Testosterone is a vasoactive hormone that mediates vasodilatory features of several psychiatric disorders.28 It may mitigate depressive behaviors by inducing the expression of brain-derived neurotrophic factor and promoting neurogenesis in the hippocampus. Testosterone may also interact directly with the androgen receptor or undergo aromatization into estrogen metabolites in the brain, which activate mitogen-activated protein kinase signaling, ultimately improving emotion and mood.28–30 Consistent with this idea, giving testosterone to gonadectomized male rats reduces depressive behaviors, and this correlates with aromatization of the hormone into estrogen metabolites in the dentate gyrus.9 Whatever the protective mechanisms of testosterone, they may decrease with age, since levels of the hormone fall as men grow older.31,32 Additionally, the inflammatory cytokine (c-reactive protein, CRP) levels were found to be significantly increased in patients with MDD,33 and testosterone levels were reversely associated with CRP.34 These findings suggest that there is an association between testosterone levels and MDD risk. Furthermore, the mean leukocyte counts significantly decreased with increasing testosterone in middle-aged and elderly men,35 and TNC could induce synthesis of proinflammatory cytokines, such as IL-6, which resulted in T-helper (Th)17 cell differentiation.36 It is stated that a relationship between TNC levels and MDD risk is introduced. Thus, the alterations of CRP and lymphocyte factor levels in MDD could partly explain the associations observed for testosterone and TNC.
The inverse correlation between TNC and testosterone in our study is consistent with previous studies showing that both molecules affect inflammation. Testosterone exerts anti-inflammatory effects partly by down-regulating the expression of the inflammatory factors MCP-1, TNF-α, and IL-6 in macrophages.37 TNC, for its part, affects the accumulation, apoptosis, and necrosis of macrophages.38 In addition, testosterone may directly alter the expression and secretion of TNC, as shown in certain cancer cells.39–42 Thus, reduced circulating testosterone levels may induce the expression of TNC in neurons or microglia of the central nervous system, which may contribute to MDD.
In men with MDD, circulating TNC concentrations negatively correlate with testosterone concentrations. TNC up-regulation in the presence of testosterone deficiency may help drive MDD progression. Thus, exogenous androgen should be supplemented in time to reduce the production of neuroinflammatory cytokines induced by the upregulation of TNC expression in the population with testosterone deficiency and prevent the occurrence or development of MDD. Moreover, the inverse relation between the two markers (testosterone and TNC) or the upregulation of TNC expression by androgen deficiency will be further studied in cell or animal models in vitro.
In summary, to the best of our knowledge, this is the first-ever research on depressed patients to define the relationship between serum testosterone levels and TNC. Decreased serum levels of testosterone and increased concentrations of TNC were found in patients with MDD, and this might contribute to the abnormal neurological physiology. These changes in serum levels arise independently and may be correlated to the risk for developing MDD.
Limitation
Unfortunately, our cross-sectional data do not allow us to explore whether changes in testosterone levels cause changes in TNC levels, which should be tested in the future longitudinal work, preferably with a larger sample. We cannot exclude the possibility that our results are affected by unrecognized confounders, although we did adjust for numerous variables taken from the literature.
Acknowledgments
We thank the Wuhan Univ, Renmin Hospital, to provide for the technical consultation. This study was supported by the Fundamental Research Funds for the Central Universities (No. 2042020kf0064).
Disclosure
The authors declare that they have no conflict of interests for this work.
References
1. Chiriţă AL, Gheorman V, Bondari D, Rogoveanu I. Current understanding of the neurobiology of major depressive disorder. Rom J Morphol Embryol. 2015;56:651–658.
2. Cuijpers P, Beekman AT, Reynolds CF
3. Ostinelli EG, Zangani C, Giordano B, et al. Depressive symptoms and depression in individuals with internet gaming disorder: a systematic review and meta-analysis. J Affect Disord. 2021;284:136–142.
4. Ferhat L. Chevassus au Louis N, Jorquera I, et al. Transient Increase of Tenascin-C in Immature Hippocampus: Astroglial and Neuronal Expression. J Neurocytol Actions. 1996;25:53–66.
5. Bot M, Chan MK, Jansen R, et al. Serum proteomic profiling of major depressive disorder. Transl Psychiatry Actions. 2015;5:e599.
6. Giltay EJ, van der Mast RC, Lauwen E, Heijboer AC, de Waal MWM, Comijs HC. Plasma testosterone and the course of major depressive disorder in older men and women. Am J Geriatr Psychiatry. 2017;25:425–437.
7. Justin M, Johnson MD, Lisa B, Nachtigall MD, Theodore A, Stern MD. The effect of testosterone levels on mood in men: a review. Psychosomatics. 2013;54:509–514.
8. Carrier N, Saland SK, Duclot F, He H, Mercer R, Kabbaj M. The anxiolytic and antidepressant-like effects of testosterone and estrogen in gonadectomized male rats. Biol Psychiatry. 2015;78:259–269.
9. Yarrow JF, Wronski TJ, Borst SE. Testosterone and adult male bone: actions independent of 5α-reductase and aromatase. Exerc Sport Sci Rev. 2015;43:222–230.
10. Peng R, Dai W, Li Y. Neuroprotective effect of a physiological ratio of testosterone and estradiol on corticosterone-induced apoptosis in PC12 cells via Traf6/TAK1 pathway. Toxicol in Vitro. 2018;50:257–263.
11. Krysiak R, Kowalcze K, Okopień B. The effect of vitamin D on thyroid autoimmunity in euthyroid men with autoimmune thyroiditis and testosterone deficiency. Pharmacol Rep. 2019;71:798–803.
12. Pektas SD, Cinar N, Duman DD, et al. The relationship among androgens, insulin resistance and ghrelin polymorphisms in post-adolescent male patients with severe acne vulgaris. Postepy Dermatol Alergol. 2020;37:800–809.
13. Bianchi VE. The anti-inflammatory effects of testosterone. J Endocr Soc. 2018;3:91–107.
14. Xue Y, van der Laak JA, Latijnhouwers MA, Smedts F, van der Poel HG, de la Rosette JJ. Transient tenascin enhancement is an early event after androgen ablation in rat prostate. Urol Res. 1999;27:9–15.
15. Peng R, Dai W, Li Y. High serum levels of tenascin-C are associated with suicide attempts in depressed patients. Psychiatry Res. 2018;268:60–64.
16. Anjum S, Qusar MMAS, Shahriar M, Islam SMA, Bhuiyan MA, Islam MR. Altered serum interleukin-7 and interleukin-10 are associated with drug-free major depressive disorder. Ther Adv Psychopharmacol. 2020;10:2045125320916655.
17. Han SB, Lee SH, Ha IH, Kim EJ. Association between severity of depressive symptoms and chronic knee pain in Korean adults aged over 50 years: a cross-sectional study using nationally representative data. BMJ Open. 2019;9:e032451.
18. Peng R, Li Y. Low serum thyroid-stimulating hormone levels are associated with lipid profile in depressive patients with long symptom duration. J Affect Disord. 2017;217:99–104.
19. Wang LG, Huangfu XQ, Tao B, Zhong GJ, Le ZD. Serum tenascin-C predicts severity and outcome of acute intracerebral hemorrhage. Clin Chim Acta. 2018;481:69–74.
20. Matsumoto KI, Aoki H. The roles of Tenascins in cardiovascular, inflammatory, and heritable connective tissue diseases. Front Immunol. 2020;11:609752.
21. Mi Z, Halfter W, Abrahamson EE, Klunk WE, Mathis CA, Mufson EJ. Tenascin-C is associated with cored amyloid-β plaques in Alzheimer disease and pathology burdened cognitively normal elderly. J Neuropathol Exp Neurol. 2016;75:1190.
22. Vasanthi M, Adole PS, Pandit VR, Vinod KV. Assessment of serum tenascin-C and growth differentiation factor-15 among type 2 diabetes mellitus patients with and without acute coronary syndrome. J Med Biochem. 2020;39:460–466.
23. Berns EJ, Álvarez Z, Goldberger JE, Boekhoven J, Kessler JA, Kuhn HG. A tenascin-C mimetic peptide amphiphile nanofiber gel promotes neurite outgrowth and cell migration of neurosphere-derived cells. Acta Biomater. 2016;37:50–58.
24. Haage V, Elmadany N, Roll L, Faissner A, Gutmann DH, Semtner M. Tenascin C regulates multiple microglial functions involving TLR4 signaling and HDAC1. Brain Behav Immun. 2019;81:470–483.
25. Faissner A, Roll L, Theocharidis U. Tenascin-C in the matrisome of neural stem and progenitor cells. Mol Cell Neurosci. 2017;81:22–31.
26. Yuan W, Zhang W, Yang X, Zhou L, Hanghua Z, Xu K. Clinical significance and prognosis of serum tenascin-C in patients with sepsis. BMC Anesthesiol. 2018;18:170.
27. Hamza O, Kiss A, Kramer AM, et al. Tenascin C promotes valvular remodeling in two large animal models of ischemic mitral regurgitation. Basic Res Cardiol. 2020;115:76.
28. Shen T, Wang W, Zhou W, et al. MAPK4 promotes prostate cancer by concerted activation of androgen receptor and AKT. J Clin Invest. 2021;131:e135465.
29. Trentani A, Kuipers SD, Ter Horst GJ, Den Boer JA. Selective chronic stress-induced in vivo ERK1/2 hyperphosphorylation in medial prefrontocortical dendrites: implications for stress-related cortical pathology? Eur J Neurosci. 2002;15:1681–1691.
30. Carrier N, Kabbaj M. Extracellular signal-regulated kinase 2 signaling in the hippocampal dentate gyrus mediates the antidepressant effects of testosterone. Biol Psychiatry. 2012;71:642–651.
31. Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Norman PE. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography-tandem mass spectrometry in a population-based cohort of older men. J Clin Endocrinol Metab. 2012;97:4030–4039.
32. Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598.
33. Xia QR, Liang J, Cao Y, Shan F, Liu Y, Xu YY. Increased plasma nesfatin-1 levels may be associated with corticosterone, IL-6, and CRP levels in patients with major depressive disorder. Clin Chim Acta. 2018;480:107–111.
34. Rastrelli G, Di Stasi V, Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9(1):88–98.
35. Park B, Lee YJ. Inverse association of testosterone and sex hormone binding globulin levels with leukocyte count in middle-aged and elderly men. Exp Gerontol. 2019;117:38–44.
36. Machino-Ohtsuka T, Tajiri K, Kimura T, et al. Tenascin-C aggravates autoimmune myocarditis via dendritic cell activation and Th17 cell differentiation. J Am Heart Assoc. 2014;3(6):e001052.
37. Yamazaki H, Kushiyama A, Sakoda H, Fujishiro M, Yamamotoya T, Nakatsu Y. Protective effect of sex hormone-binding globulin against metabolic syndrome: in vitro evidence showing anti-inflammatory and lipolytic effects on adipocytes and macrophages. Mediators Inflamm. 2018;2018:3062319.
38. Wang Z, Wei Q, Han L, Cao K, Lan T, Xu Z. Tenascin-c renders a proangiogenic phenotype in macrophage via annexin II. J Cell Mol Med. 2018;22:429–438.
39. Soares HD, Potter WZ, Pickering E, Kuhn M, Immermann FW, Shera DM. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch Neurol. 2012;69:1310–1317.
40. Duval F, Mokrani MC, Erb A, Gonzalez Lopera F, Alexa C, Proudnikova X. Chronobiological hypothalamic-pituitary-thyroid axis status and antidepressant outcome in major depression. Psychoneuroendocrinology. 2015;59:71–80.
41. Cai C, Luo J, Liu Q, et al. Claspin overexpression promotes tumor progression and predicts poor clinical outcome in prostate cancer. Genet Test Mol Biomarkers. 2021;25:131–139.
42. Maetzler W, Deleersnijder W, Hanssens V, Bernard A, Brockmann K, Marquetand J. GDF15/MIC1 and MMP9 cerebrospinal fluid levels in Parkinson’s disease and lewy body dementia. PLoS One. 2016;11:e0149349.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.