Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Association study of genetic variants of the ANGPTL3 gene and susceptibility to ischemic stroke
Authors Gong Q, Ye L, Gui H, Liu J, Li H , Sun Q
Received 11 May 2019
Accepted for publication 3 August 2019
Published 24 October 2019 Volume 2019:15 Pages 3015—3020
DOI https://doi.org/10.2147/NDT.S215387
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Qi Gong,1,* Liping Ye,2,* Huiwen Gui,1 Jing Liu,1 Huanyin Li,1 Qian Sun1
1Department of Neurology, Minhang Branch, Zhongshan Hospital, Fudan University, Shanghai 201199, People’s Republic of China; 2Nursing Department, Minhang Branch, Zhongshan Hospital, Fudan University, Shanghai 201199, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huanyin Li
Department of Neurology, Minhang Branch, Zhongshan Hospital, Fudan University, 170 Shenlong Road, Minhang District, Shanghai 201199, People’s Republic of China
Tel/Fax +86 216 492 3400
Email [email protected]
Background: Stroke ranks as the third-leading cause of years of life lost worldwide. ANGPTL3 plays important roles in lipid metabolism, atherosclerosis, and occurrence of stroke. The purpose of this study was to evaluate associations of genetic variants in the ANGPTL3 gene with ischemic stroke (IS) risk.
Methods: A case–control study was conducted to evaluate the associations of tag single-nucleotide polymorphisms (SNPs) of the ANGPTL3 gene and risk of IS, as well as serum lipid levels. Dual-luciferase reporter assays in the HEK293T cell line was conducted to evaluate the promoter activity of ANGPTL3 rs6690733.
Results: We found rs6690733 (C vs A: OR 1.34, 95% CI 1.13–1.59; P=0.001) and rs12563308 (C vs T: OR 0.77, 95% CI 0.64–0.93, P=0.007) were significantly associated with susceptibility to IS. Even corrected for Bonferroni adjustment, the two variants were still significant (0.007×4=0.028). Carriers of the minor allele of SNP rs6690733 had significantly higher levels of TC and LDL-C, while carriers of the minor allele of SNP rs12563308 had significantly lower levels of TC and LDL-C (all P<0.05). For rs6690733, the luciferase assay showed that promoter activity was significantly increased by 67% of plasmids containing the minor C allele compared with the major A allele in HEK293 cells.
Conclusion: Our study revealed genetic variants of the ANGPTL3 gene could contribute to susceptibility to IS through participating in the regulation of lipid metabolism.
Keywords: ischemic stroke, ANGPTL3, genetic, atherosclerosis
Introduction
A major threat to health and quality of human life, stroke has raised public concern.1 According to the 2017 Global Burden of Diseasestudy, stroke causes 6.1673 million deaths annually and is the third-leading cause of years of life lost worldwide.2 In China, stroke (over 2 million new cases annually) has been associated with the highest disability.3 The rising global burden of stroke-related disability provided the impetus to direct our research focus toward risk factors and effective measures of stroke prevention.4 Ischemic stroke (IS) accounts for about 87% of all strokes, while atherosclerosis is a major cause of IS.5,6 It is estimated that extracranial and intracranial large-vessel atherosclerosis account for about 20% of IS cases.7 Therefore, exploration of related mechanisms of atherosclerosis is of great significance for the prevention and treatment of IS.
ANGPTL3, an endogenous inhibitor of lipoprotein lipase and endothelial lipase, is involved in the metabolic regulation of triglycerides, LDL-C, and HDL-C, as well as atherosclerosis in mice and humans.8,9 Exon sequencing has revealed that ANGPTL3 mutations play an important role in LDL-C metabolism in humans.10 A large-scale epidemiological study also identified that genetic and therapeutic antagonism of ANGPTL3 in humans and mice was associated with decreased levels of all three major lipid fractions and decreased odds of atherosclerotic cardiovascular disease.11 In the current study, we hypothesized that genetic variants of the ANGPTL3 gene could also influence susceptibility to IS, one major consequence of abnormal lipid metabolism. We conducted this case–control study in a Chinese population, with functional validation experiments in vitro to test our hypotheses.
Methods
Study subjects
Consecutive IS patients were prospectively screened for enrollment, with the diagnosis confirmed by two neurologists. Diagnosis of IS was based on clinical history and neurological examination of patients and confirmed by brain computed-tomography imaging and basal laboratory tests. Patients were excluded if they had a history of stroke, had received treatment before admission, including statins, or had systemic diseases. Healthy controls were recruited from people without a history of stroke, myocardial infarction, or systemic diseases receiving health examinations in our hospital during the same study period. Finally, 989 IS patients and 990 healthy volunteers were included in this study. All participants were interviewed face to face using a structured questionnaire. Venous blood (5 mL) was collected from each study subject by venipuncture in EDTA-containing tubes, and genomic DNA was isolated from fresh blood samples using a TianAmp blood DNA kit (Tiangen Biotech, Beijing, China) and stored at −80°C until further use. This study was approved by the Ethics Committee of the Minhang Branch of Zhongshan Hospital. Written informed consent was obtained from every participant, and the study was conducted in accordance with the Declaration of Helsinki. The STROBE checklist is given in Supplementary file 1.
Single-nucleotide polymorphism selection and genotyping
With Haploview 4.2 software, tag single-nucleotide polymorphisms (tagSNPs) within the ANGPTL3 gene and its 10 kb flanking region were selected according to the criteria of minor-allele frequency >5% in the Chinese Han population in 1,000-genome phase III data. Linkage-disequilibrium r2 values should be <0.8 for candidate SNPs. Finally, four candidate SNPs (rs12048208, rs6690733, rs12563308, and rs72641123) were included in this case–control study. Genotyping was conducted with TaqMan on an ABI Prism 7900HT fast real-time PCR system (Applied Biosystems). For quality control, 5% of the samples were randomly selected for repeated genotyping. Repeatability of results was 100%.
Plasmid constructs, cell culture, and luciferase assays
To construct reporter plasmids with ANGPTL3 promoters, promoter fragments containing rs6690733 were amplified and subcloned into KpnI and XhoI restriction sites upstream of the luciferase gene in a pGL3 basic vector (Promega, Madison, WI, USA). The recombinant plasmids were verified by DNA sequencing. Then, HEK293T cells were grown in DMEM supplemented with 10% FBS. Transfections with 800 ng of each ANGPTL3 reporter plasmid (pGL3-basic, pGL3-G, and pGL3-A) were conducted using Lipofectamine 3000 (Invitrogen) for each cell line. Luciferase assays were performed 24 hours later using a dual-luciferase reporter–assay system (Promega) according to the manufacturer’s instructions. Three independent experiments were performed for each reporter.
Statistical analysis
Differences in the distribution of demographic variables between IS cases and healthy controls were evaluated by Pearson’s χ2 or Student’s t-test. The distribution of genotypes for the four tagSNPs was evaluated for violation of Hardy–Weinberg equilibrium by Pearson's χ2 test. ORs and 95% CIs from logistic regression analyses were calculated to estimate the association between genetic polymorphisms of the ANGPTL3 gene and risk of IS adjusted for age, sex, smoking and drinking status, body-mass index, hypertension, diabetes, and hypercholesterolemia. P<0.05 (two-sided) was considered statistically significant, and all analyses were conducted with SPSS version 22.
Results
General characteristics of participants
Distributions of general characteristics of the study population are presented in Table 1. In brief, there were no statistical differences in distributions of age, sex, smoking status, drinking status, or body-mass index) between IS patients and healthy controls (P<0.05). However, IS cases were more likely to be hypertension, diabetes, and hypercholesterolemia patients (P<0.001).
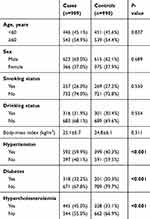 |
Table 1 Distributions of selected variables in ischemic stroke cases and healthy controls |
Associations between genetic variants of the ANGPTL3 gene and susceptibility to IS
Genotype distributions of ANGPTL3 polymorphisms and their associations with IS risk are shown in Table 2. TGenotype frequencies of rs12048208, rs6690733, rs12563308, and rs72641123 in the controls were concordant with Hardy–Weinberg equilibrium (P>0.05). For tagSNPs, we found rs6690733 (C vs A: OR 1.34, 95% CI 1.13–1.59; P=0.001), and rs12563308 (C vs T: OR 0.77, 95% CI 0.64–0.93; P=0.007) were significantly associated with susceptibility to IS. Even corrected for Bonferroni adjustment, the two variants were still significant (0.007×4=0.028). For rs6690733, compared with major AA homozygotes, both AC heterozygotes (OR 1.35, 95% CI 1.10–1.66) and minor CC homozygotes (OR 1.81, 95% CI 1.07–3.04) were associated with increased susceptibility to IS. For rs12563308, compared with major TT homozygotes, both TC heterozygotes (OR 0.82, 95% CI 0.67–1.00) and minor CC homozygotes (OR 0.43, 95% CI 0.18–1.00) were associated with decreased susceptibility to IS. We did not find significant associations for variants rs12048208 and rs7264112.
 |
Table 2 Genetic variants of the ANGPTL3 gene and susceptibility to ischemic stroke (IS) |
Associations of ANGPTL3 rs6690733 and rs12563308 and serum-lipid levels in controls
To further explore the effect of ANGPTL3variants on lipid metabolism, we evaluated associations of ANGPTL3 rs6690733 and rs12563308 and serum-lipid levels in controls. As shown in Table 3, we found that carriers of the minor allele of SNP rs6690733 had significantly higher levels of TC and LDL-C, while carriers of the minor allele of SNP rs12563308 had significantly lower levels of TC and LDL-C (all P<0.05).
 |
Table 3 Associations of ANGPTL3 variants and serum-lipid levels in controls |
Promoter-activity analysis of ANGPTL3 rs6690733
To evaluate the promoter activity of variant rs6690733, we performed in vitro luciferase promoter assays. As shown in Figure 1, assays showed that promoter activity was significantly increased by 67% of plasmids containing the minor C allele compared with the major A allele in HEK293 cells. This suggested that the C allele has higher transcription activity than the A allele.
Discussion
We explored associations between genetic variants of the ANGPTL3 gene and susceptibility to IS in a large-scale case–control study in a Chinese population. We found that rs6690733 and rs12563308 were significantly associated with susceptibility to IS. Also, carriers of the minor allele of SNP rs6690733 had significantly higher levels of TC and LDL-C, while carriers of the minor allele of SNP rs12563308 had significantly lower levels of TC and LDL-C. Further dual-luciferase reporter assays showed that the rs6690733 C allele had lower levels of luciferase activity than the rs6690733 A allele. Our findings indicate that ANGPTL3 rs6690733 and rs12563308 may contribute to susceptibility to IS in the Chinese population through participating in the regulation of lipid metabolism.
ANGPTL3 has been mapped to the 1p31 region, and is expressed principally in the liver.12 In 2002, Koishi et al9 first that reported ANGPTL3 could regulate lipid metabolism in mice. It can decrease very low–density lipoprotein–triglyceride clearance by inhibition of lipoprotein lipase and stimulate endothelial cell adhesion and migration via integrin αvβ3, and induces blood-vessel formation.13,14 For these reasons, it was thought to be a new drug target for treatment of dyslipidemia very early.15 Clinical studies revealed that plasma ANGPTL3 was associated with arterial wall thickness, uremic dyslipidemia, hepatic triglyceride lipase, and rheumatic diseases.16–18 Genetic variants of ANGPTL3 have also been evaluated in many different kinds of diseases.11,19–24 Járomi et al25 evaluated the association of ANGPTL3 rs12130333 (minor-allele frequencyin Chinese was 0.024) with risk of IS in 459 Caucasian stroke patients and 168 control subjects using PCR and restriction fragment length–polymorphism methods and got null results, which might have been caused by low statistical power. Bokor et al26 found that the ANGPTL3 rs11207997 polymorphism (which was in high linkage disequilibrium with rs6690733) was associated with lower plasma HDL-C in adolescents. In a previous study by Li et al,27 rs12563308 SNP was associated with a decreased risk of coronary artery disease (dominant — OR 0.69, 95% CI 0.45–0.94, P=0.011; log-additive — OR 0.73, 95% CI 0.49–0.89, P=0.009), but not susceptibility to IS. This might have been caused by limited statistical power. In our study, we not only found rs12563308 was significantly associated with decreased risk of IS but also detected carriers of the minor allele of SNP rs12563308 had significantly lower levels of TC and LDL-C.
This study had several strengths. First, to the best of our knowledge, we confirmed the function of rs6690733 and rs12563308 in both lipid metabolism and susceptibility to IS. Second, using Quanto 1.2.4 software, we found our studies had 94.1% statistical power to detect such an association between rs6690733 and susceptibility to IS. Third, the dual-luciferase reporter assay showed that the rs6690733 C allele had lower levels of luciferase activity than the rs6690733 A allele. Several limitations remained in our study. First, Neyman bias might have been present. Second, potential selection bias for this hospital-based case–control study was unavoidable.
Conclusion
Our study reveals that genetic variants of the ANGPTL3 gene can contribute to susceptibility to IS in the Chinese population through participating in the regulation of lipid metabolism. Future exploration of the functional mechanism of the ANGPTL3 gene and its biological function in lipid metabolism should be conducted to determine the etiology of IS.
Author contributions
All authors contributed to data analysis and drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ekker MS, Boot EM, Singhal AB, et al. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol. 2018;17(9):790–801. doi:10.1016/S1474-4422(18)30233-3
2. Collaborators GBDCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi:10.1016/S0140-6736(18)32203-7
3. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. doi:10.1016/S1474-4422(18)30500-3
4. Pandian JD, Gall SL, Kate MP, et al. Prevention of stroke: a global perspective. Lancet. 2018;392(10154):1269–1278. doi:10.1016/S0140-6736(18)31269-8
5. Borne Y, Fagerberg B, Persson M, et al. Cadmium, carotid atherosclerosis, and incidence of ischemic stroke. J Am Heart Assoc. 2017;6:12. doi:10.1161/JAHA.117.006415
6. Sundstrom J, Soderholm M, Borne Y, et al. Eosinophil cationic protein, carotid plaque, and incidence of stroke. Stroke. 2017;48(10):2686–2692. doi:10.1161/STROKEAHA.117.018450
7. Marulanda-Londono E, Chaturvedi S. Stroke due to large vessel atherosclerosis: five new things. Neurol Clin Pract. 2016;6(3):252–258. doi:10.1212/CPJ.0000000000000247
8. Tikka A, Jauhiainen M. The role of ANGPTL3 in controlling lipoprotein metabolism. Endocrine. 2016;52(2):187–193. doi:10.1007/s12020-015-0838-9
9. Koishi R, Ando Y, Ono M, et al. Angptl3 regulates lipid metabolism in mice. Nat Genet. 2002;30(2):151–157. doi:10.1038/ng814
10. Musunuru K, Pirruccello JP, Do R, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010;363(23):2220–2227. doi:10.1056/NEJMoa1002926
11. Dewey FE, Gusarova V, Dunbar RL, et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377(3):211–221. doi:10.1056/NEJMoa1612790
12. Conklin D, Gilbertson D, Taft DW, et al. Identification of a mammalian angiopoietin-related protein expressed specifically in liver. Genomics. 1999;62(3):477–482. doi:10.1006/geno.1999.6041
13. Shimizugawa T, Ono M, Shimamura M, et al. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J Biol Chem. 2002;277(37):33742–33748. doi:10.1074/jbc.M203215200
14. Camenisch G, Pisabarro MT, Sherman D, et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J Biol Chem. 2002;277(19):17281–17290. doi:10.1074/jbc.M109768200
15. Naoumova RP, Betteridge DJ. A new drug target for treatment of dyslipidaemia associated with type 2 diabetes and the metabolic syndrome? Lancet. 2002;359(9325):2215–2216.
16. Hatsuda S, Shoji T, Shinohara K, et al. Association between plasma angiopoietin-like protein 3 and arterial wall thickness in healthy subjects. J Vasc Res. 2007;44(1):61–66. doi:10.1159/000098153
17. Shoji T, Hatsuda S, Tsuchikura S, et al. Plasma angiopoietin-like protein 3 (ANGPTL3) concentration is associated with uremic dyslipidemia. Atherosclerosis. 2009;207(2):579–584. doi:10.1016/j.atherosclerosis.2009.05.023
18. Hayashi Y, Jinnin M, Makino T, et al. Serum angiopoietin-like protein 3 concentrations in rheumatic diseases. Eur J Dermatol. 2012;22(4):500–504. doi:10.1684/ejd.2012.1774
19. Yang Y, Yang S, Jiao X, et al. ANGPTL3 mutations in unrelated Chinese Han patients with familial hypercholesterolemia. Curr Pharm Des. 2019. doi:10.2174/1381612825666190228000932
20. Jiang S, Qiu GH, Zhu N, Hu ZY, Liao DF, Qin L. ANGPTL3: a novel biomarker and promising therapeutic target. J Drug Target. 2019;1–9. doi:10.1080/1061186X.2019.1616296
21. Luo F, Guo Y, Fang Z, Li X. Concerns on the genetic or therapeutic antagonism of ANGPTL3. J Am Coll Cardiol. 2017;70(16):2099. doi:10.1016/j.jacc.2017.04.053
22. Tikka A, Metso J, Jauhiainen M. ANGPTL3 serum concentration and rare genetic variants in Finnish population. Scand J Clin Lab Invest. 2017;77(8):601–609. doi:10.1080/00365513.2017.1379608
23. Hanson RL, Leti F, Tsinajinnie D, et al. The Arg59Trp variant in ANGPTL8 (betatrophin) is associated with total and HDL-cholesterol in American Indians and Mexican Americans and differentially affects cleavage of ANGPTL3. Mol Genet Metab. 2016;118(2):128–137. doi:10.1016/j.ymgme.2016.04.007
24. Sumegi K, Jaromi L, Magyari L, et al. Functional variants of lipid level modifier MLXIPL, GCKR, GALNT2, CILP2, ANGPTL3 and TRIB1 genes in healthy Roma and Hungarian populations. Pathol Oncol Res. 2015;21(3):743–749. doi:10.1007/s12253-014-9884-5
25. Járomi L, Csöngei V, Polgár N, et al. Triglyceride level-influencing functional variants of the ANGPTL3, CILP2, and TRIB1 loci in ischemic stroke. Neuromolecular Med. 2011;13(3):179–186. doi:10.1007/s12017-011-8149-7
26. Legry V, Bokor S, Cottel D, et al. Associations between common genetic polymorphisms in angiopoietin-like proteins 3 and 4 and lipid metabolism and adiposity in European adolescents and adults. J Clin Endocrinol Metab. 2009;94(12):5070–5077. doi:10.1210/jc.2009-0769
27. Li WJ, Yin RX, Cao XL, Chen WX, Huang F, Wu JZ. DOCK7-ANGPTL3 SNPs and their haplotypes with serum lipid levels and the risk of coronary artery disease and ischemic stroke. Lipids Health Dis. 2018;17(1):30. doi:10.1186/s12944-018-0677-9
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

