Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Association Plasma Aβ42 Levels with Alzheimer’s Disease and Its Influencing Factors in Chinese Elderly Population
Authors Wu Y , Wang Z, Yin J , Yang B, Fan J, Cheng Z
Received 16 May 2022
Accepted for publication 17 August 2022
Published 24 August 2022 Volume 2022:18 Pages 1831—1841
DOI https://doi.org/10.2147/NDT.S374722
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yue Wu1 *, Zhiqiang Wang2 *, Jiajun Yin,3 Bixiu Yang,2 Jie Fan,1 Zaohuo Cheng1
1Department of Geriatric Psychiatry, The Affiliated Wuxi Mental Health Center of Jiangnan University, Wuxi, People’s Republic of China; 2Department of Clinical Psychology, The Affiliated Wuxi Mental Health Center of Jiangnan University, Wuxi, People’s Republic of China; 3Brain Science Basic Laboratory, The Affiliated Wuxi Mental Health Center of Jiangnan University, Wuxi, People’s Republic of China
* These authors contributed equally to this work
Correspondence: Zaohuo Cheng; Jiajun Yin, Department of Geriatric Psychiatry, The Affiliated Wuxi Mental Health Center of Jiangnan University, 156 Qianrong Road, Wuxi, 214151, People’s Republic of China, Tel +86 510 830 30359, Fax +86 510 830 12201, Email [email protected]; [email protected]
Background and Purpose: Intracerebral Aβ protein deposition is an important pathological mechanism of Alzheimer’s disease (AD) and is one of the indicators of early diagnosis of AD. However, invasive lumbar puncture and Aβ PET are difficult to perform in primary units, resulting delays in early diagnosis of AD. In recent years, it has been found that plasma Aβ can reflect the pathological state of AD in early stage, but the results are not consistent. The objective of this study was to explore the association between plasma Aβ 42 levels and AD cognitive impairment and its influencing factors in Chinese elderly population, so as to provide guidance for the clinical application of plasma Aβ 42 as a blood biomarker of AD.
Methods: This is a cross-sectional study based on the community population. Plasma samples were collected from 604 healthy controls (HC), 508 mild cognitive impairment (MCI) and 202 dementia with Alzheimer’s type (DAT) patients from three cities. We analyzed the correlation between plasma Aβ 42 levels and cognitive function and the influence of confounding factors on the relationship between plasma Aβ 42 levels and AD. The independent influencing factors of plasma Aβ 42 levels were determined by covariance and linear regression analysis.
Results: Our results suggest that there is a special linear relationship between plasma Aβ 42 and cognitive impairment of AD in Chinese elderly population, with Aβ 42 levels slightly decreased in early AD and significantly increased in moderate-to-severe AD (P< 0.01). There are many factors influencing the association between plasma Aβ 42 levels and AD cognitive impairment, and sample source, gender and BMI are independent influencing factors of plasma Aβ 42.
Conclusion: This indentifies that plasma Aβ 42 may be a peripheral biomarker for AD screening in Chinese elderly population, but it is necessary to establish standardized detection methods and establish different demarcation criteria for various influencing factors.
Keywords: amyloid-beta 42, Alzheimer’s disease, association analysis, influencing factors, Chinese elderly population
Introduction
With the global population ages, the number of Alzheimer’s disease (AD) is rising sharply and is expected to reach 152 million by the year 2050, which will bring severe challenges to social development.1 AD has no effective treatment at present. Any measure that can detect and monitor pathological changes at an early stage of disease will help prevent or delay the onset of disease and reduce the public health burden.2 Since George proposed that amyloid beta (Aβ) accumulation might be the pathological mechanism of AD in 1984,3 this hypothesis has been increasingly supported by advances in pathology, neurochemistry and genetics4,5 and has dominated the revision of AD prevention protocols and diagnostic criteria for more than 30 years.6–8 The core AD biomarker, Aβ is produced by many cell types in vivo, including neurons and glial cells in the central nervous system, platelets, leukocytes and other cells such as skeletal muscle and vascular wall smooth muscle cells in the peripheral system, and so on. Aβ42 toxic protein is the main cause of the pathogenesis of AD. It was found that there was a large amount of Aβ in the plasma of AD patients, mainly represented by Aβ42, but less in normal controls. Peripheral circulation Aβ42 is mainly derived from platelets and its production mechanism is similar to neurons. Recent studies have found that Aβ can be bidirectionally transported and interact with the brain and peripheral circulation. Peripheral Aβ42 not only reflects the pathological changes of central nervous system but also may play a positive role in the development of AD. Platelets may be a potential source of the amyloid deposits in meningeal vessels and brain parenchyma.9,10 When the blood–brain barrier (BBB) is damaged due to aging or vascular changes, peripheral Aβ42 is likely to enter the brain from the circulation and deposit in the extracellular space of cerebral cortex and vascular wall, and induce other AD pathology, such as tau hyper-phosphorylation, increased neuroinflammatory response and functional deficits of hippocampal neurons.
AD is a continuous pathophysiological process in which amyloidosis develops long before clinical symptoms appear. Both the International Working Group (IWG) in 2014 and the A/T/N classification system of the National Institute on Aging-Alzheimer Association (NIA-AA) in 2018 included biomarkers in the diagnosis of AD, which is a major breakthrough in the field of AD research.7,8 The ideal AD biomarkers should be reliable, repeatable, less invasive and more economical. Cerebrospinal fluid (CSF) Aβ42 is currently the “gold standard” biomarker for AD, however lumbar puncture remains invasive technique and difficult to implement in early screening or large studies. Meanwhile, the amyloid-PET test is very expensive and impractical for population screening. Relevant studies have shown that some biomarkers as Aβ in blood can reflect the pathological state of AD in the early stage, and their application to the clinic may break the current difficulties in the diagnosis and treatment of AD. In the past decades, numerous AD cohort studies have been carried out in Europe and the United States, strongly supporting the important role of plasma biomarkers such as Aβ42 and Tau proteins in the development of AD biomarkers.11,12 However, due to various reasons, such as the short half-life of Aβ in blood, technical problems of detection, disease-related factors, heterogeneity of study design, statistical ability, ethnic differences and sample size, studies on the correlation between blood Aβ and AD pathology and on the diagnosis and prediction of AD are inconsistent. Therefore, it is necessary to establish a prospective, multi-ethnic, multi-center, large-sample cognitive cohort for further study. Currently, there are few large-sample studies on plasma Aβ42 in Chinese populations. Therefore, in this study, we investigated the association between plasma Aβ42 levels and cognitive impairment of AD in 1314 Chinese population aged 50 years and older in the community, and analyzed the factors influencing the correlation between Aβ42 concentration and cognitive impairment.
Materials and Methods
Subjects
This study recruited adults and elderly volunteers aged 50 and above from communities and psychiatric hospital memory clinics from three cities: Wuxi, Jiang-Yi and Hangzhou. All the participants underwent clinical interviews and relevant neurocognitive evaluation, as well as necessary laboratory or imaging examinations. Based on the interview and test results, 1314 valid samples were obtained, including 881 in Wuxi, 248 in Hangzhou and 185 in Jiang-Yi, after excluding the serious physical, neurological and mental disorders that may cause cognitive impairment. The 1314 volunteers were divided into three groups: 604 health control (HC), 508 MCI patients and 202 dementia with Alzheimer’s type (DAT), of which 150 DAT patients were from memory clinics, and the other DAT patients, HC and MCI subjects from community volunteers. The diagnosis of MCI and AD is based on the core clinical criteria recommended by the National Institute on Aging and the Alzheimer’s Association (NIA-AA) workgroup.13,14 There were significant differences in general demographic and clinical data among the three-city groups, including gender, age, education, family, occupation, clinical diagnosis and Clinical Dementia Rating (CDR) scores.15 However, we did not observe significant differences in plasma levels of Aβ42 among the three-city groups. Sample sources and basic information are shown in Table 1.
 |
Table 1 Sample Source and Basic Data |
According to the declaration of Helsinki, all subjects or their guardians signed an informed consent form before participating in the study. This study was approved by the Ethics Committee of Wuxi Mental Health Center.
Procedure
Neurocognitive and Psychological Assessment
The assessments involved three primary sections: (1) subjective cognitive impairment screening; (2) objective cognitive impairment assessment; and (3) related mental rating. The brief elderly cognitive screening questionnaire screening (BECSI)16 was used to screen subjective cognitive impairment of volunteers in the community. It contains of 13 items aimed at assessing four functional domains, including memory function, temporal orientation, work efficiency, and mental pathology, with total score of more than 4 points indicating subjective cognitive impairment. The Clinical Dementia Rating (CDR), Alzheimer’s Dementia Assessment Scale-cognitive subscale (ADAS-cog),17 and Mini Mental State Examination (MMSE)18 were used to evaluate overall objective cognitive impairment. The criteria for normal cognition were CDR = 0, or ADAS-cog: 0–9, or MMSE: 28–30; mild cognitive impairment were CDR = 0.5, or ADAS-cog: 10–15, or MMSE: 20–27 and severe cognitive impairment were CDR ≥1, or ADAS-cog≥16, or MMSE <20. The clinical grading of AD was mainly based on CDR score, with CDR = 1 for mild AD and CDR = 2–3 for moderate-to-severe AD. Other related psychological rating scales included Activity of Daily Living Scale (ADL),19 Hachinski Ischemic Scale (HIS)20 and Hamilton Depression Scale (HAMD).21
Clinical Interview and Examination
Our clinical interview and examination process had 4 primary sections: (1) social demographic questions: name, gender, age, education, marriage, family status, occupation, etc.; (2) medical history collection and psychiatric examination: memory and cognitive impairment, mental status examination, medicine, family history and individual medical history; (3) physical examination, such as height, weight, heart rate, blood pressure, vision and hearing, etc., with emphasis on neurological examination, such as sensory symmetry, motor function, muscle strength, muscle tension, language function, gait and balance, tremor, etc.; (4) necessary auxiliary examinations: electrocardiograms (ECG), electroencephalograms (EEG), brain computed tomography (CT), blood biochemistry tests, etc.
Plasma Aβ42 Assays and APOE Genotyping
(1) Fasting venous blood was collected from all participants using 2% EDTA-coated vacuums and collected in followed by centrifugation at speeds 3000 rpm for 30 minutes at room temperature. Plasma and leukocytes were collected, respectively, in plastic vials and stored at −80℃ for further analyses; (2) Biomarker measurements were performed at Nanjing Amory Biotechnology Co., LTD. The quantitative sandwich ELISA kit (R&D Systems, Inc. Minneapolis, America) was used to quantify plasma Aβ42 levels. One hundred microliter standard and sample were pipetted to the microplate coated with monoclonal antibody specific for human Amyloid β in concentration order. Following a washing away unbound substances, the cold Human Amyloid β (aa1-42) Conjugate was added to the wells. Repeated the aspiration/wash 3 times, 200μL of Substrate Solution was added to each well. After incubating at room temperature for 30 minutes, 50μL of Stop Solution was added to each well. The color development was stopped, and the intensity of the color was measured. A standard curve was created from which the concentration of Aβ42 was read; (3) DNA extraction and APOEε4 genotyping were performed by Wuxi Biowing Applied Biotechnology Co., Ltd. Leukocyte DNA was extracted using blood genotyping DNA extraction kit (Tiangen Biotech, Beijing, China), and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was used to analyze APOE genotype.
Statistical Analysis
All data were processed with SPSS24.0 software (IBM Corporation, Armonk, NY, USA). Comparisons among groups were performed using Pearson Chi-square test for nominal data or analysis of variance for numeric data, including demographic, clinical and laboratory data, and cognitive scores. Due to the skewed distribution of plasma Aβ42 levels, the data were log-transformed for statistical testing. Pearson correlation coefficients between plasma Aβ42 levels and CDR, ADAS-Cog and MMSE were calculated. ANOVA was used to compare the plasma Aβ42 levels of HC, MCI and DAT subjects after classification by gender, age, education level, occupation, sample origin, APOE ε4 genotype and BMI, and ANCOVA observes the influence of each classification variable on plasma Aβ42 levels. Covariance analysis and linear regression analysis were used to determine the influencing factors of plasma Aβ42. Significant P-value was reported as P<0.05.
Results
Participant Characteristics
A total of 1314 participants were enrolled in this study, including 604 elderly with normal cognition (HC group), 508 patients with MCI (MCI group) and 202 patients with DAT (DAT group). All participants completed clinical interview and examination, neurocognitive and psychological assessment, laboratory tests and plasma Aβ42 assays, of which 990 participants completed body mass index (BMI) and 971 completed APOEε4 genotyping. The demographic, clinical and laboratory data of the three groups are shown in Table 2. Except for gender, blood pressure, blood glucose, BMI, triglyceride, thyroid stimulating hormone, folic acid and vitamin B12, there were significant differences among the three groups in demographic, clinical and laboratory data (P < 0.05 for all). Based on different stages of the disease, DAT patients were divided into mild AD and moderate-to-severe AD, and there were statistically significant differences in Aβ42 levels between HC, MCI, mild AD and moderate-to-severe AD (P<0.001). Post hoc (LSD) analysis showed that the Aβ42 levels in moderate-to-severe AD group (3.87 ± 1.10) were higher than those in the mild AD group (3.40 ± 1.05), MCI group (3.47 ± 1.04) and HC group (3.43 ± 1.08) (P < 0.001). There were no significant differences among mild AD, MCI group and HC group (P > 0.05).
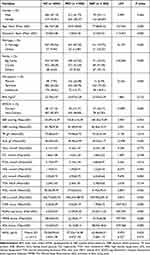 |
Table 2 Demographic, Clinical and Laboratory Data and Cognitive Scores of Three Groups |
Relationship Between Plasma Aβ42 and Cognitive Function
According to CDR, ADAS-cog and MMSE scores, subjects were divided into normal cognitive group, mild cognitive impairment group and severe cognitive impairment group. The plasma Aβ42 differences among the three groups were compared by the ANOVA (age and education as covariates) and the correlation between Aβ42 and cognitive scores was calculated by Pearson correlation analysis (shown in Table 3). After eliminating the effects of age and education, in the CDR groups, participants with severe cognitive impairment had a significantly higher Aβ42 levels compared to those with mild cognitive impairment and normal cognition (P=0.019), and there was a significant correlation between Aβ42 levels and CDR scores (r=0.093); In the ADAS-cog or MMSE groups, no difference for Aβ42 levels were observed among different cognitive function groups, and no correlation was detected between Aβ42 levels and ADAS-cog scores or MMSE scores (P > 0.05 for all).
 |
Table 3 Association Between Aβ42 and Cognitive Function |
Factors Influencing the Association Between Aβ42 and Cognitive Impairment
Stratified by demographic and clinical characteristics (Table 4), when the subjects were female, low education, APOEε4+/ε4-, aged 51–64 and 65–74 years, and samples from Wuxi, the plasma Aβ42 levels in the DAT group were significantly higher than those in the MCI and HC groups (P<0.05 for all), and in physical occupation participants, Aβ42 levels in the MCI and DAT group were higher than those in HC group, while low BMI subjects, Aβ42 levels in the MCI group were higher than those in DAT group (P<0.05). There were no significant differences in Aβ42 levels between HC, MCI and DAT groups in other demographic and clinical characteristic stratifications (P> 0.05 for all).
 |
Table 4 Factors Influencing the Association Between Plasma Aβ42 and Cognitive Impairment |
The Combined Effects of Related Factors on Aβ42
The combined effects of diagnosis, gender, age, education, occupation, and sample source on plasma Aβ42 were examined by covariance analysis and linear regression analysis. As shown in Table 5, both covariance and linear regression showed that effects of diagnosis, gender and sample source were statistically significant (P< 0.05), but the three factors could only explain 1.7% or 1.6% Aβ42 variation. Neither covariance nor linear regression analysis, the effects of age, education, and occupation were not statistically significant (P> 0.05).
 |
Table 5 Combined Effects of Related Factors on Plasma Aβ42 |
Discussion
In this study, we found that the relationship between plasma Aβ42 levels and cognitive impairment of AD was not a simple linear relationship. The level of Aβ42 decreased slightly in the early stage of AD and increased significantly in moderate-to-severe AD, that is, the overall trend of plasma level of Aβ42 increased with the progression of AD, which was consistent with other reports.22–25 A follow-up study by Mayeux et al found that compared with individuals who never developed AD, patients with AD at baseline and those who developed AD during the follow-up had significantly higher plasma Aβ42 levels and high plasma Aβ42 levels may also be associated with mortality in patients with AD.26 The main sources of plasma Aβ42 are platelets and peripheral tissues, and a small part is CSF Aβ42 transported through the blood–brain barrier. Receptor for Advanced Glycation End Products (RAGE) and LDL Receptor-related Protein 1 (LRP1) are transporters of Aβ across the BBB. Studies have shown that the expression and function of RAGE on the BBB of AD patients were significantly enhanced, while LRP1 significantly decreased. In the early AD stage, a large amount of Aβ42 was transported from plasma to cerebrospinal fluid due to the intensification of RAGE mediated inward mobility,27 resulting in decreased peripheral Aβ42 levels. As the disease progresses, the inward transport of Aβ42 is blocked by cerebral amyloid vascular disease (CAA).28 Meanwhile, the scavenging capacity of Aβ42 in liver and kidney and the phagocytic capacity of monocytes decreased gradually. The decrease of intracerebral transport and peripheral clearance may result in significantly elevated plasma Aβ42 levels in patients with moderate-to-severe AD. Hanon et al found that CSF Aβ42 level increased in early AD and decreased with the progression of the disease. They believed that the possible cause was related to plasma Aβ42 transport, which was consistent with our view.29
Recently, Jiao et al reported that plasma levels of Aβ42 were significantly correlated with MoCA scores in AD patients and HC elderly but not with MMSE.30 In this study, Aβ42 levels was positively correlated with CDR score, but not with MMSE or ADAS-cog score, which may be related to the higher efficacy of CDR in screening for AD than the other two scales. CDR is a semi-structured assessment scale, which can effectively identify objective cognitive impairment and severity grade through comprehensive analysis of interviews with the subjects and their informants.31 Its sensitivity and specificity for screening dementia are 95% and 100%, respectively,15,32 both higher than MMSE (92.5%, 79.1%) and ADAS-cog (73.7%, 92.4%).17,18
Assessing the association between cognitive function and plasma biomarkers is an important part of AD protein biomarker research. We confirmed the difference of plasma Aβ42 levels in different clinical stages of AD and the association between plasma Aβ42 and CDR. Meanwhile, we also found that the changes of plasma Aβ42 levels at different stages of AD were affected by gender, age, education level, occupation, sample origin, APOEε4 genotype and BMI. Interestingly, our data showed that women and low cognitive reserve had a similar effect on the relationship between plasma amyloid levels and cognitive decline, with a slight decline in MCI followed by a significant increase in AD, while there is no significant difference in plasma Aβ42 at different stages of AD in male or those with medium or higher education level. The reason may be related to the fact that the education level of elderly female in China is generally lower than that of male. According to the “cognitive reserve hypothesis”, compensatory factors may reduce the correlation between AD pathology and clinical symptoms in highly educated people, and improving cognitive reserve may reduce the risk of Alzheimer’s disease.33 In our study, the effects of age on plasma Aβ42 levels and cognitive impairment in AD were selective. Plasma Aβ42 levels were significantly higher in AD patients aged 50–64 and 65–74 years than in HC and MCI patients, while no significant changes were observed in 75 years and older. It may be related to the decrease of peripheral platelets producing exogenous Aβ due to the relatively weakened function of bone marrow platelets, vascular endothelial injury and increased platelet consumption during aging in the elderly.34 In manual labor group, plasma Aβ42 levels were significantly higher in patients with AD and MCI than in normal controls, which may be related to the relatively low education level or simple lifestyle of manual workers. A number of large multimodal intervention trials have shown that healthy lifestyle interventions could affect Aβ metabolism by increasing brain-derived neurotrophic factor (BDNF), decreasing inflammatory markers (TNF-α and IL-6) and improving insulin sensitivity or cortisol regulation, thus reducing the risk of progression to AD in patients with subject cognitive decline (SCD) and MCI.35,36
Wuxi, Jiang-Yi and Hangzhou are all located in southern China with similar economies, culture and living habits. Plasma Aβ42 levels of participants in the three cities were measured in the same laboratory, but there was no significant change in plasma Aβ42 levels in the other two cities except Wuxi at different stages of AD. This may be related to the different detection time points of samples in different cities. It has been reported that the plasma of AD patients has been hypothesized to have a tendency to foster Aβ42 aggregation compared to healthy subjects, with 87.5% of patients having detected elevated plasma Aβ42 levels over time from baseline at various time points within 24 hours.37 The ε4 allele of APOE is currently the major genetic risk factor identified for AD. Our results suggested that in APOE ε4 carriers, the levels of Aβ42 in AD patients were extremely significantly higher than that in normal control and MCI patients (P< 0.001). Animal studies have shown that APOE-mediated receptor pathway may be the main pathway for Aβ clearance. Peripheral Aβ can be rapidly cleared from plasma via liver and kidney, and its clearance rate is affected by APOE genotype. Compared with APOE ε2 and APOE ε3, ApoEε4 subtype has poor binding to Aβ and the weakest clearance efficiency for Aβ, leading to an increase in plasma Aβ levels.38 Obesity is a risk factor for AD, and total cholesterol can enhance APP protein’s “amyloid processing” pathway and the production of Aβ42 by increasing BACE1 activity and inhibiting the activity of α-secretase.39 Many studies have confirmed that the level of Aβ42 is positively correlated with BMI.40 Our data showed that BMI has a significant effect on plasma Aβ42 levels, and plasma Aβ42 levels increased with BMI in the HC and DAT groups. Interestingly, in low BMI group, Aβ42 levels were significantly lower in AD patients than those in MCI patients and normal controls. Previous studies have reported that when the body adipose tissue cannot be maintained within the physiological range, adipose tissue can secrete and release adipose factors, such as leptin, adiponectin, interleukin, etc., some of these factors slow the formation of Aβ, and some promote the accumulation and deposition of Aβ, but the specific mechanism remains to be further elucidated.41,42
Due to the complexity of the pathological mechanism of AD and the instability of plasma Aβ, demographic and clinical characteristics of subjects were stratified to avoid bias when exploring cross-sectional associations between Aβ42 levels and AD. However, this project is an investigation study based on community grassroots level. Most of the samples are normal elderly people in the community, affected by informed consent and limited by community health resources, and we did not conduct amyloid PET or CSF fluid tests. Therefore, there were certain limitations in our study.
Conclusion
Taken together, there is a special linear relationship between plasma Aβ42 and cognitive impairment of Alzheimer’s disease in community elderly adults. There are many possible factors influencing the association between plasma Aβ42 and AD cognitive impairment, and sample source, gender and BMI are independent influencing factors of plasma Aβ42, although they explain only a small amount of variation. We suggest that plasma Aβ42 may be a peripheral biomarker for AD screening in Chinese elderly population, but it is necessary to further standardize testing procedures, establish cut-off values based on different demographic and clinical characteristics, and conduct cohort studies with long-term follow-up.
Acknowledgments
The authors thank the participating volunteers, patients and their guardians. This study was supported by the Social Development key Projects in Jiangsu Province (BE2015615), Wuxi Municipal Health Commission (Q202131) and Science and Technology Development Foundation of Nanjing Medical University (NMUB2020292).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no actual or potential conflict of interest.
References
1. Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. World Alzheimer Report 2015 The Global Impact Of Dementia An Analysis Of Prevalence, Incidence, Cost, And Trends. London: Alzheimer’s Disease International; 2015.
2. Hampel H, Shen Y, Walsh DM, et al. Biological markers of amyloid β-related mechanisms in Alzheimer’s disease. Exp Neurol. 2010;223(2):334–346. doi:10.1016/j.expneurol.2009.09.024
3. Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120(3):885–890. doi:10.1016/s0006-291x(84)80190-4
4. Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi:10.1126/science.1566067
5. Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539–547. doi:10.1212/WNL.0000000000002923
6. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of department of health and human services task force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi:10.1212/wnl.34.7.939
7. Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG- criteria. Lancet Neurol. 2014;13(6):614–629. doi:10.1016/S1474-4422(14)70090-0
8. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer´s disease. Alzheimers Dement. 2018;14(5):535–562. doi:10.1212/WNL.0000000000002923
9. Bu XL, Xiang Y, Jin WS, et al. Blood-derived amyloid-β protein induces Alzheimer’s disease pathologies. Mol Psychiatry. 2018;23(9):1948–1956. doi:10.1038/mp.2017.204
10. Rosen RF, Fritz JJ, Dooyema J, et al. Exogenous seeding of cerebral β-amyloid deposition in βAPP-transgenic rats. J Neurochem. 2012;120(5):660–666. doi:10.1111/j.1471-4159.2011.07551.x
11. Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F. Plasma amyloid β as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol. 2012;69(7):824–831. doi:10.1001/archneurol.2011.1841
12. Zhang S, Huang SY, An XB, Zeng L, Ai J. Medical histories of control subjects influence the biomarker potential of plasma aβ in Alzheimer’s disease: a meta-analysis. J Mol Neurosci. 2020;70(6):861–870. doi:10.1007/s12031-020-01510-1
13. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi:10.1212/wnl.34.7.939
14. Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi:10.1016/j.jalz.2011.03.008
15. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi:10.1212/wnl.43.11.2412-a
16. Wu Y, Xu WW, Cheng ZH, Wu B, Tang L, Zhou XQ. Brief elderly cognitive screening inventory: development, reliability and validity. Chin J Geront. 2016;36(5):1211–1213. doi:10.3969/j.issn.1005-9202.2016.05.086
17. Yang HY, Cheng ZH, Li ZM, et al. Validation study of the Alzheimer’s Disease assessment scale-cognitive subscale for people with mild cognitive impairment and Alzheimer’s disease in Chinese communities. Int J Geriatr Psychiatry. 2019;34(11):1658–1666. doi:10.1002/gps.5179
18. Folstein MF, Robins LN, Helzer JE. The mini-mental state examination. Arch Gen Psychiatry. 1983;40(7):812. doi:10.1001/archpsyc.1983.01790060110016
19. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. doi:10.1093/geront/9.3_Part_1.179
20. Hachinski VC, Lassen NA, Marshall J. Multi-infarct dementia. A cause of mental deterioration in the elderly. Lancet. 1974;2(7874):207–210. doi:10.1016/s0140-6736(74)91496-2
21. Ballesteros J, Bobes J, Bulbena A, et al. Sensitivity to change, discriminative performance, and cutoff criteria to define remission for embedded short scales of the Hamilton depression rating scale (HAMD). J Affect Disord. 2007;102(1–3):93–99. doi:10.1016/j.jad.2006.12.015
22. Lue LF, Sabbagh MN, Chiu MJ, et al. Plasma levels of Aβ42 and tau identified probable alzheimer’s dementia: findings in two cohorts. Front Aging Neurosci. 2017;9:226. doi:10.3389/fnagi.2019.00222
23. Teunissen CE, Chiu MJ, Yang CC, et al. Plasma amyloid-β (Aβ42) correlates with cerebrospinal fluid Aβ42 in alzheimer’s disease. J Alzheimers Dis. 2018;62(4):1857–1863. doi:10.3233/JAD-170784
24. Lue LF, Pai MC, Chen TF, et al. Age-dependent relationship between plasma Aβ40 and Aβ42 and total tau levels in cognitively normal subjects. Front Aging Neurosci. 2019;11:222. doi:10.3389/fnagi.2019.00222
25. Chiu MJ, Yang SY, Chen TF, et al. Synergistic association between plasma Aβ1-42 and p-tau in Alzheimer’s disease but not in Parkinson’s disease or frontotemporal dementia. ACS ChemNeurosci. 2021;12(8):1376–1383. doi:10.1021/acschemneuro.1c00010
26. Mayeux R, Honig LS, Tang MX, et al. Plasma Aβ40 and Aβ42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61(9):1185–1190. doi:10.1212/01.WNL.0000091890.32140.8F
27. Pyun JM, Kang MJ, Ryoo N, et al. Amyloid metabolism and amyloid-targeting blood-based biomarkers of Alzheimer’s Disease. J Alzheimers Dis. 2020;75(3):685–696. doi:10.3233/JAD-200104
28. Roher AE, Kuo YM, Esh C, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer’s disease. Mol Med. 2003;9(3–4):112–122. doi:10.1007/BF03402043
29. Hanon O, Vidal JS, Lehmann S, et al. Plasma amyloid levels within the Alzheimer’s process and correlations with central biomarkers. Alzheimers Dement. 2018;14(7):858–868. doi:10.1016/j.jalz.2018.01.004
30. Jiao F, Yi F, Wang Y, et al. The validation of multifactor model of plasma Aβ42 and total-tau in combination with moca for diagnosing probable Alzheimer disease. Front Aging Neurosci. 2020;12:212. doi:10.3389/fnagi.2020.00212
31. Huang HC, Tseng YM, Chen YC, Chen PY. Diagnostic accuracy of the Clinical Dementia Rating Scale for detecting mild cognitive impairment and dementia: a bivariate meta-analysis. Int J Geriatr Psychiatry. 2021;36(2):239–251. doi:10.1002/gps.5436
32. Jiang XJ, Wu Y, Liu XW, Tang L, Feng W, Cheng ZH. Validity of the clinical dementia rating for early screening of Alzheimer’s disease in community. Chin J Behav Med Brain Sci. 2021;30(6):554–559. doi:10.3760/cma.j.cn371468-20210401-00178
33. Yaffe K, Weston A, Graff-Radford NR, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305(3):261–266. doi:10.1001/jama.2010.1995
34. Franchini M. Hemostasis and aging. Crit Rev Oncol Hematol. 2006;60(2):144–151. doi:10.1016/j.critrevonc.2006.06.004
35. Nascimento CMC, Pereira JR, de Andrade LP, et al. Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Curr Alzheimer Res. 2014;11(8):799–805. doi:10.2174/156720501108140910122849
36. Jensen CS, Hasselbalch SG, Waldemar G, Simonsen AH. Biochemical markers of physical exercise on mild cognitive impairment and dementia: systematic review and perspectives. Front Neurol. 2015;26(6):187. doi:10.3389/fneur.2015.00187
37. Yang YH, Huang LC, Hsieh SW, Huang LJ. Dynamic blood concentrations of Aβ1-40 and Aβ1-42 in Alzheimer’s disease. Front Cell Dev Biol. 2020;8:768. doi:10.3389/fcell.2020.00768
38. Sharmana MJ, Moricic M, Honec E, et al. APOE genotype results in differential effects on the peripheral clearance of amyloid-β42 in APOE knock-in and knock-out mice. J Alzheimers Dis. 2010;21(2):403–409. doi:10.3233/JAD-2010-100141
39. Mosera VA, Pikea CJ. Obesity and sex interact in the regulation of alzheimer’s disease. Neurosci Biobehav Rev. 2016;67:102–118. doi:10.1016/j.neubiorev.2015.08.021
40. Luciano R, Barraco GM, Muraca M, et al. Biomarkers of Alzheimer disease, insulin resistance, and obesity in childhood. Pediatrics. 2015;135(6):1074–1081. doi:10.1542/peds.2014-2391
41. Niedowicz DM, Studzinski CM, Weidner AM, et al. Leptin regulates amyloid β production via the ϒ-secretase complex. Biochim Biophys Acta. 2013;1832(3):439–444. doi:10.1016/j.bbadis.2012.12.009
42. Dukic L, Simundic AM, Martinic-Popovic I, et al. The role of human kallikrein 6, clusterin and adiponectin as potential blood biomarkers of dementia. ClinBiochem. 2016;49(3):213–218. doi:10.1016/j.clinbiochem.2015.10.014
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
