Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Association of Versican Gene Polymorphisms with Intracranial Aneurysm Susceptibility in the Eastern Chinese Population
Authors Zhu L , Yu C, Zhou S, Xue M, Chen J , Wu M, Dong S, Huang G, Chang Y, Zhang M
Received 28 September 2021
Accepted for publication 30 November 2021
Published 7 December 2021 Volume 2021:17 Pages 3531—3537
DOI https://doi.org/10.2147/NDT.S338311
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Lei Zhu,1 Chuanqing Yu,1 Shuping Zhou,2 Min Xue,1 Jie Chen,3 Meijun Wu,4 Shuyang Dong,1 Guanmin Huang,5 Yueyue Chang,1 Mei Zhang1
1Department of Neurology, First Affiliated Hospital of Anhui University of Science and Technology, First People’s Hospital of Huainan, Huainan, 232007, People’s Republic of China; 2Department of Gastroenterology, First Affiliated Hospital of Anhui University of Science and Technology,First People’s Hospital of Huainan, Huainan, 232007, People’s Republic of China; 3Department of Neurosurgery, General Hospital of Huainan Oriental Hospital Group, Huainan, 232007, People’s Republic of China; 4Department of Comprehensive Health Care, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, 310000, People’s Republic of China; 5Department of Neurosurgery, First Affiliated Hospital of Anhui University of Science and Technology, First People’s Hospital of Huainan, Huainan, 232007, People’s Republic of China
Correspondence: Chuanqing Yu; Mei Zhang
Department of Neurology, First Affiliated Hospital of Anhui University of Science and Technology, First People’s Hospital of Huainan, 203 Huaibin Road, Huainan, 232007, Anhui Province, People’s Republic of China
Tel +86-18949669880
; +86-18949669978
Email [email protected]; [email protected]
Objective: The proteoglycan versican (VCAN) plays an important role in extracellular matrix (ECM) assembly, and diminished maintenance of the ECM has been increasingly regarded as an important factor in the development of intracranial aneurysms (IAs). Previous studies have revealed that single-nucleotide polymorphisms (SNPs) of the VCAN gene are associated with susceptibility to IAs in European or Japanese populations. However, the association between IA susceptibility and VCAN SNPs in the Eastern Chinese population remains unclear. This study aimed to investigate the associations of the SNPs rs251124, rs2287926, and rs173686 with IA susceptibility in the Eastern Chinese population.
Methods: A total of 162 patients with IA and 182 controls were enrolled in this study. The study was conducted between January 2017 and December 2020. SNP genotyping for rs251124, rs2287926, and rs173686 was performed using Kompetitive Allele Specific PCR (KASP) after DNA extraction. The SNP data were analysed with CFX Manager Software version 3.1 (Bio-Rad).
Results: rs251124 and rs173686 were significantly associated with susceptibility to IA. The frequency of rs251124-TT in IA was higher than in controls (OR =1.26, 95% CI: 1.07– 1.49; P< 0.01), and its risk mainly came from the T allele. Furthermore, logistic regression analysis showed that the T/T genotype and T allele of rs251124 were independent risk factors for IA (OR=1.726, 95% CI: 1.136– 2.263; P=0.011). Moreover, the G/G genotype and G allele of rs173686 were associated with increased IA susceptibility (OR=2.52, 95% CI: 1.261– 5.037; P=0.009).
Conclusion: The SNPs rs251124 and rs173686 were strongly associated with genetic susceptibility to IA in the Eastern Chinese population; however, no such association was found in the SNP rs2287926 of VCAN. Our findings suggest that the VCAN gene is an IA susceptible gene that should be further studied as a screening marker for IAs.
Keywords: intracranial aneurysm, susceptibility, VCAN gene, Eastern Chinese population, SNP
Introduction
Intracranial aneurysm (IA) is a complex disease characterised by pathological dilatation of the cerebral arteries. IA may rupture, leading to subarachnoid haemorrhage (SAH) and significant morbidity and mortality, one of the most devastating neurological events.1 Although the pathogenesis of IA has been explored for many years, the mechanisms of its formation, growth, and rupture remain largely unknown. Various theories have been proposed to develop IA, which is generally believed to be a multifactorial disorder that occurs due to the interaction of environmental and genetic factors.2,3 Environmental factors, such as alcohol consumption, smoking, sex, and hormonal background of the patient, and hypertension seem to be common risk factors.4,5 In addition, there have been many studies on genetic markers for the risk of IA,6,7 which vary according to ethnicity and may not be generalisable to different populations.
Extracellular matrix (ECM) remodelling plays an important role in maintaining the structure and integrity of intracranial arteries. Disruption of the ECM of the arterial wall is a likely factor in the pathogenesis of IA.8 The VCAN gene, located at 5q12-q14, has 15 exons encoding a large 372.82 kDa chondroitin sulphate proteoglycan in the ECM, which plays a key role in maintaining ECM function.9 It has also been implicated in cell proliferation, cell adhesion, cell migration, and invasion. The VCAN gene produces four subtypes, V0, V1, V2, and V3, by alternate splicing of exons 7 and 8. The two largest exons, 7 and 8, encode glycosaminoglycan (GAG) attachment sites, GAG alpha and GAG beta, respectively.9,10 The latter contains a proteoglycan class with heparin sulphate. The loss of function of similar protein-binding domains may lead to the rupture of these components in certain proteins in the ECM, softening the artery wall, forming an aneurysm.
VCAN is a candidate gene for IA because it plays a vital role in ECM assembly and is localised in a previously implicated locus for IA on chromosome 5q.11 However, polymorphisms can have various effects in these gene regions that can be race-specific (since polymorphisms are known to be race-specific). Previous linkage studies have indicated that the three VCAN SNPs, rs251124, rs173686, and rs2287926, are associated with aneurysms.10,12,13 Nevertheless, replication studies in different ethnicities have provided conflicting results regarding IAs.10,12,14,15 Considering the genetic differences among different races and regions and the uncertainty of the correlation between VCAN gene polymorphism and IA incidence in the Chinese population, we aimed to explore the association of the VCAN gene variants rs251124, rs173686, and rs2287926 with IA prevalence in the Eastern Chinese population.
Patients and Methods
Ethics Approval and Informed Consent
This study complied with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board and Ethics Committee of the First Affiliated Hospital of Anhui University of Science and Technology. All participants provided written informed consent (Approval No. 2019B20,2019-1-1).
Study Population
This case-controlled prospective study included 162 patients with sporadic IAs and 182 age-matched controls admitted to the First Affiliated Hospital of Anhui University of Science and Technology between January 2017 and December 2020. All cases with confirmed IAs based on digital subtraction angiography (DSA) were retrospectively confirmed by a neuroradiologist and two highly qualified cerebrovascular neurosurgeons. Patients were excluded if they had a family history of IA, fusiform and dissection aneurysms, or traumatic and infectious aneurysms. A total of 182 controls were symptomatically normal and did not have a medical or family history of IA or SAH. Controls subjects were sex- and age-matched individuals, diagnosed negative for IA by DSA or CTA from the same hospital, and matched to IA patients for the area of residence to eliminate the effect of population stratification by heterogeneity.
SNP Selection and Genotyping
Candidate SNPs were selected by searching for SNPs in previous studies and genome-wide association studies (GWAS) that showed significant associations with IA. DNA was extracted from peripheral blood leukocytes using the TIANamp Blood DNA Extraction Kit (TIANGEN Biotech Co., Ltd., Beijing, China). The DNA samples were stored at –80 °C until use. SNP genotyping for rs251124, rs2287926, and rs173686 was performed using Kompetitive Allele Specific PCR (KASP). The SNP data were analysed with CFX Manager Software version 3.1 (Bio-Rad).
Statistical Analysis
General clinical features of the case and control groups were described as mean ± standard deviation (SD) and compared using Student’s t-test for continuous variables. Genotype and allele frequencies were computed and checked for deviation from the Hardy–Weinberg equilibrium (ihg2.helmholtz-muenchen.de/cgi-bin/hw/hwa1.pl). Categorical variables were presented as proportions and were compared using the chi-square test or Fisher’s exact test (two-tailed). Further stratification of the patients based on sex and aneurysm rupture status was performed to understand the role of the VCAN variant within the sexes and between the ruptured aneurysm and non-ruptured aneurysm groups. The association of SNPs with the risk of sporadic IA was investigated using logistic regression analysis. Statistical significance was set at P<0.05. All statistical analyses were performed using GraphPad Prism 8.02 (San Diego, CA, USA) and SPSS software (version 21.0; IBM Corp., Armonk, NY, USA).
Results
Characteristics of Study Population
A total of 162 IA patients and 182 control subjects were included in this study. The demographic characteristics of patients and controls are shown in Table 1. In the case group, 72.8% of the patients had a single aneurysm. The aneurysms were primarily located in the internal carotid artery (38.3%), followed by the middle cerebral artery (19.1%), anterior communicating artery (13.6%), and other locations. Additional details are provided in Table 2.
 |
Table 1 Characteristics of Cases vs Controls |
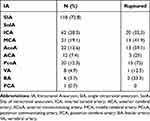 |
Table 2 Clinical Characteristics of Intracranial Aneurysm |
SNP Analysis
The distribution of the SNP genotypes was consistent with the Hardy–Weinberg equilibrium. As shown in Table 3, there were significant differences in the genotype and allele frequency distributions of rs251124 and rs173686 between the IA cases and controls (P<0.05). A significant association was observed with rs251124 at the allelic (OR=1.489, CI: 1.089–2.044; P=0.0137) and genotypic (P=0.0415) levels. The frequency of rs251124-TT in IA patients was higher than in controls (χ2= 6.364, P=0.0415). The risk allele frequencies of rs173686 were significantly different between patients with IA and controls (OR=1.558, CI: 1.115–2.171; P=0.0096). For SNP rs2287926, there was no statistically significant difference in genotype or allele frequencies between the patient and control groups (Table 3).
 |
Table 3 Genotype and Allele Frequencies of SNPs of VCAN |
Table 4 shows the genotypes of the SNPs and their associations with IA. Logistic regression analysis showed that the T/T genotype and T allele of rs251124 were independent risk factors for IA (OR=1.726, 95% CI: 1.136–2.263; P=0.011). Similarly, for the SNP rs173686, G allele carriers were also at a significantly higher risk of IA (OR=2.52, 95% CI: 1.261–5.037; P=0.009).
 |
Table 4 Logistic Regression Model of Genotype Analysis |
Table 5 shows no significant difference in genotype frequencies between the ruptured and unruptured aneurysm groups at either of the two SNPs rs251124 and rs173686 (P>0.05).
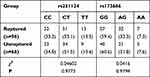 |
Table 5 Genotypic Results of Ruptured and Unruptured Aneurysms |
Table 6 shows no significant difference in genotype frequencies among aneurysm groups at either of the two SNPs rs251124 and rs173686 (P> 0.05).
 |
Table 6 Genotype Frequencies of SNPs rs251124 and rs173686 at Different Locations |
Table 7 suggests that the rs251124 T allele and rs173686 G allele contribute to a significantly higher risk for males and females at both allelic and genotypic levels when stratified based on sex (P<0.05).
 |
Table 7 Comparison of the Genotype and Allele Frequencies of rs251124 and rs173686 Within Males and Females in Cases and Control |
Discussion
There are various theories about the development of aneurysms, most of which reflect an imbalance in the ECM remodelling. ECM remodelling plays an important role in maintaining the structure and integrity of intracranial arteries and its reduction is a prominent feature of cerebral aneurysms. The VCAN gene localised on 5q12–q14 has 15 exon codes9 for a large 372.82 kDa chondroitin sulphate proteoglycan found abundantly in the ECM and plays various roles in maintaining ECM functions. Some studies have shown that a decreased density of smooth muscle cells results in decreased production of CSPG2 (also known as versican), which in turn weakens the vascular wall. VCAN (CSPG2) also plays an important role in the assembly of ECM, and diminished maintenance of the ECM has been increasingly regarded as an important factor in the development of IA.16
This study investigated the associations between three SNPs in the VCAN gene and IA susceptibility in the Eastern Chinese population. The results showed that rs251124T/T genotype and T allele and rs173686 G/G genotype and G allele increased the risk of IA formation. No significant differences in genotype or allele frequencies between the case and control groups were detected at the rs2287926 SNP. Our findings further support the VCAN gene as an IA susceptibility gene.
The VCAN gene (also known as chondroitin sulphate proteoglycan [CSPG2]) predicts the risk of aneurysm development and subsequent rupture.12,15,16 Nevertheless, the association between the SNPs of VCAN and the risk of IA remains controversial due to contrary reports. For example, Sathyan et al10 found that the minor allele T of rs251124 showed an increased risk of IA. The CT genotype has been defined as a risk factor for the occurrence and rupture of aneurysms in a South Indian population. In addition, rs2287926 further substantiates the potential role of VCAN in the pathogenesis of IA.
Moreover, no confirmed association between rs173686 at genotypic and allelic levels and IA in the South Indian population has been reported. However, in a Dutch population, the VCAN (CSPG2) genes were identified as susceptibility genes for IA, and the rs173686 polymorphism has also been linked to IA.12 The association between the rs173686 polymorphism and IA risk has also been observed in a Kazakh population.14 Similarly, another study replicated the association of rs251124 with the risk of IAs in a Japanese IA population.15 In contrast, replication studies reported a lack of association between the CSPG2 variants (rs173686 and rs251124) with IA susceptibility.15 The above studies suggest that race and geography may influence these contradictory results. On the other hand, this may be explained by the heterogeneity of ethnic origin and/or small sample size.
Consistent with previous studies and meta-analyses,10,12,13 we found that rs251124 was associated with sporadic IAs, and rs251124T/T genotype and T allele were risk factors for IA in the Eastern Chinese population, but not in agreement with the results of Sun et al,15 who found no confirmed association in a study of Han Chinese. Additionally, our study found that the rs173686 G/G genotype and G allele significantly increased the risk of IA formation, which was similar to the results of studies conducted among European,12 Kazakh,13 and Japanese populations,14 but inconsistent with the results of Sun et al.15 The contrary results indicate that individuals from the same ethnic group also have different susceptibilities. Sun et al15 reported that the two SNPs rs173686 and rs251124 of the CSPG2 gene were not susceptible to IAs in Northern Chinese (Beijing) Han nationality. Alternatively, we observed that rs173686 and rs251124 highly increased the risk of IA formation in the Eastern Chinese population. To the best of our knowledge, the occurrence of IA is the result of the combined effects of genetic and environmental factors. One of the underlying causes of this discrepancy might be regional differences. Our research subjects included patients from eastern China; the possibility of different genetic backgrounds and surroundings caused by ethnic and regional differences might affect the experimental results. In addition, our study group was relatively small; therefore, our results require validation in a large-scale population. Overall, it has been hypothesised that genetic heterogeneity among diverse populations can lead to such paradoxical results.
In addition, our study included patients with ruptured and unruptured IAs. For either of the two SNPs rs173686 and rs251124, there was no statistically significant difference in genotype or allele frequencies between the two groups studied, suggesting that no associations were found in the SNPs rs173686 and rs251124 with IA rupture susceptibility in the Eastern Chinese population. To the best of our knowledge, this is the first study to investigate the association between the VCAN gene and IA rupture in a Chinese population.
Moreover, our study found no significant difference in genotype frequencies among aneurysm groups at either SNP rs251124 or rs173686 (P> 0.05). This suggests that the locations of aneurysm distribution may have nothing to do with SNP variation. Furthermore, subgroup analysis by sex showed that rs251124 T allele and rs173686 G allele contributed to high risk for both males and females at both allelic and genotypic levels, implying that sex has no significant effect on SNP variation. Another possible reason for this result is that the sample size was small.
This study had some limitations. It included only Eastern Chinese patients; therefore, the sample size was relatively small and may not be adequate for assessing the effect of these SNPs on the formation of IAs. Thus, additional studies using different populations are warranted to further validate ethnic and regional differences in the impact of the VCAN polymorphism on IA risk. Moreover, molecular biology experiments and reliable animal models are required to explore the specific mechanisms further.
Conclusion
This study revealed that rs251124 and rs173686 are genetic risk factors for IA formation in the Eastern Chinese population. No association was found between SNP rs2287926 and IA. Our findings suggest that the VCAN gene is an IA susceptible gene, which warrants further study as a screening marker for intracranial aneurysms.
Acknowledgments
The authors thank Key project of Education Department of Anhui Province (KJ2019A0096) and Huainan science and technology planning project (2016A26(3)) for supporting this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hitchcock E, Gibson WT. A review of the genetics of intracranial berry aneurysms and implications for genetic counseling. J Genet Couns. 2017;26(1):21–31. doi:10.1007/s10897-016-0029-8
2. Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120(3):472–495. doi:10.1161/CIRCRESAHA.116.308398
3. Ruigrok YM, Rinkel GJ. From GWAS to the clinic: risk factors for intracranial aneurysms. Genome Med. 2010;2(9):61. doi:10.1186/gm182
4. Cho SM, Marquardt RJ, Rice CJ, et al. Cerebral microbleeds predict infectious intracranial aneurysm in infective endocarditis. Eur J Neurol. 2018;25(7):970–975. doi:10.1111/ene.13641
5. Chen Y, Zhang Y, Chao YJ, et al. Stent-assisted coiling embolization of middle cerebral artery trifurcation wide-necked aneurysms. Eur Rev Med Pharmacol Sci. 2017;21(19):4346–4349.
6. Tromp G, Weinsheimer S, Ronkainen A, Kuivaniemi H. Molecular basis and genetic predisposition to intracranial aneurysm. Ann Med. 2014;46(8):597–606. doi:10.3109/07853890.2014.949299
7. Bourcier R, Redon R, Desal H. Genetic investigations on intracranial aneurysm: update and perspectives. J Neuroradiol. 2015;42(2):67–71. doi:10.1016/j.neurad.2015.01.002
8. Ruigrok YM, Rinkel GJ, Wijmenga C. Genetics of intracranial aneurysms. Lancet Neurol. 2005;4:179–189. doi:10.1016/S1474-4422(05)70021-1
9. Iozzo RV, Naso MF, Cannizzaro LA, et al. Mapping of the versican proteoglycan gene (CSPG2) to the long arm of human chromosome 5 (5q12–5q14). Genomics. 1992;14(4):845–851. doi:10.1016/S0888-7543(05)80103-X
10. Sathyan S, Koshy LV, Balan S, et al. Association of Versican (VCAN) gene polymorphisms rs251124 and rs2287926 (G428D), with intracranial aneurysm. Meta Gene. 2014;2:651–660. doi:10.1016/j.mgene
11. Onda H, Kasuya H, Yoneyama T, et al. Genomewide-linkage and haplotype-association studies map intracranial aneurysm to chromosome 7q11. Am J Hum Genet. 2001;69(4):804–819. doi:10.1086/323614
12. Ruigrok YM, Rinkel GJ, Wijmenga C. The versican gene and the risk of intracranial aneurysms. Stroke. 2006;37(9):2372–2374. doi:10.1161/01.STR.0000236499.55301.09
13. Zholdybayeva EV, Medetov YZ, Aitkulova AM, et al. Genetic risk factors for intracranial aneurysm in the Kazakh population. J Mol Neurosci. 2018;66(1):135–145. doi:10.1007/s12031-018-1134-y
14. Ruigrok YM, Rinkel GJ, Wijmenga C, et al. Association analysis of genes involved in the maintenance of the integrity of the extracellular matrix with intracranial aneurysms in a Japanese cohort. Cerebrovasc Dis. 2009;28(2):131–134. doi:10.1159/000223438
15. Sun H, Zhang D, Zhao J. Chondroitin sulfate proteoglycan 2 (CSPG2) gene polymorphisms rs173686 and rs251124 are not associated with intracranial aneurysms in Chinese Han nationality. Ups J Med Sci. 2007;112(3):289–295. doi:10.3109/2000-1967-201
16. Ruigrok YM, Rinkel GJ, Van’t Slot R, et al. Evidence in favor of the contribution of genes involved in the maintenance of the extracellular matrix of the arterial wall to the development of intracranial aneurysms. Hum Mol Genet. 2006;15:3361–3368. doi:10.1093/hmg/ddl412
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
