Back to Journals » OncoTargets and Therapy » Volume 9
Association of TIMP-2-418G/C and TIMP-2-303G/A with gastric cancer: a meta-analysis
Authors Yang Y, Xiong Y, Li J, wu C, Jiang J
Received 2 June 2016
Accepted for publication 1 August 2016
Published 3 November 2016 Volume 2016:9 Pages 6801—6808
DOI https://doi.org/10.2147/OTT.S114113
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Yan Yang, Yuqi Xiong, Jing Li, Changping Wu, Jingting Jiang
Department of Tumor Biological Treatment, The Third Affiliated Hospital, Soochow University, Changzhou, Jiangsu, People’s Republic of China
Background: According to the relevant reports, TIMP-2 polymorphism might be associated with the susceptibility to gastric cancer. Owing to the inconclusive results from the published studies based on the association between TIMP-2 single nucleotide polymorphisms (SNPs) and gastric cancer susceptibility, a meta-analysis was conducted to investigate the correlation between the TIMP-2 SNPs and the risk of gastric cancer.
Materials and methods: PubMed, Embase, CNKI and Wanfang databases were systematically searched to cover all the studies based on the association of two SNPs with the susceptibility to gastric cancer published before April 2016. Pooled odds ratios (ORs) and 95% confidence intervals were calculated for all genetic models.
Results: A total of six case–control studies on TIMP-2-418G/C and four studies on TIMP-2-303G/A were included. No obvious association was found between TIMP-2-418G/C polymorphism and the risk of gastric cancer in all the genetic models. On the other hand, TIMP-2-303G/A polymorphism had a significant association with increased risk of gastric cancer in homozygote recessive and allele comparisons, and similar results were observed in subgroups of Asian populations, but there were inadequate data to completely verify the association between TIMP-2-303G/A and gastric cancer.
Conclusion: TIMP-2-418G/C polymorphism is not correlated with the risk of gastric cancer, while TIMP-2-303G/A is a risk factor for gastric cancer, especially in Asian populations. However, owing to the limited cases, the results of TIMP-2-303G/A should be thoroughly examined and validated with large-scale and well-designed studies.
Keywords: TIMP-2, gastric cancer, genetic polymorphism, meta-analysis
Introduction
As is universally acknowledged, gastric cancer is a critical illness threatening human health and existence. According to the statistics, gastric cancer ranks the fourth and the third in morbidity and mortality among all cancers, respectively.1 The year 2012 had witnessed 951,600 new gastric cancer cases and 723,100 deaths, with the mortality rate being as high as 76%, accounting for 8% of the cancer cases and 10% of cancer deaths, respectively.2 Gastric cancer, distal gastric cancer in particular, is associated with a low 5-year survival rate, and its morbidity and mortality are in continuous growth in most developing countries.3 Most of the patients with gastric cancer are diagnosed at an advanced stage at the first time they consult a doctor. As a consequence, early detection is particularly important in the diagnosis of gastric cancer.4 Affected by many factors, gastric cancer has a complex genesis and development process. Currently, the relationship between single nucleotide polymorphisms (SNPs) and gastric cancer remains unclear, but it may help to explain the genetic susceptibility of patients with risk of gastric cancer.
Matrix metalloproteinase (MMP) is a class of Zn2+- and Ca2+-dependent endogenous proteolytic enzyme that is also a membrane-spanning protein and can degrade the extracellular matrix and the basement membrane under physical conditions.5 It is extensively considered that MMPs play an important role in promoting the growth, invasion, and metastasis of tumor cells.6 TIMP is a natural inhibitor of MMPs when it combines with them. Under normal conditions, MMPs and TIMPs maintain a relative state of equilibrium in vivo and sustain the integrity of the extracellular matrix and the basement membrane, so that the role of inhibiting the invasion and metastasis of tumor cells can be achieved. It has been shown that MMP-2 and TIMP-2 play a vital role in the invasion and metastasis of gastric cancer.7,8 Genetic polymorphism, especially SNP, is the most common genetic variation in human beings.9
As indicated in some studies, TIMP-2-418G/C and TIMP2-303G/A, which locate in the promoter and third exon region of the TIMP-2 gene polymorphism, respectively, may alter their own gene transcription activity or spatial conformation and influence their expression in the body, thereby affecting the occurrence of tumor.10–12 It has been reported that these two polymorphisms are associated with the risk of lung cancer, breast cancer, head and neck cancer, endometrial cancer, colon cancer and other malignant tumors; however, the relationship between the two SNPs and the risk of gastric cancer is still unknown.13–15 So far, no meta-analysis has been conducted to investigate the correlation between them, which can account for the reason why a meta-analysis should be performed to assess the association between TIMP-2-418G/C and TIMP-2-303G/A polymorphisms and the susceptibility to gastric cancer.
Materials and methods
Search strategy
Two independent investigators systematically searched PubMed, Embase, CNKI and Wanfang databases for studies published before April 20, 2016. The following keywords were used: “TIMP-2” or “tissue inhibitor matrix metalloproteinase-2”, “polymorphisms” or “genetic polymorphisms” or “mutation” or “variation” or “genotype” or “single nucleotide” and “stomach neoplasm” or “gastric neoplasm” or “stomach cancer” or “gastric cancer” or “cancer of the stomach”. No limitations were placed on language. Only published studies with full texts were enrolled, and meeting or conference abstracts were not covered.
Inclusion and exclusion criteria
Eligible studies should meet the following criteria: 1) studies that scrutinized the association of TIMP-2-418G/C, TIMP-2-303G/A polymorphisms and the risk of gastric cancer that has been confirmed by histopathological examinations; 2) research methodology based on an unrelated case–control design; and 3) detailed genotype data for estimating odds ratio (OR) and 95% confidence interval (CI). Exclusion criteria were: 1) duplication of earlier publications; 2) comments, reviews and editorials; 3) family-based studies of pedigrees; and 4) studies with no detailed genotype data.
Data extraction
Two investigators independently evaluated and extracted the data. For each study, the following information was extracted: first author, year of publication, ethnicity, genotyping method, Hardy–Weinberg equilibrium (HWE) and the quality assessment of the studies. Any controversy on the baseline information was consulted with a third author. Two authors checked the extracted data and reached an agreement on the obtained data. If there was any objection, they would review the raw data of the included studies and have a discussion to reach an agreement. If a disagreement still existed, the third investigator would be involved to verdict the dissent (Table 1).
  | Table 1 Main characteristics of all studies included in the meta-analysis |
Quality assessment
Two investigators independently evaluated and scored the qualities of the included studies in accordance with the Newcastle–Ottawa Quality Assessment Scale, which is a star-rating system. The quality of the studies was evaluated by examining three items: selection of case and controls, comparability of groups and ascertainment of exposure. Nine stars are defined as the full score; five to nine stars are usually considered to be a high methodological quality while zero to four stars are considered to be a poor quality. The quality of all enrolled studies is shown in Table 1.
Statistical analysis
We organized and reported our meta-analysis on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.16 HWE was assessed for each study by chi-squared test in the control group, and P<0.05 was considered a significant departure from HWE. OR and 95% CIs were calculated to evaluate the correlation of TIMP-2-418/303 SNPs and the susceptibility to gastric cancer. Heterogeneity was assessed by Q statistic (significance level of P<0.05) and I2 statistic (>50% as evidence of significant inconsistency).17 A fixed-effects model (if P-value >0.05) or a random-effects model (if P-value <0.05) was used for pooling the results.18 Sensitivity analysis was also implemented to assess the effect of each study on the combined ORs by deleting each study in each turn. Besides, subgroup analyses were stratified by ethnicity. Publication bias was checked by Begg’s funnel plots and Egger’s regression test.19,20 An asymmetric plot and the P-value of Egger’s test <0.05 was considered a significant publication bias. All statistical analyses were implemented using Stata 12.0 software (StataCorp LP, College Station, TX, USA). All P-values were two sided, and the statistical significance level was considered as P-value <0.05 for this meta-analysis.
Results
Characteristics of studies
In total, 85 studies were obtained from PubMed, Embase, CNKI and Wanfang databases (PubMed: 32; Embase: 48; CNKI: 2; Wanfang: 3). The literature selection process is shown in Figure 1. All searched articles were thoroughly reviewed by reading the titles and abstracts, and the full texts for the potentially relevant research articles were further inspected for their adequacy for this meta-analysis. We included only case–control studies reporting the frequency of all five genotypes, and studies investigating the levels of TIMP-2 mRNA or protein expression or relevant review articles were excluded from this study.
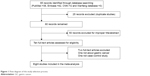  | Figure 1 Flow diagram of the study selection process. |
Evaluation of heterogeneity
To test the heterogeneity among the selected publications, Q-test and I2 statistics were employed. Heterogeneity was observed in all the genetic models, ie, allele (C vs G or A vs G), homozygous (CC vs GG or AA vs GG), heterozygous (GC vs GG or GA vs GG), dominant (CC + GC vs GG or AA + GA vs GG) and recessive (CC vs GG + GC or AA vs GG + GA). As a consequence, random-effects model was mostly applied to analyze the data for TIMP-2-418G/C, while fixed-effects model was applied to analyze the TIMP-2-303G/A data (Table 2).
  | Table 2 Genotypic distribution of TIMP-2-418G/C gene polymorphism |
Publication bias
Begg’s funnel plot and Egger’s test were implemented to assess the publication bias for TIMP-2-418G/C and gastric cancer susceptibility. The funnel plots failed to reveal any obvious asymmetry in all genotypes in the overall population. Neither Begg’s test nor Egger’s test showed statistical evidence for publication bias in our meta-analysis (P>0.05). Neither Begg’s funnel plot nor Egger’s test was performed to assess the association between TIMP-2-303G/A and gastric cancer susceptibility owing to the limited number of included studies.
Sensitivity analysis
To assess the stability of our meta-analysis results, we implemented sensitivity analysis to define whether the inclusion criteria of this meta-analysis influenced the results. Sensitivity analysis was performed to examine the influence set by the individual study on the pooled ORs for TIMP-2-418G/C and TIMP-2-303G/A by deleting each study once in every genetic model. We observed no significant difference after the omission of any study for all the five models, implying that our results are statistically reliable.
Association between TIMP-2-418G/C polymorphism and gastric cancer susceptibility
The association between TIMP-2-418G/C polymorphism and the risk of gastric cancer was analyzed in six independent studies. For the heterogeneity, this result should be treated with caution. Random-effects model was used in the allele model, homozygote model, dominant model and recessive model due to the presence of heterogeneity, and fixed-effects model was used in the heterozygote model. Overall, significant association was not identified in any genetic model (C vs G: OR =1.131, 95% CI 0.840–1.521, PH=0.417; CC vs GG: OR =1.576, 95% CI 0.602–4.127, PH=0.355; GC vs GG: OR =1.038, 95% CI 0.875–1.231, PH=0.670; GC + CC vs GG: OR =1.098, 95% CI 0.853–1.414, PH=0.467; CC vs GC + GG: OR =1.543, 95% CI 0.620–3.842, PH=0.351; Table 3).
  | Table 3 Statistics to test publication bias and heterogeneity in the meta-analysis |
Association between TIMP-2-303G/A polymorphism and gastric cancer susceptibility
No significant heterogeneity was identified by Q-test and I2 statistic in any of the genetic models; therefore, fixed-effects model was used in all the models. A significant increased risk of gastric cancer was observed in homozygote comparison (AA vs GG: OR =0.498, 95% CI 0.332–0.748, PH=0.001; Figure 2), recessive comparison (AA vs GA + GG: OR =0.499, 95% CI 0.335–0.742, PH=0.001; Figure 3) and allele comparison (A vs G: OR =0.799, 95% CI 0.683–0.934, PH=0.005; Figure 4). No significant association was found in heterozygote comparison (GA vs GG: OR =0.926, 95% CI 0.757–1.132, PH=0.453) or dominant comparison (GA + AA vs GG: OR =0.844, 95% CI 0.697–1.023, PH=0.083; Table 3).
  | Figure 2 Forest plot of homozygote comparison of TIMP-2-303G/A polymorphism for overall comparison (AA vs GG). |
  | Figure 3 Forest plot of recessive comparison of TIMP-2-303G/A polymorphism for overall comparison (AA vs GA + GG). |
  | Figure 4 Forest plot of allele comparison of TIMP-2-303G/A polymorphism for overall comparison (A vs G). |
Next, subgroup analysis was conducted according to ethnicity. In Asians, a significant increased risk of gastric cancer was observed in homozygote comparison (AA vs GG: OR =0.476, 95% CI 0.314–0.772, PH=0.000), recessive comparison (AA vs GA + GG: OR =0.476, 95% CI 0.314–0.722, PH=0.000) and allele comparison (A vs G: OR =0.811, 95% CI 0.687–0.957, PH=0.013). In addition, no significant association was found in heterozygote comparison (GA vs GG: OR =1.000, 95% CI 0.803–1.244, PH=0.997) or dominant comparison (GA + AA vs GG: OR =0.884, 95% CI 0.719–1.088, PH=0.899). As for Caucasian populations, no obvious association was found between TIMP-2-303G/A polymorphism and the risk of gastric cancer in all the genetic models (Table 3).
Discussion
In this meta-analysis, eight qualified case–control studies including 1,265 cases and 1,671 controls for TIMP-2-418G/C and four studies including 947 cases and 946 controls for TIMP-2-303G/A were analyzed. Consistent with the previous meta-analyses focused on TIMP-2-418G/C polymorphism and cancer risk, no evidence was found for the association between TIMP-2-418G/C polymorphism and gastric cancer susceptibility in any genetic models, and similar results were discovered in the subgroup analysis by ethnicity. On the other hand, TIMP-2-303G/A polymorphism had a significant association with increased risk of gastric cancer in homozygote recessive and allele comparisons, and similar results were observed in the subgroups of Asian populations. However, this finding should be interpreted with caution for limited sample heterogeneity.
TIMP-2 genes are located in human chromosome 17q23–17q25, which participate in the regulation of MMP2 activity and have a promoting effect on cell growth.21,22 TIMP-2 gene is a specific tissue inhibiting factor for MMP2 gene, which could combine with MMP2 enzymes and active enzyme in a ratio of 1:1 and then cause inactivation of the latter. The balance between MMP2 and TIMP-2 has important significance in maintaining the integrity of the basement membrane and the normal function of MMP-2.
TIMP-2-418G/C polymorphism could destroy the combination of the transcription factor Spl, which may cause a decline in genetic transcription activity.11 We have collected the studies on the association of TIMP-2-418G/C polymorphism with the risk of gastric cancer, but the results were not quite similar. Reports by Yang et al23 and Zhang et al24 showed that TIMP-2-418G/C polymorphism had an increased risk of developing gastric cancer and might be correlated with gastric cancer susceptibility.23 But in the mean time, reports from Wu et al,25 Chen et al26 and Sun27 showed that no association had been found between -418G/C polymorphism and the risk of gastric cancer. Kubben et al28 did not report the relationship between −418G/C polymorphism and gastric cancer risk. Our results indicated that −418G/C SNP had no association with the risk of gastric cancer in any genetic models. During the subgroup analysis, we found that ethnicity had no effect on the association between TIMP-2-418G/C polymorphism and gastric cancer in any of the genetic models, suggesting -418G/C SNP was not associated with gastric cancer in either Asian or Caucasian. As for sensitivity analysis of TIMP-2-418G/C, although the included studies differed from one another in various aspects, the association could be driven by none of the single study.
TIMP-2-303G/A is located in the third exon of TIMP-2 gene, which belongs to synonymous mutation. Although synonymous mutation does not alter coded amino acid, nucleotide sequence was changed, which might have an influence on posttranscriptional processing, cut-grafting order of signal and important bases in promoter region. The -303G/A locus polymorphism of TIMP-2 may destroy the combination of transcription factor Spl, which may decrease the transcriptional activity of genes.29 Zhang et al’s24 and Lin et al’s30 works proved that TIMP-2-303G/A variants might be correlated with gastric cancer susceptibility, while no significant correlation was observed in Alakus et al’s12 and Kubben et al’s28 works. This work proved that TIMP-2-303G/A was correlated with gastric cancer susceptibility in homozygote recessive and allele models. For TIMP-2-303G/A, when we focused on Asian population through the subgroup analysis, positive association was observed in all the genetic models, while the study conducted in Caucasians showed no significant association in any of the genetic models. This could be explained by factors such as the different life styles and ethnicity. As to sensitivity analysis of TIMP-2-303G/A, the association could be driven by none of the single study as well.
Our meta-analysis has some advantages. First, this is the first meta-analysis focused on the association between TIMP-2 polymorphisms and the susceptibility to gastric cancer compared with the former meta-analyses about TIMP-2 polymorphism. Second, another TIMP-2 polymorphism of TIMP-2-303G/A was also explored. Third, there was no obvious evidence of publication bias through qualitative funnel plot and quantitative Egger linear regression, which indicated that our results are statistically robust. Fourth, no limitation was made in the literature search, thus selection bias was well controlled. In spite of the significant findings from our current analysis, we still have to acknowledge some of the limitations of this analysis. First of all, the number of included studies for TIMP-2-303G/A polymorphism limited further analysis due to the shortage of original studies. In addition, one study did not conform to HWE expectations for TIMP-2-418G/C. Finally, lacking individual data of environmental exposure limited our further studies of gene–environment interactions.
Conclusion
This meta-analysis suggested that TIMP-2-418G/C polymorphism might not be associated with the susceptibility to the risk of gastric cancer in the overall population, while TIMP-2-303G/A polymorphism had a significant association with increased risk of gastric cancer, especially in Asians. However, there were inadequate data to completely verify the association of TIMP-2-303G/A polymorphism and the risk of gastric cancer. Taking into account the limitations mentioned earlier, it is necessary to implement more elaborately designed studies with a larger sample size in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
Nagini S. Carcinoma of the stomach: a review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156–169. | ||
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. | ||
Nicolas C, Sylvain M, Come L, et al. Trends in gastric cancer incidence: a period and birth cohort analysis in a well-defined French population. Gastric Cancer. 2016;19:508–514. | ||
Kim HS, Lee H, Jeung HC, et al. Advanced detection of recent changing trends in gastric cancer survival: up-to-date comparison by period analysis. Jpn J Clin Oncol. 2011;41:1344–1350. | ||
Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. | ||
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. | ||
Nomura H, Sato H, Seiki M, Mai M, Okada Y. Expression of membrane-type matrix metalloproteinase in human gastric carcinomas. Cancer Res. 1995;55:3263–3266. | ||
Alakus H, Grass G, Hennecken JK, et al. Clinicopathological significance of MMP-2 and its specific inhibitor TIMP-2 in gastric cancer. Histol Histopathol. 2008;23:917–923. | ||
International Human Genome Sequencing, C. Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. | ||
Price SJ, Greaves DR, Watkins H. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: role of Sp1 in allele-specific transcriptional regulation. J Biol Chem. 2001;276:7549–7558. | ||
De Clerck YA, Darville MI, Eeckhout Y, Rousseau GG. Characterization of the promoter of the gene encoding human tissue inhibitor of metalloproteinases-2 (TIMP-2). Gene. 1994;139:185–191. | ||
Alakus H, Afriani N, Warnecke-Eberz U, et al. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. World J Surg. 2010;34:2853–2859. | ||
Zhou Y, Yu C, Miao X, et al. Functional haplotypes in the promoter of matrix metalloproteinase-2 and lung cancer susceptibility. Carcinogenesis. 2005;26:1117–1121. | ||
Yu C, Zhou Y, Miao X, Xiong P, Tan W, Lin D. Functional haplotypes in the promoter of matrix metalloproteinase-2 predict risk of the occurrence and metastasis of esophageal cancer. Cancer Res. 2004;64:7622–7628. | ||
Zhou Y, Yu C, Miao X, et al. Substantial reduction in risk of breast cancer associated with genetic polymorphisms in the promoters of the matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 genes. Carcinogenesis. 2004;25:399–404. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W264. | ||
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. | ||
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. | ||
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. | ||
Seo DW, Li H, Guedez L, et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. | ||
Hayakawa T, Yamashita K, Ohuchi E, Shinagawa A. Cell growth-promoting activity of tissue inhibitor of metalloproteinases-2 (TIMP-2). J Cell Sci. 1994;107(pt 9):2373–2379. | ||
Yang L, Gu HJ, Zhu HJ, et al. Tissue inhibitor of metalloproteinase-2 G-418C polymorphism is associated with an increased risk of gastric cancer in a Chinese population. Eur J Surg Oncol. 2008;34:636–641. | ||
Zhang DY, Wang J, Zhang GQ, Chu XQ, Zhang JL, Zhou Y. Correlations of MMP-2 and TIMP-2 gene polymorphisms with the risk and prognosis of gastric cancer. Int J Clin Exp Med. 2015;8:20391–20401. | ||
Wu CY, Wu MS, Chen YJ, et al. Clinicopathological significance of MMP-2 and TIMP-2 genotypes in gastric cancer. Eur J Cancer. 2007;43:799–808. | ||
Chen F, Yang SJ, Tian Z, et al. Single nucleotide polymorphisms of MMP-2 and TIMP-2 for risk of gastric cancer. Mod Prev Med. 2014;41(1):130–133. | ||
Sun DL. Correlation of MMP-2 and TIMP-2 Polymorphism to Gastric Cardiac Adenocarcinoma and Esophageal Squamous Cell Carcinoma. Hebei: Hebei Medical University; 2009. | ||
Kubben FJ, Sier CF, Meijer MJ, et al. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. Br J Cancer. 2006;95:744–751. | ||
Palei AC, Sandrim VC, Amaral LM, et al. Association between matrix metalloproteinase (MMP)-2 polymorphisms and MMP-2 levels in hypertensive disorders of pregnancy. Exp Mol Pathol. 2012;92:217–221. | ||
Lin XD, Chen G, Li C, et al. Association of polymorphism in matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 with genetic susceptibility of gastric cancer. Zhonghua Yu Fang Yi Xue Za Zhi. 2011;45:711–716. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
