Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Association of the Monocyte-to-High-Density Lipoprotein Cholesterol Ratio with Gastrointestinal Involvement of Immunoglobulin a Vasculitis
Authors Shao X, Li H, Chen T, Chen Y, Qin X, Liu L, Luo X , Chen J
Received 18 November 2022
Accepted for publication 19 January 2023
Published 5 February 2023 Volume 2023:16 Pages 359—367
DOI https://doi.org/10.2147/CCID.S398134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Xinyi Shao,1,* Hao Li,2,* Tingqiao Chen,1 Yangmei Chen,1 Xue Qin,1 Lin Liu,1 Xiaoyan Luo,2 Jin Chen1
1Department of Dermatology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, 400010, People’s Republic of China; 2Department of Dermatology, Children’s Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing, 400016, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoyan Luo, Department of Dermatology, Children’s Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, No. 136, Zhongshan Second Road, Yuzhong District, Chongqing, 400016, People’s Republic of China, Tel +86 13012381671, Email [email protected] Jin Chen, Department of Dermatology, the First Affiliated Hospital of Chongqing Medical University, No. 1 Youyi Road, Yuzhong District, Chongqing, 400010, People’s Republic of China, Tel +86 15023188592, Email [email protected]
Objective: To illustrate the association of monocyte to high-density lipoprotein cholesterol ratio (MHR) and other serum indicators with the pathogenesis and prognosis of immunoglobulin A vasculitis (IgAV) patients in different subgroups.
Methods: A total of 158 adult patients and 113 healthy controls were enrolled, and the clinical presentation and laboratory indexes were comprehensively assessed.
Results: IgAV patients show significantly elevated levels of inflammatory parameters and lipid profiles compared to healthy controls (P < 0.05). Higher levels of the MHR and other normal inflammatory indicators were found in patients with Gastrointestinal (GI) involvement compared to other subgroups. And in group with GI involvement, significantly higher white blood cell (WBC), neutrophil, complement 4 (C4), NLR (neutrophil-to-lymphocyte ratio) and PLR (platelet-to-lymphocyte ratio) levels and lower levels of apolipoprotein-a (Apo-a) were observed. Their correlation analysis demonstrated positive results between MHR level and white blood cell (WBC) count (r = 0.416, P = 0.034), D-Dimer (r = 0.464, P = 0.026) and monocyte (r = 0.947, P < 0.001). And the time until first remission of skin purpura was shown positively correlated with their age (r = 0.456, P = 0.043), C-reactive protein (CRP) level (r = 0.641, P = 0.018), D-Dimer level (r = 0.502, P = 0.040) while negatively correlated with albumin (Alb) level (r=− 0.626, P = 0.003) and low-density lipoprotein (LDL) level (r=− 0.478, P = 0.033).
Conclusion: Our study suggests that those biomarkers represented for inflammatory responses, lipid profile and immunological functions have significant differences in the subgroups of adult IgAV patients. In addition, we also found that MHR level may serve as a potential biomarker for the pathogenesis and prognosis of IgAV patients with GI involvement.
Keywords: monocyte-to-high-density lipoprotein cholesterol ratio, immunoglobulin A vasculitis, endothelial dysfunction, gastrointestinal involvement, inflammation
Introduction
Immunoglobulin A vasculitis (IgAV), also known as Henoch Schonlein Purpura (HSP), is a kind of leukocytoclastic vasculitis characterized by IgA1-dominant immune deposition at vessel walls.1 Although it is relatively rare in adults, it leads to a worse clinical presentation and prognosis compared to children.2–4 In addition to some patients only presented with skin purpura, IgAV patients may have different organs involved, which led to different prognosis accordingly5 and gastrointestinal (GI) involvement represents major risk of mortality in acute adult IgAV and often shows severe hemorrhage and bowel perforation.6
Increased levels of oxidative stress and inflammatory markers have been reported by several studies in IgAV patients, such as tumor necrosis factor ɑ (TNF-ɑ), interleukin 6 (IL-6), interleukin 1β (IL-1β), monocyte-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio (NLR) and CRP.7–9 However, so far these markers were not assessed in different subgroups of IgAV.
Endothelial dysfunction induced by vasculitis is considered crucial in the vascular involvement.10 Previous study has suggested oxidative stress as well as inflammatory activities as major contributors to the endothelial dysfunction in IgAV.11 Recently, monocyte to high-density lipoprotein cholesterol ratio (MHR) has been proved to be a novel indicator for diseases such as behcet’s disease, ischemic stroke and atherosclerosis.12–14 It is related to inflammatory responses and oxidative stress. However, few studies have investigated the significance of MHR level in different subgroups of IgAV so far.
In this study, we retrospectively analyzed all IgAV patients in our hospital for approximately 10 years to evaluate whether the MHR level and other serum indicators are associated with the pathogenesis and the prognosis of IgAV patients.
Materials and Methods
Participants
Subjects included 158 adults with a history of IgAV diagnosed at the First Affiliated Hospital of Chongqing Medical University, between January 2013 and April 2022. Patients initially diagnosed as IgAV according to the European League Against Rheumatism (EULAR) standard were included.15 The definitions used for the assessment of the skin, joint, renal and gastrointestinal involvement are provided in Supplement Table 1. Patients were excluded when they were complicated with other systemic vasculitis, immunologic comorbidities, heart failure, coronary artery disease, moderate to severe valvular heart disease, renal and hepatic failure, active hepatobiliary disease, active infectious disease, hematologic disorders or malignancy. Another 113 age and sex matched healthy volunteers from the institution served as the healthy controls.
All of the patients enrolled were classified into five subgroups according to the clinical manifestation: group with only skin involved, group with joint involved, group with renal involved, group with gastrointestinal involved and group with multiple system involved (Supplement Table 2). The Ethics Committee of The First Affiliated Hospital of Chongqing Medical University approved this retrospective study (ID: 2022-k204). The research protocol complies with the Declaration of Helsinki. As a retrospective study, informed consent was waived from all enrolled patients.
Data Collection
All data was collected from electronic medical records including the following: gender, age, body mass index (BMI), disease duration, clinical manifestations of IgAV, laboratory indexes (white blood cell [WBC], platelet [PLT], lymphocyte, monocyte, neutrophil, neutrophil-to-lymphocyte ratio [NLR] and platelet-to-lymphocyte ratio [PLR] values, C-reactive protein [CRP], alanine aminotransaminase [ALT], aspartate aminotransferase [AST], complement 3 [C3], complement 4 [C4], albumin [Alb], total cholesterol, triacylglycerol [TG], low-density lipoprotein [LDL], high-density lipoprotein [HDL], apolipoprotein-a [Apo-a], apolipoprotein-b [Apo-b], MHR, D-Dimer (D-D), immunoglobulin [IgA, IgG, IgM]) and the time until first remission of skin purpura. Patients missing details of serological markers were excluded.
Statistical Analysis
The measurement data of normal distribution were described by mean±SD. Analysis of variance and independent sample t-test were used for inter-group comparison. The measurement data of skewed distribution were described by median and interquartile interval, and the comparison between groups was conducted by Kruskal Wallis test and Mann Whitney U-test. Counting data were described by case number and rate, and chi square test was used for comparison between groups. Multivariate logistic regression model was used to explore the association of MHR with GI of IgAV patients. Spearman rank correlation analysis was used to explore the correlation between variables. The variables with P < 0.05 in the single factor were included in the multivariate analysis, and the stepwise method was used to screen the variables. The inclusion criterion was P < 0.05, and the exclusion criterion was P > 0.05. A two-side p-value of <0.05 was considered statistically significant. All statistical analyses were performed using SAS9.4 (Copyright ©2016 SAS Institute Inc. Cary, NC, USA).
Results
The baseline demographic, clinical cohort characteristics, disease features and laboratory parameters of the study population are described in Table 1. Age, sex, BMI, AST and creatinine levels in all groups are found comparable. Compared to healthy controls, IgAV patients had dramatically increased levels of WBC, ALT, triglyceride, Lp (a) and MHR (P < 0.05). However, they showed significantly lower levels of Alb, total cholesterol, HDL, LDL, Apo (a) (P < 0.05). The result of multivariate analysis showed that the levels of Alb, ALT and MHR were different between IgAV patients and controls (Supplement Table 3).
 |
Table 1 Comparison of Clinical Characteristics Between Healthy Controls and IgAV Patients |
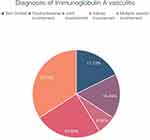
Among the 158 patients, 17.72% of them did not show extra-cutaneous involvement, merely 6.96% of them showed joints involvement, 16.46% of them showed GI involvement, 24.68% of them showed renal involvement, and 34.18% of them showed multi-systemic involvements (Figure 1). The clinical characteristics and laboratory parameters of IgAV patients among different subgroups are described in Supplement Table 4. The average disease duration of patients was 19.5 days. Among them, the group with renal involvement had the longest disease duration with an average duration of 90 days, while the group with joints involvement had the shortest, with only an average duration of 7 days.
In subgroup analysis, we found that the levels of TG were the highest in patients with renal involvement and the lowest in patients with joints involvement (P < 0.05). In terms of immunological analysis, the levels of C4 and IgG were different among these subgroups. In terms of the group with GI involved, we found that the level of MHR was increased significantly when compared to other groups. Therefore, we focused on comparing the differences of clinical indicators between the group with GI involved and the others. Statistical analysis showed that the level of WBC, Neutrophil, NLR and PLR were increased significantly in the group with GI involved when compared to other groups. However, in the lipid profile, they showed significantly lower Apo (a) levels. Although the levels of other lipid indicators showed a downward trend, there was no statistical difference (Table 2). For IgAV patients with GI involved, the C4 level was significantly higher than other subgroups (P < 0.05). Although there was no statistical difference, the level of Alb was decreased compared to the renal involvement group. Notably, Elevated MHR level was also found in GI involved IgAV patients, and further multivariate analysis also verified this result (Supplement Table 5). Correlation analysis showed positive result of the MHR level with WBC count (r = 0.416, P = 0.034), D-Dimer (r = 0.464, P = 0.026) and monocyte (r = 0.947, P < 0.001) (Table 3).
 |
Table 2 Comparison of Clinical Characteristics Between Gastrointestinal Involvement and Other IgAV Patients |
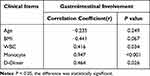 |
Table 3 Correlation Analysis Between MHR and Clinical Characteristics of Gastrointestinal Involvement in IgAV |
Additionally, the correlation analysis between various clinical parameters and the time until first remission of skin purpura were also carried out by Spearman correlation. In GI involvement patients, the time until first remission of skin purpura was showed positively correlated with their age (r = 0.456, P = 0.043), CRP level (r = 0.641, P = 0.018), D-Dimer level (r = 0.502, P = 0.040) while negatively correlated with Alb level (r=−0.626, P = 0.003) and LDL level (r=−0.478, P = 0.033) (Table 4). However, no correlations between the time until response to the therapy and other inflammation-related laboratory parameters (WBC, NLR, neutrophils, lymphocytes, monocytes, TG, HDL) (P > 0.05) were found.
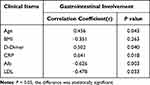 |
Table 4 Correlation Analysis Between Time to First Remission and Clinical Characteristics of Gastrointestinal Involvement in IgAV |
Discussion
IgAV is a kind of vasculitis mostly involving small vessels, characterized by IgA-complexes deposition on small vascular walls. The prevalence of IgAV in adult is 0.1 to 1.8 per 100,000 individuals and is significantly lower than that in children, which has an incidence of 3 to 26 per 100,000 children every year.1–4 However, higher risk of systemic complications in adults was found, among which most notable risk is the chronic renal failure and GI involvement.2–4 Genetic factors, both abnormal innate and acquired immunity, inflammation, galactose-deficient IgA1 immunocomplexes, COVID-19 as well as anti-COVID vaccines are considered to be involved in the pathogenesis of IgAV.11,16 Endothelial cells distribute on the inner surfaces of blood vessels as well as lymphatic vessels. They have proven to play a crucial role in the metabolism and endocrine function and they are also the primary targets of external stimuli and endogenous immune activity. Therefore, Injuries to endothelial cells are recognized as a physiological feature of IgA vasculitis.17
Since inflammation state and oxidative stress have been regarded as important determinants of endothelial cell damage. Clinicians have been investigating their potential role in the pathology of IgAV and the prediction of prognosis. In this study, we found that the parameters of inflammation and oxidative stress showed some difference among different subgroups, suggesting that further subgroup analysis is needed in IgAV patients.
Because of poor prognosis of IgAV-related GI involvement, identifying serum predictors is important for appropriate application of aggressive treatment. In the subgroup analysis, we found that inflammatory parameters were significantly higher in the group of GI involvement such as WBC, neutrophil, NLR and PLR. They also showed higher level of the MHR and significantly lower levels of Apo (a) compared to other subgroups. Thus, those results suggested that inflammation and dyslipidemia are playing significant roles in developing GI involvement in IgAV.
Monocyte activation and their further differentiation into lipid-laden macrophages have been proven important roles in inflammatory activities. Abnormal activation of monocytes can further interact with damaged or activated endothelium and produce multiple types of pro-inflammatory factors as well as pro-oxidant mediators. Thereafter, foam cells are formed when monocytes-differentiated macrophages take up oxidized low-density lipoproteins and other lipids.18–20 Conversely, HDL-C functions as anti-inflammatory and antioxidant factor. It inhibits the migration of macrophage and remove cholesterol debris from them. Besides, it also increases the expression of endothelial nitric oxide synthase and further enhance vasodilation.21–23 Therefore, monocytes act as stimulatory factor, while the HDL-C functions as inhibitory factor during the inflammatory and oxidative processes.
Recently, as a novel inflammatory biomarker, MHR has drawn intensive attention and is widely available in clinical practice. Previous studies have proved an association between increased MHR level and the systemic inflammation and oxidative stress, and MHR could be used as predictive marker for behcet’s disease, ischemic stroke and atherosclerosis.12–14 For this reason, MHR is suggested to be a novel inflammation-based marker than other hematological parameters, as it indicates a balance during inflammatory reactions. Our study showed that IgAV patients with GI involvement have the highest MHR level. Besides, their MHR level was positively correlated with WBC count (r = 0.416, p = 0.034). Thus, these results suggested a potential correlation of increased MHR level with the development of GI involvement in IgAV.
CRP is an acute phase protein that is elevated during infection. However, there are controversies about the relationship between CRP and IgAV-related GI involvement6,24 In our study, IgAV patients with GI involvement showed a trend towards an increase in the level of CRP compared to other groups, suggesting elevated CRP level may be related to the development of GI involved symptoms in IgAV. Besides, the level of CRP has positive correlation with the time until first remission of skin purpura in the GI involvement group, thus suggesting that CRP is associated with disease severity and anti-infective therapy plays a critical role in the prognosis of IgAV patients with GI involvement.
Additionally, albumin is another important acute phase response protein which is also a well-known antioxidant in vivo. Its levels are significantly reduced in multiple inflammatory diseases and hypoalbuminemia has been considered associated with poor prognosis in diseases such as vasculitis.25 Several studies suggested potential relationship between decreased Alb with developing IgAV nephritis (IgAVN).26 Interestingly, our results showed downward trend of Alb level in IgAV patients with GI involvement even when compared to IgAVN patients (Supplement Table 4). The result further proved that it is necessary to be alert to the possibility of the loss of albumin in gastrointestinal system in IgAV patients. Therefore, it suggested that the levels of albumin in blood and urine should be closely monitored.
However, several limitations exist in our study. First, the retrospective design of the study holds restriction to the control of treatment method. Second, this study was conducted in a single center and included a relatively limited number of patients. Nevertheless, our data provide a better understanding of the disease course of adult IgAV patients with GI involvement and the utility of MHR level as a prognostic marker.
Conclusion
In conclusion, our study suggests that there are differences in serum markers between IgAV patients and healthy controls. Subgroup analysis showed that inflammation markers, lipid markers and immunological markers have significant difference in subgroups. It suggested potential differences in the pathogenesis of different subgroups, and it provided directions for future research in IgAV. Last but not least, MHR is potentially a novel inflammatory biomarker, which was significantly decreased in IgAV patients with GI involvement. Our study also suggested MHR level to be a potential marker for the pathogenesis and prognosis of IgAV patients with GI involvement.
Abbreviations
MHR, monocyte to high-density lipoprotein cholesterol ratio; IgAV, immunoglobulin A vasculitis; GI, Gastrointestinal; TG, triacylglycerol; Apo-a, apolipoprotein-a; Apo-b, apolipoprotein-b; WBC, white blood cell; CRP, C-reactive protein; Alb, albumin; low-density lipoprotein, LDL; TNF-ɑ, tumor necrosis factor ɑ; IL-6, interleukin 6; IL-1β, interleukin 1β; NLR, neutrophil-to-lymphocyte ratio; EULAR, the European League Against Rheumatism; BMI, body mass index; PLT, platelet; ALT, alanine aminotransaminase; AST, aspartate aminotransferase; C3, complement 3; C4, complement 4; high-density lipoprotein, HDL; D-D, D-Dimer, IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the First Affiliated Hospital, Chongqing Medical University (ID: 2022-k204). We confirm that we will keep the privacy of the participants and data strictly confidential.
Acknowledgments
The authors would like to thank the patients who participated in this study. This study was supported by the National Natural Science Foundation of China (n82073462).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (n82073462).
Disclosure
All authors declare no potential conflicts of interest, including any relevant financial interests, activities, relationships, or affiliations for this work.
References
1. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi:10.1002/art.37715
2. Nossent J, Raymond W, Keen H, Inderjeeth C, Preen DB. Hospitalisation rates and characteristics for adult and childhood immunoglobulin A vasculitis in Western Australia. Intern Med J. 2019;49(4):475–481. doi:10.1111/imj.14065
3. García-Porrúa C, Calviño MC, Llorca J, Couselo JM, González-Gay MA. Henoch-Schönlein purpura in children and adults: clinical differences in a defined population. Semin Arthritis Rheum. 2002;32(3):149–156. doi:10.1053/sarh.2002.33980
4. Kang Y, Park JS, Ha YJ, et al. Differences in clinical manifestations and outcomes between adult and child patients with Henoch-Schönlein purpura. J Korean Med Sci. 2014;29(2):198–203. doi:10.3346/jkms.2014.29.2.198
5. Sunderkötter CH, Zelger B, Chen KR, et al. Nomenclature of cutaneous vasculitis: dermatologic addendum to the 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheumatol. 2018;70(2):171–184. doi:10.1002/art.40375
6. Audemard-Verger A, Pillebout E, Amoura Z, et al. Gastrointestinal involvement in adult IgA vasculitis (Henoch-Schönlein purpura): updated picture from a French multicentre and retrospective series of 260 cases. Rheumatology. 2020;59(10):3050–3057. doi:10.1093/rheumatology/keaa104
7. Hočevar A, Tomšič M, Jurčić V, Perdan Pirkmajer K, Rotar Ž. Predicting gastrointestinal and renal involvement in adult IgA vasculitis. Arthritis Res Ther. 2019;21(1):302. doi:10.1186/s13075-019-2089-2
8. Berthelot L, Jamin A, Viglietti D, et al. Value of biomarkers for predicting immunoglobulin A vasculitis nephritis outcome in an adult prospective cohort. Nephrol Dial Transplant. 2018;33(9):1579–1590. doi:10.1093/ndt/gfx300
9. Nagy GR, Kemény L, Bata-Csörgő Z. Neutrophil-to-lymphocyte ratio: a biomarker for predicting systemic involvement in adult IgA vasculitis patients. J Eur Acad Dermatol Venereol. 2017;31(6):1033–1037. doi:10.1111/jdv.14176
10. Balta S. Endothelial dysfunction and inflammatory markers of vascular disease. Curr Vasc Pharmacol. 2021;19(3):243–249. doi:10.2174/18756212MTA1oOTYh3
11. Song Y, Huang X, Yu G, et al. Pathogenesis of IgA vasculitis: an up-to-date review. Front Immunol. 2021;12:771619. doi:10.3389/fimmu.2021.771619
12. Acikgoz N, Kurtoğlu E, Yagmur J, Kapicioglu Y, Cansel M, Ermis N. Elevated monocyte to high-density lipoprotein cholesterol ratio and endothelial dysfunction in Behçet disease. Angiology. 2018;69(1):65–70. doi:10.1177/0003319717704748
13. Omar T, Karakayalı M, Yesin M, Alaydın HC, Karabağ Y, Gümüşdağ A. Monocyte to high-density lipoprotein cholesterol ratio is associated with the presence of carotid artery disease in acute ischemic stroke. Biomark Med. 2021;15(7):489–495. doi:10.2217/bmm-2020-0705
14. Zhou Y, Wang L, Jia L, et al. The monocyte to high-density lipoprotein cholesterol ratio in the prediction for atherosclerosis: a retrospective study in adult Chinese Participants. Lipids. 2021;56(1):69–80. doi:10.1002/lipd.12276
15. Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: ankara 2008. Part II: final classification criteria. Ann Rheum Dis. 2010;69(5):798–806. doi:10.1136/ard.2009.116657
16. Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med. 2022;9:996288. doi:10.3389/fmed.2022.996288
17. Xu S, Han S, Dai Y, Wang L, Zhang X, Ding Y. A review of the mechanism of vascular endothelial injury in immunoglobulin a vasculitis. Front Physiol. 2022;16(13):833954. doi:10.3389/fphys.2022.833954
18. Ghattas A, Griffiths HR, Devitt A, Lip GY, Shantsila E. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62(17):1541–1551. doi:10.1016/j.jacc.2013.07.043
19. Combadière C, Potteaux S, Rodero M, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C (hi) and Ly6C (lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117(13):1649–1657. doi:10.1161/CIRCULATIONAHA.107.745091
20. Wu TT, Zheng YY, Chen Y, Yu ZX, Ma YT, Xie X. Monocyte to high-density lipoprotein cholesterol ratio as long-term prognostic marker in patients with coronary artery disease undergoing percutaneous coronary intervention. Lipids Health Dis. 2019;18(1):180. doi:10.1186/s12944-019-1116-2
21. Goswami B, Rajappa M, Singh B, Ray PC, Kumar S, Mallika V. Inflammation and dyslipidaemia: a possible interplay between established risk factors in North Indian males with coronary artery disease. Cardiovasc J Afr. 2010;21(2):103–108.
22. Ansell BJ, Navab M, Hama S, et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108(22):2751–2756. doi:10.1161/01.CIR.0000103624.14436.4B
23. Kuvin JT, Rämet ME, Patel AR, Pandian NG, Mendelsohn ME, Karas RH. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am Heart J. 2002;144(1):165–172. doi:10.1067/mhj.2002.123145
24. Karadağ ŞG, Çakmak F, Çil B, et al. The relevance of practical laboratory markers in predicting gastrointestinal and renal involvement in children with Henoch-Schönlein Purpura. Postgrad Med. 2021;133(3):272–277. doi:10.1080/00325481.2020.1807161
25. Ahn SS, Yoon T, Song JJ, Park YB, Lee SW. Serum albumin, prealbumin, and ischemia-modified albumin levels in patients with ANCA-associated vasculitis: a prospective cohort study. PLoS One. 2022;17(7):e0271055. doi:10.1371/journal.pone.0271055
26. Jauhola O, Ronkainen J, Koskimies O, et al. Clinical course of extrarenal symptoms in Henoch-Schonlein purpura: a 6-month prospective study. Arch Dis Child. 2010;95(11):871–876. doi:10.1136/adc.2009.167874
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.