Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Association of Serum Renalase Levels and Renalase rs10887800 Polymorphism with Unstable Angina Pectoris Patients Having Metabolic Syndrome
Authors Izadpanah P, Asadian F, Jangjou A
Received 2 June 2020
Accepted for publication 19 August 2020
Published 15 September 2020 Volume 2020:13 Pages 3249—3259
DOI https://doi.org/10.2147/DMSO.S265773
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Peyman Izadpanah,1 Fatemeh Asadian,2 Ali Jangjou3
1Cardiology Department, Shiraz University of Medical Sciences, Shiraz, Iran; 2Department of Medical Laboratory Sciences, School of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran; 3Emergency Medicine Department, Shiraz University of Medical Sciences, Shiraz, Iran
Correspondence: Ali Jangjou
Emergency Medicine Department, Shiraz University of Medical Sciences, Shiraz, Iran
Tel +98-9173157555
Email [email protected]
Purpose: An increased risk of cardiovascular mortality and morbidity has been linked with metabolic syndrome (MetS), described as the secondary risk reduction target. These patients are predisposed to high complication levels such as unstable angina-pectoris (USAP) by MetS. As with the role of renalase in the regulation of blood pressure (BP), the study was carried out to determine the levels of renalase circulation in patients with USAP and MetS (USAP+MetS), as well as the association of renalase gene (RNLS) rs10887800 polymorphism and USAP and MetS susceptibility.
Patients and Methods: A total of 134 patients with USAP+MetS and 134 control subjects were recruited in this case-control study.
Results: Renalase was found to have a significantly higher level in USAP+MetS patients (23.28 ± 4.09 μg/dL) than in healthy ones (20.81 ± 2.73 μg/dL) (P < 0.001). Also, it was shown that renalase sensitivity and specificity values for the early diagnosis of USAP and MetS seemed to be 53.7% and 76.9, respectively. Moreover, the value for renalase area under curve (AUC) was 0.654 (95% CI: 0.58– 0.72). The frequency of rs10887800 AG and GG genotypes of RNLS gene was significantly higher in USAP+MetS patients than in control subjects, suggesting that this genotype might be a risk factor against USAP+MetS (OR = 2.114 [95% CI 1.113– 4.016]; P = 0.022) and (OR = 2.057 [95% CI 1.011– 4.186]; P = 0.047), respectively.
Conclusion: The present results showed that renalase serum levels increased in USAP and MetS patients. Moreover, the RNLS rs10887800 was reported to be associated with a higher risk of USAP+MetS.
Keywords: cardiovascular diseases, metabolic syndrome, polymorphism, renalase, rs10887800, unstable angina pectoris
Introduction
Metabolic syndrome (MetS) is a variety of conditions which occur simultaneously and include abdominal obesity, hyperglycemia, dyslipidemia, and hypertension. Such conditions lead to an increased risk of heart disease, stroke, and diabetic status.1 MetS is clinically important in terms of detecting a subgroup of patients who have in common a physiopathological state which makes them suffer from chronic diseases.2 Unfortunately, the prevalence of MetS has increased in recent years. According to an enormous number of studies, the prevalence of MetS in a variety of countries ranged from 12.8% to 41.7%, while in Iran it was shown to vacillate between 22% and 31%.3,4 Moreover, unlike the developed countries, Iran has seen a 20% to 45% increase in the level of cardiovascular mortality over the past 20 years,5,6 which is partially due to the prevalence of MetS. In addition, MetS causes a threefold rise in the risk of diabetes; thus, the crucial role the diagnosis of this syndrome plays in the prevention of diabetes and cardiovascular diseases (CVDs).7
Ischemic heart diseases are comprised of a vast variety of conditions ranging from asymptomatic ischemia, exertion-induced angina, and unstable angina pectoris (USAP), to acute myocardial infarction (MI).8 Being on the top of this list, USAP causes disability and some other risks which are greater than those created by chronic stable angina pectoris (SAP), but less than those of acute MI. Not only is USAP very prevalent, but it is also regarded as a quite serious form of acute coronary syndrome (ACS). Furthermore, it accounts for huge numbers of hospitalization in the United States, that is, more than an annual number of 750,000 hospitalized cases, from which 70,000 patients develop MI, leading to their sudden death.1,9,10 USAP is mainly diagnosed in a clinical way and on the basis of symptom recognition.11 Inflammatory mediators are inextricably linked to the sequence of events that contribute to formation, progression, and fracture of atherosclerotic plaque. This understanding has spurred examination of many inflammation markers as prospective instruments for the prediction of the risk of CVD, of which renalase is the most persistently researched. Therefore, the incidence of USAP and MetS is determined by an intricate association between genetic, metabolic, and environmental factors.12,13
CVDs have been reported to witness a rise in the level of catecholamines. Also, it is shown that catecholamines, namely norepinephrine, epinephrine, and dopamine, are involved in regulating blood pressure (BP).12 Intracellular enzymes, including catechol-O‐methyltransferase, monoamine oxidase‐A (MAO‐A), and MAO‐B, are involved in the degradation of catecholamines. Renalase, which was developed by Xu et al,13 is a truly innovative monoamine oxidase enzyme as well as a soluble dinucleotide-dependent amine oxidase flavin adenine. Research has revealed that the increase in the level of plasma catecholamines greatly increases renalase performance which supports renalase’s contribution to circulation of catecholamine degradation, as well as controlling BP and cardiac activity.14
A considerable array of evidence has shown that USAP and MetS are partially determined in a genetic manner.15–18 An understanding of the precise genetic factors inherent in development of USAP and MetS may possibly explain why USAP and MetS features often co-occur within the same particular person. It is noteworthy that many genetic variants have been investigated to determine the genes influencing the development of USAP and MetS with contradictory results; nevertheless, no study has been conducted on the possible implications of the renalase gene (RNLS) polymorphisms and USAP and/or MetS. RNLS on chromosome 10q23.33 is identified as a human renalase enzyme that encodes genes which has 309, 469 base pairs (bp), and 10 exons.14
A theory which has attempted to describe the elevated BP in MetS, likely to be included in USAP pathogenesis, has been proposed in this regard. Based on the prior research, the increased activity of sympathetic vasoconstrictors is a crucial mechanism that enhances vascular resistance. With respect to the involvement of renalase in BP regulation, in the current study, we assessed firstly the association between single-nucleotide polymorphism (SNP) of RNLS rs10887800 at intron 6 close to the exon/intron border and susceptibility of USAP and MetS (USAP+MetS) in southern Iran. Additionally, we sought to determine the renalase circulation levels in USAP and MetS patients.
Materials and Methods
Subjects
This case-control research was conducted on 268 participants who referred to Emergency Units of Namazi and Al-Zahra Heart Hospitals affiliated to Shiraz University of Medical Sciences, Shiraz, Iran. The research involved 134 subjects with USAP+MetS (55 men and 79 women, mean age = 56.88 ± 9.25 years), and 134 healthy subjects (64 men and 70 women, mean age = 53.50 ± 8.50 years). Patients who suffered from chest pain volunteered to take part in the research, so they were physically checked by cardiologist and/or an emergency physician. Patients that fulfilled the following therapeutic requirements were regarded as USAP cases: admission to hospital with angina pectoris or a comparable ischemic pain with at least one of three attributes (1) having taken place at rest (or with relatively low exertion), commonly lasting >10 minutes; (2) being severe and new (ie, within 4–6 weeks); and/or (3) having happened with a crescendo trend (ie, clearly more severe, prolonged, or frequent than before). In addition, MetS was taken into consideration in compliance with the scientific criteria set by the American Heart Association.19 Participants with three or more of the following three factors are identified as MetS: 1) high waist circumference (WC) (85 and 80 cm in men and women, respectively); 2) high triglycerides (TG) (≥150 mg/dL (1.7 mmol/l)) or drug therapy; 3) high-density lipoprotein cholesterol (HDL-c) (<40 mg/dL (1.0 mmol/L) in males (<50 mg/dL (1.3 mmol/L) in females) or drug therapy; 4) high BP (systolic blood pressure (SBP) 130 mmHg and/or diastolic blood pressure (DBP) 85 mmHg) or taking antihypertensive medication; 5) fasting glucose (≥100 mg/dL (5.56 mmol/L)) or drug therapy.19
Subjects of insufficient medical and demographic information, discontented participants and those under hormone replacement therapy or afflicted with congenital heart disease, chronic kidney disease (CKD), respiratory diseases, strokes, cancers, autoimmune diseases, thyroid disease and chronic hepatic condition were all excluded from the study. After exclusion, 134 patients with USAP+MetS and 134 healthy subjects were included in the data analyses. This research was approved by the local Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (IR.sums.med.rec.1398.661), and was undertaken based on the principles of the Helsinki Declaration. We received a signed informed consent.
Anthropometric and Biochemical Measurements
BP of all subjects was measured from the right arm using a mercury sphygmomanometer after at least a five-minute rest and was repeated at a minimum five-minute interval, and then the mean pressure was used. The WC was evaluated at the level of the iliac crest and at the end of a normal expiration of the subject with a measuring tape in a horizontal plane around the abdomen. The weight of participants was gauged using the lowest possible clothing while being barefoot with a 100g accuracy digital scale. The height of subjects was measured by a measuring tape with an accuracy of 1cm while standing beside the wall, and their shoulders in normal position.
At the time of admission, 10 milliliter fasting blood samples were extracted from each patient, of which 5cc was preserved in tubes that included ethylenediaminetetraacetic acid (EDTA) anticoagulant for DNA extraction at −20°C and the remaining 5cc was centrifuged at room temperature for a few minutes (at 2500 rpm for 7 min). The serum samples were then kept at −70°C until assessment of the renalase. Regular laboratory tests were used to measure the fasting blood glucose (FBG), TG, and HDL-c.
Measurement of Renalase
The concentration of serum renalase was determined by means of an enzyme-linked immunosorbent assay (ELISA) kit (USCN Life Science Inc., Wuhan, China) comprised of commercially available FAD-dependent amine oxidase, as directed by the manufacturer. Note that renalase serum amounts were measured in μg/mL.
Genotype Determination
Genomic DNA from the whole blood samples (obtained from EDTA-anticoagulated blood) was collected using the “salting-out” process. The polymerase chain reaction-restriction fragment length polymorphism (PCR‐RFLP) method outlined below was also used to establish genotypes of RNLS rs10887800 polymorphism. Bagci et al20 reported the primers’ sequences that were used to amplify DNA fragments. The PCR parameters were as follows: initial denaturation at 95°C for 5 minutes accompanied by 30 amplification cycles comprising 95°C denaturation, 60°C annealing, 72°C extension (30 seconds each), and 5 minutes ultimate extension at 72°C. PCR products (10 μL) were digested at 37°C for 1 hour with 5 U of PstI restriction endonuclease (Jena Bioscience, Jena, Germany) and particles were segregated in the TBE 0.5 X buffer by electrophoresis with 1.6% (w/v) ethidium bromide-stained agarose gel. They were later envisioned by ultraviolet light. The RNLS 10887800A allele had no PstI cleavage site and produced only a 554‐bp fragment. The RNLS 10887800G, however, had two PstI cleavage site, creating 139‐bp and 415‐ digested fragments (Figure 1).
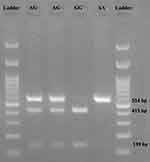 |
Figure 1 Gel electrophoresis of RNLS rs10887800 gene polymorphism. AA genotype (139 bp); AG genotype (139 bp, 415 bp, and 554 bp); GG genotype (139 bp and 415 bp). |
Statistical Analysis
All the statistical procedures were executed by means of the statistical package for the social sciences (SPSS) version 23.0 for Windows (SPSS Inc., Chicago, IL). Note that a p-value lower than 0.05 was set as significant. The Kolmogorov–Smirnov test was employed to examine the assumption of normality. All continuous variables were mentioned as mean ± standard deviation (M±SD) and categorical variables in the form of numbers (percentages). Comparisons of anthropometric indices, laboratory biomarkers, and renalase serum levels between the two groups of participants were made through Chi-square test for categorical variables and the Student’s t-test or Mann–Whitney U-test for continuous ones. The discrepancies between subgroups were studied utilizing Kruskal–Wallis with post-hoc Mann–Whitney U-tests as well as Chi-square/Fisher’s exact tests (applying the Bonferroni correction). Deviation from the Hardy–Weinberg equilibrium (HWE) was tested by the Chi-square test. Moreover, correlations between MetS components and renalase were assessed as necessary through the Spearman test and, also, to achieve a renalase cutoff value for USAP+MetS prediction, receiver operating characteristic (ROC) curve was used. Finally, the logistic regression model was used to investigate the association between the study groups with allele and genotype frequencies. Crude and adjusted odds ratios (OR) were estimated through simple and multiple models adjusted for age, sex, BMI, SBP, DBP, FBS, TG, HDL-c, and WC.
Results
Table 1 outlines the medical characteristics for patients and healthy subjects. There were no significant age discrepancies (56.88 ± 9.25 years for the case and 53.50 ± 8.50 years for controls) and sex differences between the case and control groups (P= 0.09 and P= 0.32, respectively), while BMI was significantly higher in USAP+MetS patients than in control patients (P < 0.001). All the components of MetS varied significantly between the two categories (P < 0.001).
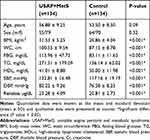 |
Table 1 Comparison of Anthropometric Indices, Laboratory Biomarkers, and Renalase Serum Levels Between USAP+MetS and Control Subjects |
Serum renalase levels between the two groups can be seen in Figure 2. Renalase has been shown to have significantly higher levels in patients with USAP+MetS (23.28 ± 4.09 μg/dL) compared to the healthy ones (20.81 ± 2.73 µg/dL) (P < 0.001). As can be seen in Figure 3, there were no significant differences in renalase levels between men and women in each group (P = 0.13 for case group and P = 0.07 for control one), while men exhibited higher levels of renalase compared with women.
 |
Figure 2 Comparison of renalase serum levels between unstable angina pectoris and metabolic syndrome (USAP+MetS) patients and control individuals. |
 |
Figure 3 Comparison of renalase serum levels between unstable angina pectoris and metabolic syndrome (USAP+MetS) and control groups divided by gender. |
Table 2 demonstrates the comparison of demographic factors, clinical laboratory parameters and serum renalase levels between MetS components. Other factors, apart from sex (P = 0.50) including BMI, WC, FBG, TG, HDL, SBP, DBP, and renalase differed significantly between MetS components (P = 0.002 for age and P < 0.001 for all comparisons). Besides, no significant correlation was found between the components of MetS and renalase (data are not shown).
 |
Table 2 Comparison of Anthropometric Indices, Laboratory Biomarkers, and Renalase Serum Levels Among MetS Components |
Figure 4 illustrates the comparison of renalase serum levels among MetS components. It was also found that zero (20.43 ± 2.58 μg/dL) and one (20.86 ± 3.00 μg/dL) MetS component groups significantly differ from four and five (23.63 ± 3.86 μg/dL) MetS components (P = 0.01 and P = 0.004) in terms of renalase serum levels.
We also described the ROC curve by comparing the specificity and sensitivity of renalase for USAP+MetS diagnoses (Figure 5). Renalase sensitivity and specificity for the early diagnosis of USAP and MetS seemed to be 53.7% and 76.9%, respectively. Renalase area under curve (AUC) was 0.654 (95% CI: 0.58–0.72). The findings indicated that the ideal serum renalase cut-off value for USAP+MetS diagnosis was 22.45 μg/dL.
Table 3 depicts the allelic and genotypic levels of polymorphism in participants with and without USAP+MetS. The frequency of the RNLS genotype rs10887800 AG was significantly higher in USAP+MetS patients than in control subjects and this genotype might also constitute a risk factor against USAP+MetS (OR = 2.11 [95% CI 1.11–4.01]; P = 0.02). Similarly, the prevalence of the RNLS rs10887800 GG genotype in USAP+MetS patients demonstrated a significantly higher number relative to control subjects, implying that this genotype possibly functions as a risk factor against USAP+MetS (OR = 2.05 [95% CI 1.01–4.18]; P = 0.04). In the adjusted model, we also found that the prevalence of RNLS rs10887800 G allele was greater in USAP+MetS patients compared to the control group (57% vs 49%, respectively) (OR = 1.57 [95% CI 1.04–2.36]; P = 0.03). In the adjusted dominant genetic model, the AG + GG genotypes were correlated with 4.14 times higher odds of USAP+MetS (OR = 4.14, [95% CI = 1.29–13.23], P = 0.01). Although the crude recessive model showed no significant association with USAP+MetS risk (P= 0.49), adjusted model demonstrated an increased association with USAP+MetS risk (OR = 1.57 [95% CI 1.04–2.36]; P = 0.01). In addition, there were no significant discrepancies in HWE deviation of genotypic frequencies either in the USAP+MetS or in the control groups (P > 0.05).
 |
Table 3 Genotype and Allele Frequencies of RNLS Rs10887800 Polymorphism in Subjects with USAP+MetS and Control Groups |
A summary of mean values for the clinical and anthropometric variables analyzed in USAP+MetS and control subjects according to RNLS rs10887800 genotypes (Tables 4 and 5, respectively). There were no significant differences in the variables tested (MetS components) in terms of the three RNLS rs10887800 genotypes in both studied groups (P > 0.05).
 |
Table 4 Comparison of Mean Values of the Examined Parameters According to RNLS Rs10887800 Genotypes in USAP+MetS Subjects |
 |
Table 5 Comparison of Mean Values of the Examined Parameters According to RNLS Rs10887800 Genotypes in Control Subjects |
Discussion
The manifestation, development and severity of USAP and MetS rely on hereditary predisposition in combination with metabolic and environmental factors.13 The detection of MetS genetic/metabolic risk factors is therefore important for USAP and other threatening diseases to be predicted at risk. As one of the metabolic factors that cause different metabolic diseases, renalase is regarded as an innovative indicator that interferes with different metabolic mechanisms. The current research thus aimed to exam the probable association of the circulating level of renalase and its RNLS rs10887800 polymorphism with USAP and MetS. The key results of the current study revealed that higher renalase levels correlate with USAP and MetS. In addition, both AG and GG genotypes of RNLS rs10887800 polymorphism displayed a risk factor impact with an elevated risk of USAP and MetS in 2.114 and 2.057 times. Even though there were significant associations between MetS components and renalase levels, no significant relationship was reported between MetS components and RNLS rs10887800 genotypes.
MetS has found to be related to an increased likelihood of cardiovascular mortality and morbidity and has since been established as a secondary risk reduction target.21 In acute coronary syndrome (ACS), MetS incidence increases to approximately 50%, and MetS makes it possible for such patients to suffer from higher complication levels (eg, heart failure, stroke, and mortality).22,23 Several studies have examined the impact of MetS on MI, but none involved individuals with pure non-ST segment myocardial infarction (NSTEMI) and an unstable angina pectoris (USAP). As the main aim of the current study, the investigation of renalase circulating level was performed as a probable predictor biomarker in diagnosing the USAP patients by MetS baseline. Renalase is a flavoprotein amine oxidase comprising 342 amino acids, indicated to be active in the metabolism of catecholamines. Evidence seems to suggest that this enzyme is secreted into the body by kidneys. This enzyme is, nevertheless, also present in the heart, intestine, liver, skeletal muscle, and endothelium. The enzyme plays a pivotal role in circulating the degradation of catecholamines, consequently leading to a significant drop in BP as well as other main medical issues such as MetS.24–26 According to the evidence, the plasma lacks sufficient renalase activity in humans under basal circumstances; however, its activity might be enhanced nearly 10-fold in 30 seconds through infusion of exogenous epinephrine for at least 60 minutes.14 In addition, evidence suggests that plasma renalase is stimulated in reaction to greater systolic BP, thereby implying that this enzyme plays a significant role in the minute-to-minute regulation of BP.27 Studies have shown that hypertension is associated with renalase deficiency. Tachycardia and hypertension were seen in a renalase knockout mouse model, confirming the role of renalase in regulating BP.14
Both experimental and clinical studies have confirmed the relationship between serum renalase and pressure of the blood and other metabolic factors; although the findings are still vague. Also, for patients with USAP and MetS, current results have shown higher levels of renalase than those of the control ones. Moreover, in experimental models, renalase was reported to play a role in hypertension pathogenesis and it was Zhao et al28 who first demonstrated the relationship between renalase and hypertension. In 2586 adult Chinese population, however, the data in whites have not been verified.29 Renalase deficiency in hypertension and CKD were later displayed by Ficek et al.28 Independently, Wybraniec et al29 reported that the occurrence of hypertension in a patient following surgical reparation of aortic coarctation as well as in a control group was correlated with inadequate renalase. The results of the aforementioned studies show that the regulation of the intrarenal dopaminergic system can be accounted for by the effect of renalase. The findings of this study showed the higher levels of renalase in MetS and USAP patients. Therefore, it is possible, according to the findings of the aforementioned studies, that the renalase rises in emergency conditions such as USAP are due to body metabolic changes.
Findings obtained from the current study showed that circulating renalase levels in USAP+MetS patients were significantly higher than in healthy subjects. In this respect, men have shown higher renalase levels in both USAP+MetS and control groups compared to women, even though this difference was not significant. To the best of authors’ knowledge, this is the first study to investigate the association between renalase serum levels and USAP+MetS and its components. In addition, our analysis also established the diagnostic sensitivity and specificity of renalase in USAP and MetS cases for the first time. With regard to ROC analysis, renalase could not be regarded as a good diagnostic biomarker for predicting coronary intervention outcomes in USAP patients with syndrome X. In our research, given the accepted function of catecholamines in atherosclerosis and other CVDs, it is speculated that higher renalase levels could contribute to mitigating emergency cardiovascular conditions including USAP, especially in patients with MetS. It should be noted that, like ours, several studies have shown a high serum renalase level, although diagnostic values of such biomarkers for USAP have still not been adequately made clear, highlighting the need for further research projects to confirm the current results.
The RNLS (length 309 469 bp) is located at q23.33 on chromosome 10. This gene contains 10 exons with at least four alternatively spliced isoforms. Expressed more than other renalases and having a length of 342 amino acids, renalase 1 isoform is mainly found in plasma, kidney, skeletal muscle, heart, and liver.14,28 Different SNPs including rs10887800, rs2576178, rs2296545 and rs2114406 are present in the RNLS gene, and their association with hypertensive, vascular, diabetes, and stroke has been the focus of many studies.29–31 Even though the RNLS rs10887800 polymorphism was investigated under various conditions and in different population groups, the specific mechanism through which polymorphism altered the risk of USAP and MetS remains unspecified. The renalase rs10887800 examined by SNP is placed close to the exon/intron boundary in a putative functional region. Thus, it is possible that it influenced gene regulation and expression, leading to changes in the amount of renalase levels.31,32
The number of RNLS rs10887800 GG and RNLS rs10887800 AG genotypes in this analysis was found to be significantly higher for USAP+MetS patients. Also, it has been shown that the adjusted recessive and dominant genetic models for RNLS rs10887800 polymorphism proved to play a risk factor role against the USAP and MetS. Furthermore, the current findings did not demonstrate the association between serum renalase levels and genotypes of renalase rs10887800. Stec et al31 conducted a study on the relationship between RNLS rs10887800 gene polymorphism and renalase circulation levels in patients who were being under hemodialysis. Their results showed significantly lower renalase levels in hemodialysis of the genotype rs10887800AA compared to those of GG and AG. Nevertheless, this polymorphism did not indicate any correlation with regard to the control group’s renalase concentration which was in line with findings of the current study. In the present study, RNLS rs10887800 polymorphism was shown to be associated with USAP+MetS development in such that higher risk for USAP+MetS was associated with the RNLS rs10887800 AG and GG genotypes.
The most crucial MetS parameters related with renalase functioning were the mean of SBP and DBP24,26,33 and FBG33 which are fully studied previously. In this respect, the examination of RNLS rs10887800 polymorphism in combination with MetS components showed that the genotype distributions of rs10887800 were not correlated with MetS components. While numerous studies have found a correlation between different polymorphisms with RNLS and other diseases, no published research has been reported on the likely association between MetS, USAP, and RNLS gene polymorphism. Studies conducted by Bagci et al20 on Turkish population along with that of Teimoori et al24 on an Iranian population revealed that in women with preeclampsia, the genotype rs10887800 GG was significantly higher in SBP and DBP than in the GA or AA, both of which were not in accordance with this study. In another research by Buraczynska et al,30 the relationship of renalase rs2296545, rs2576178 and rs10887800 SNPs were investigated with the gene polymorphism for patients with type 2 diabetes, hypertension and stroke, showing that polymorphism with RNLS rs10887800 G allele could be helpful in identifying diabetes patients at an increased risk of stroke. While the possible link between RNLS polymorphism and clinical laboratory parameters was not explored in their study, our findings did not show the association between levels of FBG serum and RNLS rs10887800 genotype distributions. Although the mechanism behind the impact of RNLS rs10887800 polymorphism on hypertension and consequently, on the development of USAP and MetS could not be readily apparent, they may influence renalase enzyme expression or activity. Polymorphism rs10887800 is indicated to be located near an exon/intron boundary in a putative functional region and could therefore impact on gene expression and cause changes in the renalase protein levels.28,34
In interpreting our results, one should take account of some limitations including the limited sample size that can affect results, environmental factors, and various ethnic groups in southeast Iran. Another limitation of our analysis was that we only made our observations in the acute stage; thus, no longitudinal follow-up samples were available to assess their changes over time, leading to a cross-sectional analysis with only a limited robustness. We also assessed the diagnostic usefulness of renalase in an almost small sample. While it has been shown to be a probable biomarker, further studies with greater sample sizes are highly recommended if this hypothesis is to be clarified. Moreover, our results will be more valuable if we can test renalase activity in relation to renalase polymorphism. Since different ethnic groups exist in Iran, more research on all ethnic groups are advised to accept or deny the present findings of the study with larger sample sizes.
Conclusion
To summarize, higher circulating levels of renalase were associated with USAP+MetS. Moreover, the RNLS rs10887800 polymorphism was shown to be related to USAP+MetS. Higher risk for USAP+MetS was associated with the RNLS rs10887800 AG and GG genotypes. The present study suggests the need for further studies with larger sample sizes in order to investigate the probable early diagnostic value of renalase. It also supports its measurement in patients with USAP and MetS for a more specific risk assessment.
Abbreviations
USAP+MetS, unstable angina pectoris and metabolic syndrome; BMI, body mass index; WC, waist circumference; MetS, metabolic syndrome; CKD, chronic kidney disease; TG, triglycerides; HDL-c, high density lipoprotein cholesterol; CVD, cardiovascular diseases; RNLS, renalase gene; ROC, receiver operating characteristics; AUC, area under curve; BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure; CVD, cardiovascular diseases; USAP; unstable angina pectoris; SAP, stable angina pectoris; MAO‐A, monoamine oxidase‐A; MAO‐B, monoamine oxidase‐B; PCR‐RFLP, polymerase chain reaction-restriction fragment length polymorphism; FBG, fasting blood glucose; ACS, acute coronary syndrome; MI, myocardial infarction; ELISA, enzyme-linked immunosorbent assay; SPSS, statistical package for the social sciences; SNP, single-nucleotide polymorphism.
Acknowledgments
The present study was supported by a grant from the Vice-chancellor for Research, SUMS, Shiraz, and Iran with the ethical code (IR.sums.med.rec.1398.661). The authors appreciate Shiraz University of Medical Sciences for financially supporting this research. Moreover, we sincerely thank all participants recruited in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interest for this work.
References
1. Falk E. Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death. Autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation. 1985;71(4):699–708. doi:10.1161/01.CIR.71.4.699
2. Mauro F, Tiziana S, Di Cristo F, et al. Metabolic syndrome, mediterranean diet, and polyphenols: evidence and perspectives. 2019
3. Shahbazian H, Latifi SM, Jalali MT, et al. Metabolic syndrome and its correlated factors in an urban population in South West of Iran. J Diabetes Metab Disord. 2013;12(1):11. doi:10.1186/2251-6581-12-11
4. Kaykhaei MA, Hashemi M, Narouie B, et al. Prevalence of metabolic syndrome in adult population from Zahedan, southeast Iran. Iran J Public Health. 2012;41(2):70.
5. Azizi F, Madjid M, Rahmani M, Emami H, Mirmiran P, Hadjipour R. Tehran lipid and glucose study (TLGS): rationale and design. Iran J Endocrinol Metab. 2000;2:77–86.
6. Shayestefar M, Haghighi KS, Jahanfar S, Delvarianzadeh M, Nematzadeh F, Ebrahimi MH. Assessment of the relationship between metabolic syndrome and obstructive sleep apnea in male drivers of Shahroud city in 2018: a cross sectional study. BMC Public Health. 2019;19(1):1058. doi:10.1186/s12889-019-7361-5
7. Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–1778. doi:10.2337/diacare.28.7.1769
8. Braunwald E, Jones RH, Mark DB, et al. Diagnosing and managing unstable angina. Agency for health care policy and research. Circulation. 1994;90(1):613–622. doi:10.1161/01.CIR.90.1.613
9. Hamm CW, Bleifeld W. Unstable angina: current concepts of medical management. Cardiovasc Drugs Ther. 1988;2(3):333–339. doi:10.1007/BF00054640
10. Pokras R Detailed diagnoses and procedures for patients discharged from short-stay hospitals, United States; 1985. 1987
11. Makki N, Brennan TM, Girotra S. Acute coronary syndrome. J Intensive Care Med. 2015;30(4):186–200. doi:10.1177/0885066613503294
12. Hoseini Z, Azimi‐Nezhad M, Ghayour‐Mobarhan M, et al. VEGF gene polymorphism interactions with dietary trace elements intake in determining the risk of metabolic syndrome. J Cell Biochem. 2019;120(2):1398–1406. doi:10.1002/jcb.27171
13. Xu J, Li G, Wang P, et al. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J CLIN INVEST. 2005;115(5):1275–1280.
14. Desir GV. Regulation of blood pressure and cardiovascular function by renalase. Kidney Int. 2009;76(4):366–370. doi:10.1038/ki.2009.169
15. Bellia A, Giardina E, Lauro D, et al. “The linosa study”: epidemiological and heritability data of the metabolic syndrome in a Caucasian genetic isolate. Nutr Metab Cardiovasc Dis. 2009;19(7):455–461. doi:10.1016/j.numecd.2008.11.002
16. Bosy-Westphal A, Onur S, Geisler C, et al. Common familial influences on clustering of metabolic syndrome traits with central obesity and insulin resistance: the Kiel obesity prevention study. Int J Obes. 2007;31(5):784. doi:10.1038/sj.ijo.0803481
17. Elder SJ, Lichtenstein AH, Pittas AG, et al. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J Lipid Res. 2009;50(9):1917–1926. doi:10.1194/jlr.P900033-JLR200
18. Shah SH, Hauser ER, Bain JR, et al. High heritability of metabolomic profiles in families burdened with premature cardiovascular disease. Mol Syst Biol. 2009;5(1):258. doi:10.1038/msb.2009.11
19. Alberti K, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; international. Circulation. 2009;120(16):1640–1645. doi:10.1161/CIRCULATIONAHA.109.192644
20. Bagci B, Karakus S, Bagci G, Sancakdar E. Renalase gene polymorphism is associated with increased blood pressure in preeclampsia. Pregnancy Hypertens an Int J Women’s Cardiovasc Heal. 2016;6(2):115–120.
21. Isomaa BO, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi:10.2337/diacare.24.4.683
22. National Cholesterol Education Program. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). circulation. 2002;106:3143–3421. doi:10.1161/circ.106.25.3143
23. Feinberg MS, Schwartz R, Tanne D, et al. Impact of the metabolic syndrome on the clinical outcomes of non-clinically diagnosed diabetic patients with acute coronary syndrome. Am J Cardiol. 2007;99(5):667–672. doi:10.1016/j.amjcard.2006.10.023
24. Teimoori B, Moradi-Shahrebabak M, Rezaei M, Mohammadpour-Gharehbagh A, Salimi S. Renalase rs10887800 polymorphism is associated with severe pre-eclampsia in southeast Iranian women. J Cell Biochem. 2019;120(3):3277–3285. doi:10.1002/jcb.27595
25. Desir GV, Peixoto AJ. Renalase in hypertension and kidney disease. Nephrol Dial Transplant. 2013;29(1):22–28. doi:10.1093/ndt/gft083
26. Desir GV, Tang L, Wang P, et al. Renalase lowers ambulatory blood pressure by metabolizing circulating adrenaline. J Am Heart Assoc. 2012;1(4):e002634. doi:10.1161/JAHA.112.002634
27. Boomsma F, Tipton KF. Renalase, a catecholamine-metabolising enzyme? J Neural Transm. 2007;114(6):775. doi:10.1007/s00702-007-0672-1
28. Ficek J, Małyszko J, Chudek J. Renalase and its role in the development of hypertension in patients with chronic renal failure. Przegl Lek. 2015;72(6):306–308.
29. Wybraniec MT, Mizia-Stec K, Trojnarska O, et al. Low plasma renalase concentration in hypertensive patients after surgical repair of coarctation of aorta. J AM SOC HYPERTENS. 2014;8(7):464–474.
30. Buraczynska M, Zukowski P, Buraczynska K, Mozul S, Ksiazek A. Renalase gene polymorphisms in patients with type 2 diabetes, hypertension and stroke. Neuromolecular Med. 2011;13(4):321–327. doi:10.1007/s12017-011-8158-6
31. Stec A. Rs10887800 renalase gene polymorphism influences the level of circulating renalase in patients undergoing hemodialysis but not in healthy controls. BMC Nephrol. 2017;18(1):118. doi:10.1186/s12882-017-0543-4
32. Steć A. Effect of renalase (RNLS) gene polymorphisms (rs1088700 and rs2576178) on plasma RNLS level in hemodialyzed patients affected by arterial hypertension and coronary artery disease. Polish J Public Heal. 2017;127(4):147–150. doi:10.1515/pjph-2017-0031
33. Wang F, Huang B, Li J, Liu L, Wang N. Renalase might be associated with hypertension and insulin resistance in Type 2 diabetes. Ren Fail. 2014;36(4):552–556. doi:10.3109/0886022X.2013.876352
34. Li X, Jiang W, Li L, et al. Renalase gene polymorphism in patients with hypertension and concomitant coronary heart disease. Kidney Blood Press Res. 2014;39(1):9–16. doi:10.1159/000355771
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


