Back to Journals » Cancer Management and Research » Volume 12
Association of Preoperative Neutrophil/Lymphocyte Ratio with Clinical Outcomes in Dedifferentiated Chondrosarcoma Patients
Authors Liu C, Xing Y, Jiao Q, Yang Q, Yu W, Li Y, Tao X, Yao W
Received 8 June 2020
Accepted for publication 17 July 2020
Published 3 August 2020 Volume 2020:12 Pages 6719—6726
DOI https://doi.org/10.2147/CMAR.S266671
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Chenglei Liu1 ,* Yue Xing2 ,* Qiong Jiao,3 Qingcheng Yang,4 Wenbin Yu,5 Yuncheng Li6 ,* Xiaofeng Tao,1 Weiwu Yao7
1Department of Radiology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Radiology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China; 3Department of Pathology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China; 4Department of Orthopaedics, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China; 5Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Head and Neck Surgery, Peking University Cancer Hospital & Institute, Beijing, People’s Republic of China; 6Department of Otorhinolaryngology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 7Department of Radiology, Shanghai Tongren Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weiwu Yao
Department of Radiology, Shanghai Tongren Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, People’s Republic of China
Email [email protected]
Xiaofeng Tao
Department of Radiology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
Email [email protected]
Background: Dedifferentiated chondrosarcoma (DC) is an extremely uncommon malignant bone tumor with dismal survival outcomes. The purpose of this study was to investigate whether the preoperative neutrophil/lymphocyte ratio (NLR) has the ability to predict overall survival (OS) in DC patients.
Materials and Methods: Twenty-three DC patients with surgical resection were retrospectively reviewed between 2008 and 2015. The clinical pathological information and survival data were collected for analysis. The cut-off point for NLR was defined by receiver operating curve (ROC). The impact of NLR level on OS between two groups was compared using Kaplan–Meier curves with the Log-rank test. The association between NLR and OS was calculated by univariate and multivariate Cox proportional models.
Results: From the ROC analysis, the optimal NLR cut-off point was 3.11. Patients with high NLR had a worse OS than low NLR (p = 0.003, Log-rank test). In univariate analysis, a significant association was observed between high NLR and poor OS (Hazard ratio (HR) 4.62, 95% confidence interval (CI) 1.48– 14.34, p = 0.008). After adjustment of co-variables, high NLR had more than 4 times the risk of mortality (HR 4.01, 95% CI 1.12– 14.26, p = 0.032).
Conclusion: Preoperative NLR in peripheral blood is an easily accessible and cost-effective prognostic biomarker in DC patients. A prospective study with large sample size is warranted.
Keywords: dedifferentiated chondrosarcoma, neutrophil/lymphocyte ratio, outcome, prognostic factor
Introduction
Dedifferentiated chondrosarcoma (DC) is a highly malignant and extremely uncommon bone tumor, representing about 10% of all primary chondrosarcomas.1 It is characterized by a high-grade bone sarcoma immediately adjacent to low-grade chondrosarcoma.2 The classical histologic finding is a sharp demarcation between these two components.3
Until now, the optimal therapeutic scheme for DC is the complete surgical removal of tumor with a free margin. Unlike conventional chondrosarcoma, the therapeutic regimen of DC should be similar to that of osteosarcoma according to the National Comprehensive Cancer Network (NCCN) guidelines.4 However, the role and benefit of chemotherapy are still unclear. Even with these aggressive treatments, over the last decades, the 5-year overall survival was still less than 20%, with a high local recurrence rate.3
Due to limited treatment options available, the identification of potential prognostic factors is particularly important to patients and doctors. Currently, due to the rarity of the diagnosis, the prognostic factors are only confined to clinical pathological factors, such as age, size, location, pathologic fracture, metastasis at diagnosis, margin status, and dedifferentiated subtype.5,6 However, these factors are not quite optimal and lack accuracy in estimation. Therefore, there is an imperative need for a cost-effective and widely available marker that would help to generate individual therapy and follow–up schedules.
Recently, more and more evidence indicated the involvement of systemic inflammation in the development and prognosis of various types of malignant tumors. Specifically, an elevated neutrophil/lymphocyte ratio (NLR), as a biomarker of systemic immune inflammation response, has been discovered to be an independent prognostic factor in soft–tissue sarcoma,7 lymphoma,8 bone metastasis,9 and gastrointestinal tract malignancies.10 Regarding DC patients, the baseline C-reactive protein, T cell factor 1 protein, and chondrocytic phenotype were considered to have important prognostic value.11–13 Furthermore, Nemecek et al suggested that baseline C-reactive protein was an independent predictor for overall survival in DC patients.11 However, to the best of our knowledge, very limited research has been done regarding the effects of the preoperative NLR on the prognosis for DC patients. In the clinical practice, the NLR is easily measured from peripheral blood and is cost-effective, which might be beneficial in patient stratification and risk assessment if it could be used as a prognostic biomarker. Therefore, the purpose of our research was to investigate whether the preoperative NLR has predictive value for overall survival in DC patients.
Materials and Methods
Study Subjects
This retrospective study was approved by the local ethics committee of Shanghai Sixth People’s Hospital (No.YS-2016-064), and was conducted in accordance with the ethical standards of Declaration of Helsinki. The informed consent form was obtained from each patient before the study. We ensured the patient data were kept in confidentiality. All patients with confirmed DC were retrospectively reviewed from our medical histological record system between January 2008 and December 2015. The selected subjects meet the following inclusion criteria: 1) histologically confirmed DC, 2) available preoperative laboratory parameters and treatment scheme, 3) underwent surgical resection, 4) completed follow up information on both survival time and cause of death. Six subjects were excluded due to failure to collect complete clinical data including unknown tumor stage, unknown use of chemotherapy, and lack of follow-up information. Finally, a total of 23 patients were enrolled in the current study.
The clinical database of all patients was collected from our hospital electronic medical reports system including age, sex, location, surgical margin, tumor stage, differentiated subtype, adjuvant chemotherapy, and local recurrence. The tumor sites were categorized into axial bone (pelvis and spine) or extremities (upper extremities, lower extremities). The resection margin was classified on the basis of Enneking et al.14 The tumor stage was defined by the American Joint Committee on Cancer (AJCC) criteria.15
All pathological samples were centrally reassessed by a pathologist who has extensive experience in bone tumors. The histological subtype of the dedifferentiated component was confirmed by immunohistochemistry. The absolute neutrophil and lymphocyte counts from peripheral blood were obtained 1–3 days before the first surgery. The followed-up examination of all patients was performed by telephone at regular intervals, such as 3-month intervals in 1 year, half year intervals in 2 years, and 12-month intervals thereafter. The end of follow-up was January 2018. The post-operative investigation included clinical examination and radiological analysis such as chest X-ray or CT for pulmonary metastasis and local MRI for recurrence.
Statistical Analysis
The endpoint of this study was overall survival (OS), which was defined as the period between the date of diagnosis and death from any cause. We divided the preoperative NLR into two groups using ideal cut-off point. The baseline clinical pathological characteristics between two groups were compared using chi-square (x2) or Fisher’s exact test. To reduce potential selection bias and adjust for the difference between two groups, the propensity score matching (1:1) was applied based on variables, including sex, location, metastasis at diagnosis, pathological fracture, chemotherapy, resection margins, local recurrence, AJCC tumor stage, and dedifferentiated subtype. Survival estimates between two groups were compared using Kaplan–Meier curves with the Log-rank test. The associations between prognostic factors and OS were assessed by the univariate and multivariate Cox regression model. The multivariate model was created by adjustment for co-variable that significantly associated with OS in univariate analysis. Hazard ratios (HRs) were presented with 95% confidence intervals (CIs). All statistical analyses were conducted with SPSS 26.0 (SPSS, Inc., Chicago, IL). P-value < 0.05 was believed to indicate statistical significance.
Results
The detailed clinical data and treatment scheme of all patients are summarized in Table 1. In brief, 11 (47.8%) were females and 12 (52.2%) were males. The average age was 50.57 years (range: 32 to 73 years). Fourteen (60.9%) cases were in axial bone (13 pelvis, 1 spine) and 9 (39.1%) cases were in appendicular bone (3 femur, 2 humerus, 1 finger, 1 clavicle and 1 fibula). The dedifferentiated subtypes included osteosarcoma (n=19), malignant fibrous histiocytoma (n=3) and myofibroblastic sarcoma (n=1). Among the patients, 8 (34.8%) patients presented with distant metastasis at diagnosis. All patients underwent surgery, 17 patients (73.9%) achieved wide margin. Thirteen patients (56.5%) suffered from AJCC stage ш or Ⅳ disease at baseline. Eight (34.8%) patients underwent surgery with adjuvant chemotherapy, of them 2 patients receiving neoadjuvant chemotherapy. The mean followed-up time was 16.57 months with ranging from 2 months to 67 months. The estimated survival for patients was 69.6% after 6 months, 39.1% after 1 year, and 17.4% after 5 years, respectively (Figure 1).
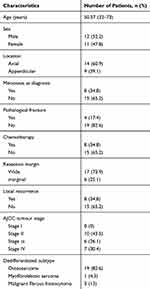 |
Table 1 Baseline Patient Characteristics |
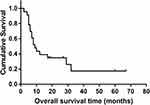 |
Figure 1 Kaplan–Meier curve for overall survival in dedifferentiated chondrosarcoma patients. |
The average neutrophil count, lymphocyte count, and NLR at baseline were 5.63x109/L (SD 3.38, range 2.0–16.8 x109/L), 1.52x109/L (SD 0.52, range 0.5–2.9 x109/L), and 4.35 (SD 4.05, range 1.3–20.6), respectively. According to the receiver operating curve (ROC) analysis, the cut–off point of the NLR was 3.11 (AUC: 0.85, 95% CI 0.67–1.00, p = 0.012). Consequently, we separated all patients into two groups: low NLR (<3.11) and high NLR (≥3.11). Ten patients (43.48%) were categorized as low NLR and 13 patients (56.52%) as high NLR. Meanwhile, we compared the difference in baseline clinical pathological characteristics between high NLR and low NLR group. For the whole cohort, more patients with advanced AJCC tumor stage (ш-Ⅳ) were observed in high NLR group (p = 0.024). In terms of chemotherapy, patients who received chemotherapy were more often seen in high NLR group (p = 0.029). No significant differences were found in sex, tumor site, surgical margin, metastasis at diagnosis, pathological fracture, local recurrence, and dedifferentiated subtype between the two groups. After we applied the propensity score matching to reduce potential selection bias, the two groups were well balanced in the baseline characters (Table 2).
 |
Table 2 Comparison of the Baseline Characters of Patients with Dedifferentiated Chondrosarcoma Between the NLR≥3.11 and NLR<3.11 Group Before and After Propensity Score Matching |
Before propensity score matching, the mean overall survival time was 10.46 months for patients with high NLR and 43.80 months for low NLR. Patients with high NLR had a worse OS than those of low NLR (log-rank, p = 0.003, Figure 2A). After propensity score matching, high NLR was also correlated with a poor OS. The 5-year overall survival in high NLR group was lower than that in the low NLR group (log-rank, p = 0.030, Figure 2B).
Our results showed the NLR was significantly different between early tumor and advanced stage (p=0.024). Compared to low AJCC stage (Ⅰ-Ⅱ), the value of NLR was significantly higher in patients with advanced stage (ш-Ⅳ) (mean: 5.19 vs 3.27, p=0.024). However, as to distant metastasis at diagnosis, NLR did not reach significant difference between the patients with and without distant metastasis at diagnosis (mean: 4.42 vs 4.24, p=0.192). Our further analysis also showed that the high NLR was significantly associated with advanced tumor stage of DC (I/II vs ш/IV) (OR, 7.8, 95% CI, 1.1–33.2). In the entire cohort, due to limited sample size, we divided all patients into two groups: low AJCC stage (Ⅰ-Ⅱ) and advanced AJCC stage (ш-Ⅳ).
For survival analysis, in the entire cohort, univariate analysis showed that the association between high NLR and poor OS was significant (HR 4.62, 95% CI 1.48–14.34, p = 0.008). In addition, primary axial location (HR 3.43, 95% CI 1.10–10.67, p = 0.033), marginal surgical resection (HR 4.52, 95% CI 1.46–13.95, p = 0.009), metastasis at diagnosis (HR 6.32, 95% CI 1.8–21.99, p = 0.004), and advanced AJCC tumor stage (ш-Ⅳ) (HR 4.41, 95% CI 1.30–13.16, p = 0.016) were also significantly associated with poor OS. However, other parameters such as chemotherapy, local recurrence, pathological fracture, and dedifferentiated subtype did not correlate with poor OS (Table 3).
 |
Table 3 The Univariate Analysis of the Associations Between Prognostic Factors and Overall Survival |
To identify whether the high NLR is an independent predictor of OS, we adjusted for co-variate including tumor primary location, initial metastasis status, surgical margin, and AJCC tumor stage. The results indicated that high NLR was an independent predictor of poor OS among DC patients with surgery (HR 4.01, 95% CI 1.12–14.26, p = 0.032) (Table 4).
 |
Table 4 Multivariate Cox Analysis Regarding Overall Survival |
Discussion
In this study, we found that preoperative NLR greater than or equal to 3.11 was strongly associated with poor clinical outcome, suggesting NLR may serve as an independent prognostic biomarker for OS in DC patients. Moreover, we further confirmed adjuvant chemotherapy did not improve overall survival for DC patients with surgery.
Over the last few years, increasing evidence showed that the systemic inflammation was closely associated with tumor biological behavior and metastasis. Inflammatory cells and tumor cells acted upon each other directly or indirectly in the tumor micro-environment. Increased leukocytes, as response to inflammatory, resulted in the unregulated proliferation, transformation, and transplantation of tumor cells by inhibiting cell apoptosis or senescence, DNA mutation, and development of angiogenesis.16 The association between high NLR and unfavorable clinical outcomes in various types of malignant tumors had been reported. JK Pine et al suggested elevated NLR could serve as an independent predictor for OS and tumor aggressive phenotype in colorectal cancer.17 In soft-tissue sarcoma study, Szkandera J et al showed patients with increased pre-operative NLR in the peripheral blood had worse clinical outcome.7,18 Similarly, tumor localized progression and metastatic relapse also associated with increased NLR.19 Moreover, in osteosarcoma patients, Xia WK et al showed high NLR was a significant association with advanced tumor stage at diagnosis.20 Our results showed that high NLR was associated with poor OS in the entire and the propensity score matched patients. Multivariable Cox regression analysis of the entire cohort showed that high NLR could independently predict poor overall survival, which was consistent with previously published data.21 In addition, our results were also in line with recently finding that the elevated baseline C-reactive protein was an independent poor predictor in DC patients.11 C-reactive protein was also identified as a biomarker of inflammation response. However, in contrast to C-reactive protein, the NLR is more cost-effective and is widely available in routine peripheral blood tests.
Although NLR was considered as a prognostic biomarker in different types of malignant neoplasm, the exact mechanism underlying these findings remains unclear. Some possible reasons have been postulated. First, an elevated NLR may break the balance between pre-tumor inflammation status and anti-tumor immune activity. Previous studies have reported that secreted various cytokines, chemokines, and inflammation proteins by tumor cells were capable of activating and recruiting neutrophils from the peripheral blood to the tumor environment. In turn, neutrophils have been discovered to mediate angiogenesis and tissue invasion by releasing matrix metalloproteinase and other pre-inflammation factors, which promotes tumor progression and metastasis.22,23 Moreover, neutrophils have been identified to be potent promoters in tumor development by facilitating genomic instability and accelerating tumor micro-spread, thereby promoting aggressive tumor behavior.16 Furthermore, a high number of neutrophils impair the tumor immune microenvironment. Previous studies have shown that neutrophils may activate T cell functions by producing ROS, NO, and arginase.16 In addition, lymphocytes were also identified as a marker of cancer immune surveillance. Previous studies have reported that increased lymphocyte count in tumor stroma was related to better prognosis in some types of cancers,24 which suggested that enhanced tumor-related immune response may control some premalignant lesion thereby caused tumor regression and improved prognosis. Thus, a reduced number of lymphocytes may be incapable of inhibiting the proliferation and migration of tumor cells. Taken together, a high NLR represents a shift in the balance of tumor homeostasis and thus results in a poor survival rate.
In this study, osteosarcoma component was the main dedifferentiated subtype of DC (82.6%). Previously published data have shown tumor aggressive behavior was determined by histopathologic subtype of dedifferentiated component.25 However, no convincing evidence has been found that adjuvant chemotherapy would improve clinical outcomes and control metastases for DC. In our present study, no significant difference in OS was found for patients with adjuvant chemotherapy after surgery or not (median: 9 months versus 8 months), which was in accordance with previous results.26 Some studies also reported that there was no obvious tumor necrosis after neoadjuvant chemotherapy.27 The underlying mechanisms were still unclear. A possible explanation may be attributed to the mesenchymal stem and progenitor cell, which expressing abundant extracellular matrix and neovascularization in tumors.28 In addition, the p-glycoprotein and multidrug resistance protein1 (MDR1) overexpression in chondrosarcoma may be an important mechanism of resistance to adjuvant chemotherapy.29,30
In line with the results from Strotman PK et al,3 our findings also showed that axial location, surgical margin, metastasis at diagnosis, and advanced AJCC tumor stage was associated with increased mortality risk. Malchenko S et al found that over 90% of DC patients developed pulmonary metastases within several months of the initial diagnosis.31 Metastasis suggests advanced AJCC tumor stage. Therefore, low-dose chest CT should be recommended as an indispensable tool for monitoring pulmonary metastases. To date, the cause of the high incidence of lung metastases in DC patients is still unclear. It may attribute to stem-like cells differentiation disorder in the process of tumor development.32
There were several limitations to the current study. First, the retrospective review with a small sample size is our main limitation, which may result in biased estimates of association. However, our preliminary findings from such a small sample size may help generate a hypothesis for testing or validation in future large prospective studies via consortia or multi-centers. Second, the pre-operative peripheral blood NLR may be influenced by unknown factors other than inflammation. Third, the prognostic factors for disease-free survival were not investigated due to the limited sample size. Fourth, we have no data available on the patients without surgery for analysis regarding the effect of NLR on overall survival for DC patients. Despite these limitations, we still carefully speculate that a high pre-treatment NLR is a potential prognosis biomarker in DC patients who underwent surgical removal.
Conclusion
In conclusion, we determined that an elevated preoperative peripheral blood NLR may independently predict overall survival in DC patients. A prospective study with large sample size is warranted. Moreover, we further confirmed that adjuvant chemotherapy could not improve the clinical outcomes in DC patients with surgery.
Data Sharing Statement
The data in this study are available on request from the corresponding author (Weiwu Yao, Email:[email protected]). The data are not publicly available due to privacy or ethical restrictions.
Disclosure
The authors declare no conflicts of interest.
References
1. Frassica FJ, Unni KK, Beabout JW, Sim FH. Dedifferentiated chondrosarcoma. A report of the clinicopathological features and treatment of seventy-eight cases. J Bone Joint Surg Am. 1986;68(8):1197–1205. doi:10.2106/00004623-198668080-00008
2. Dahlin DC, Beabout JW. Dedifferentiation of low-grade chondrosarcomas. Cancer. 1971;28(2):461–466. doi:10.1002/1097-0142(197108)28:2<461::AID-CNCR2820280227>3.0.CO;2-U
3. Strotman PK, Reif TJ, Kliethermes SA, et al. Dedifferentiated chondrosarcoma: A survival analysis of 159 cases from the SEER database (2001–2011). J Surg Oncol. 2017;116(2):252–257. doi:10.1002/jso.24650
4. Biermann JS, Adkins DR, Benjamin RS, et al. Bone cancer. J National Comprehens Cancer Network. 2010;8(6):688–712. doi:10.6004/jnccn.2010.0051
5. Grimer RJ, Gosheger G, Taminiau A, et al. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur J Cancer. 2007;43(14):2060–2065. doi:10.1016/j.ejca.2007.06.016
6. Staals EL, Bacchini P, Bertoni F. Dedifferentiated central chondrosarcoma. Cancer. 2006;106(12):2682–2691. doi:10.1002/cncr.21936
7. Szkandera J, Gerger A, Liegl-Atzwanger B, et al. The derived neutrophil/lymphocyte ratio predicts poor clinical outcome in soft tissue sarcoma patients. Am J Surg. 2015;210(1):111–116. doi:10.1016/j.amjsurg.2014.10.021
8. Wang S, Ma Y, Sun L, et al. Prognostic Significance of Pretreatment Neutrophil/Lymphocyte Ratio and Platelet/Lymphocyte Ratio in Patients with Diffuse Large B-Cell Lymphoma. BioMed res int. 2018;2018:9651254.
9. Thio Q, Goudriaan WA, Janssen SJ, Paulino Pereira NR, Sciubba DM. Prognostic role of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with bone metastases. Br J Cancer. 2018;119(6):737–743. doi:10.1038/s41416-018-0231-6
10. Liu F, Luo H. Prognostic significance of peripheral blood-derived neutrophil/lymphocyte ratio in patients with digestive cancer. J cell physiol. 2019;234(12):22775–22786.
11. Nemecek E, Funovics PT, Hobusch GM, et al. C-reactive protein: an independent predictor for dedifferentiated chondrosarcoma. J Orthopaedic Res. 2018;36(10):2797–2801. doi:10.1002/jor.24030
12. Xu X, Tang X, Guo W, Yang K, Ren T. TCF-1 participates in the occurrence of dedifferentiated chondrosarcoma. Tumour Biol. 2016;37(10):14129–14140. doi:10.1007/s13277-016-5235-3
13. Aigner T, Müller S, Neureiter D, Illstrup DM, Kirchner T, Björnsson J. Prognostic relevance of cell biologic and biochemical features in conventional chondrosarcomas. Cancer. 2002;94(8):2273–2281. doi:10.1002/cncr.10461
14. Enneking WF. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;2003(415):4–18.
15. Webber C, Gospodarowicz M, Sobin LH, et al. Improving the TNM classification: findings from a 10-year continuous literature review. Int j cancer. 2014;135(2):371–378. doi:10.1002/ijc.28683
16. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
17. Mallappa S, Sinha A, Gupta S, Chadwick SJD. Preoperative neutrophil to lymphocyte ratio >5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15(3):323–328. doi:10.1111/codi.12008
18. Szkandera J, Absenger G, Liegl-Atzwanger B, et al. Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients. Br J Cancer. 2013;108(8):1677–1683. doi:10.1038/bjc.2013.135
19. Chan JY, Zhang Z, Chew W, et al. Biological significance and prognostic relevance of peripheral blood neutrophil-to-lymphocyte ratio in soft tissue sarcoma. Sci Rep. 2018;8(1):11959. doi:10.1038/s41598-018-30442-5
20. Xia WK, Liu ZL, Shen D, Lin QF, Su J, Mao W-D. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J Surg Oncol. 2016;14(1):127. doi:10.1186/s12957-016-0889-2
21. Aggerholm-Pedersen N, Maretty-Kongstad K, Keller J, Safwat A. Serum Biomarkers as Prognostic Factors for Metastatic Sarcoma. Clin oncol. 2019;31(4):242–249. doi:10.1016/j.clon.2019.01.011
22. Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54(5):948–955. doi:10.1016/j.jhep.2010.08.041
23. Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737–744. doi:10.1038/35036374
24. Zhao Y, Ge X, He J, et al. The prognostic value of tumor-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: a systematic review and meta-analysis. j cell physiol. 2019;17(1):85.
25. Bleiweiss IJ, Kaneko M. Chondrosarcoma of the larynx with additional malignant mesenchymal component (dedifferentiated chondrosarcoma). Am J Surg Pathol. 1988;12(4):314–320. doi:10.1097/00000478-198804000-00009
26. Dickey ID, Rose PS, Fuchs B, Wold LE, Okuno SH. Dedifferentiated chondrosarcoma: the role of chemotherapy with updated outcomes. J Bone Joint Surg Am. 2004;86(11):2412–2418. doi:10.2106/00004623-200411000-00008
27. Mitchell AD, Ayoub K, Mangham DC, Grimer RJ, Carter SR, Tillman RM. Experience in the treatment of dedifferentiated chondrosarcoma. J Bone Joint Surg Br. 2000;82(1):55–61. doi:10.1302/0301-620X.82B1.0820055
28. Chow WA. Chondrosarcoma: biology, genetics, and epigenetics. F1000Research. 2018;7:1826. doi:10.12688/f1000research.15953.1
29. Chen L, Long C, Liu J, Duan X, Xiang Z. Prognostic nomograms to predict overall survival and cancer-specific survival in patients with pelvic chondrosarcoma. Cancer Med. 2019;8(12):5438–5449. doi:10.1002/cam4.2452
30. Boehme KA, Schleicher SB, Traub F. Chondrosarcoma: A Rare Misfortune in Aging Human Cartilage? The Role of Stem and Progenitor Cells in Proliferation, Malignant Degeneration and Therapeutic Resistance. Int J Mol Sci. 2018;19(1).
31. Malchenko S, Seftor EA, Nikolsky Y, et al. Putative multifunctional signature of lung metastases in dedifferentiated chondrosarcoma. Sarcoma. 2012;2012:820254. doi:10.1155/2012/820254
32. Mohseny AB, Hogendoorn PC. Concise review: mesenchymal tumors: when stem cells go mad. Stem Cells. 2011;29(3):397–403. doi:10.1002/stem.596
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

