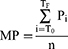Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 14
Association of Post Transplantation Anaemia and Persistent Secondary Hyperparathyroidism with Diastolic Function in Stable Kidney Transplant Recipients
Authors Hsu HC, Norton GR, Peters F, Robinson C, Dlongolo N, Solomon A, Teckie G, Woodiwiss AJ, Dessein PH
Received 5 April 2021
Accepted for publication 5 June 2021
Published 2 July 2021 Volume 2021:14 Pages 211—223
DOI https://doi.org/10.2147/IJNRD.S314313
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pravin Singhal
Hon-Chun Hsu,1,2 Gavin R Norton,1 Ferande Peters,1 Chanel Robinson,1 Noluntu Dlongolo,3 Ahmed Solomon,4 Gloria Teckie,5 Angela J Woodiwiss,1 Patrick H Dessein1,6,7
1Cardiovascular Pathophysiology and Genomics Research Unit, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; 2Nephrology Unit, Milpark Hospital, Johannesburg, South Africa; 3Rheumatology Unit, Rosebank Hospital, Johannesburg, South Africa; 4Division of Rheumatology, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa; 5Division of Nephrology, Department of Medicine, Chris Hani Baragwanath Hospital and Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa; 6Internal Medicine Department, University of the Witwatersrand, Johannesburg, South Africa; 7Internal Medicine Department, Free University and University Hospital, Brussels, Belgium
Correspondence: Patrick H Dessein
Cardiovascular Pathophysiology and Genomics Research Unit, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, 80 Scholtz Road, Norwood, 2117, Johannesburg, South Africa
Tel +27662491468
Email [email protected]
Introduction: We hypothesized that post transplantation anaemia and persistent secondary hyperparathyroidism are potential determinants of diastolic function in stable kidney transplant recipients.
Methods: We assessed traditional and non-traditional cardiovascular risk factors and determined carotid artery intima-media thickness and plaque by ultrasound, arterial function by applanation tonometry using SphygmoCor software and diastolic function by echocardiography in 43 kidney transplant recipients with a transplant duration of ≥ 6 months, no acute rejection and a glomerular filtration rate of ≥ 15 mL/min/1.73m2.
Results: Mean (SD; range) transplant duration was 12.3 (8.0; 0.5– 33.8) years. Post transplantation anaemia and persistent secondary hyperparathyroidism were identified in 27.9% and 30.8% of the patients, respectively; 67.5% of the participants were overweight or obese. In established confounder adjusted analysis, haemoglobin (partial R=− 0.394, p=0.01) and parathyroid hormone concentrations (partial R=0.382, p=0.02) were associated with E/e’. In multivariable analysis, haemoglobin (partial R=− 0.278, p=0.01) and parathyroid levels (partial R=0.324, p=0.04) were independently associated with E/e’. Waist–height ratio (partial R=− 0.526, p=0.001 and partial R=− 0.355, p=0.03), waist circumference (partial R=− 0.433, p=0.008 and partial R=− 0.393, p=0.02) and body mass index (partial R=− 0.332, p=0.04 and partial R=− 0.489, p=0.002) were associated with both e’ and E/A, respectively, in established confounder adjusted analysis. The haemoglobin-E/e’ (partial R=− 0.422, p=0.02), parathyroid hormone-E/e’ (partial R=0.434, p=0.03), waist–height ratio-e’ (partial R=− 0.497, p=0.007) and body mass index-E/A (partial R=− 0.386, p=0.04) relationships remained consistent after additional adjustment for left ventricular mass index and cardiac preload and afterload measures.
Conclusion: Haemoglobin and parathyroid hormone concentrations as well as adiposity measures are independently associated with diastolic function in kidney transplant recipients. Whether adequate management of post transplantation anaemia, persistent secondary hyperparathyroidism and excess adiposity can prevent the development of heart failure with preserved ejection fraction in kidney transplant recipients merits further investigation.
Keywords: haemoglobin, parathyroid hormone, obesity, diastolic function, kidney transplantation
Introduction
Among patients with end-stage kidney disease (ESRD), kidney transplant recipients experience a markedly better quality of life and reduced mortality rates compared to those treated with ongoing dialysis.1,2 Also, cardiovascular disease risk is increased 10- to 20-fold in dialysis patients as compared to 3- to 5-fold in kidney transplant recipients.1
Impaired diastolic function together with left ventricular hypertrophy and pronounced fibrosis comprise the most characteristic cardiac abnormalities in chronic kidney disease (CKD) patients.3 Impaired diastolic function generally results from reduced left ventricular active relaxation and increased left ventricular stiffness.3,4 Left ventricular stiffening is mediated by fibrosis and causes increased cardiac filling pressures through impaired passive relaxation.3,4
A functioning allograft in kidney transplant recipients can improve echocardiographically identified cardiac abnormalities5 and stabilize those detected by cardiac magnetic resonance imaging.6 Despite these potentially favourable effects of kidney transplantation on cardiac function and structure, ~18% of kidney transplant recipients develop de novo heart failure within 3 years subsequent to transplantation.7 This is 2 to 5 times higher than that in population-based cohorts.7 By contrast, de novo ischemic heart disease reportedly occurs at a similar frequency in kidney transplant recipients and the general population.8 Kidney transplant recipients may be particularly prone to non-atherosclerotic cardiac disease.
Impaired diastolic function underlies the development of heart failure with preserved ejection fraction (HFpEF).3,4 HFpEF is more prevalent and associated with larger mortality rates than heart failure with reduced ejection fraction (HFrEF) in patients with CKD.9,10 Hemodynamic factors that cause impaired diastolic function in CKD comprise increased preload due to volume overload and anaemia, and increased afterload mediated by heightened peripheral resistance and marked arteriosclerosis that results in arterial stiffness and enhanced wave reflection and pressure pulsatility.3,11 Other reported determinants of impaired diastolic function in CKD patients encompass aberrant bone mineral metabolism including secondary hyperparathyroidism, inflammation and oxidative stress.3,11
Impaired diastolic function may be present in more than half of patients undergoing kidney transplantation.12 However, the determinants of diastolic function in particularly stable kidney transplant recipients (transplant duration of ≥6 months, absence of acute rejection and glomerular filtration rate of ≥15 mL/min/1.73m2)13 await elucidation.
Importantly in the present context, late post-transplantation anaemia14 and persistent secondary hyperparathyroidism15 have been reported in up to 35% and 50% of kidney transplant recipients, respectively. Conceptually, anaemia and hyperparathyroidism could mediate impaired diastolic function in kidney transplant recipients. In this regard, anaemia not only increases cardiac preload and left ventricular mass but can also cause tissue hypoxia with consequent myocardial fibrosis.16–20 Secondary hyperparathyroidism can increase cardiac afterload through enhancing arteriosclerosis1,11 and directly induce cardiac hypertrophy and myocardial fibrosis.21
In this study, we hypothesized that post transplantation anaemia and persistent secondary hyperparathyroidism are potential determinants of diastolic function in stable kidney transplant recipients.
Patients and Methods
Patients
The study was performed in line with the Helsinki Declaration as revised in 2013. All organs were donated voluntarily with written informed consent, which was conducted in accordance with the Declaration of Istanbul.23 The University of the Witwatersrand Human (Medical) Research Ethics Committee (protocol number: M15-08-43) in Johannesburg, South Africa approved the study protocol. Written informed consent was obtained in each patient prior to participation. Forty-three consecutive stable kidney transplant recipients were enrolled as outpatients at the Milpark Hospital in Johannesburg, South Africa. Mean (SD; range) transplant duration was 12.3 (8.0; 0.5–33.8) years. Median (IQR) duration of dialysis prior to kidney transplantation was 2.0 (0.5–4.6) years and 4 (9.3%) patients were not dialysed prior to receiving a kidney transplant. Only 2 (4.7%) patients had a patent arteriovenous fistula. Patients with a transplant duration of <6 months, acute rejection, an estimated glomerular filtration rate22 of ≤15 mL/min/1.73m2, previously diagnosed heart failure, myocardial infarction, active infection and/or cancer were excluded. The criteria selected for stable kidney transplant status were based on a previously reported study by Ducloux et al.13
Methods
Patient characteristics that were recorded included demographic variables, traditional and non-traditional cardiovascular risk factors, carotid atherosclerosis measures, arterial function, echocardiographic parameters and systemic vascular resistance. All investigations were carried out on a single day at the time of the study.
Traditional and non-traditional or renal cardiovascular risk factors were recorded as previously reported24 and provided in the online Supplementary Methods. Intact parathyroid hormone levels were determined by an electrochemiluminescence immunoassay “ECLIA” on Cobas (Roche Diagnostics, Mannheim, Germany). This assay employs a sandwich test principle in which a biotinylated monoclonal antibody reacts with the N-terminal fragment (1–37) and a monoclonal antibody labelled with a ruthenium complexa) reacts with the C-terminal fragment (38–84). The antibodies used in this assay are reactive with epitopes in the amino acid region 26–32 and 37–42. The measurement range is 1.2 to 5000 pg/mL. The intra-assay and inter-assay coefficients of variation are each <3.5%. Vitamin D concentrations were measured using the Alinity I 25-OH Vitamin D assay (Abbott Ireland Diagnostics Division Lisnamuck, Longford, Ireland), which is a chemiluminescent microparticle immunoassay that quantifies 25-hydroxyvitamin D on the Alinity I Analyser. The measurement range is 3.5–154.2 ng/mL. The intra-assay and inter-assay coefficients of variation are each <4.6%. Mean or distending arterial blood pressure for the peripheral waveform was determined electronically by the SphygmoCor device (see below) and using the formula
where T0=start of the waveform; TF=end of waveform; Pi=pressure points and n=number of pressure points.
Atherosclerosis was assessed by carotid artery ultrasound employing a Philips CX50 POC Compact CompactXtreme Ultrasound System (Philips Medical Systems (Pty) Ltd, USA) attached to a linear array 4.0–12.0 MHz probe. The software provided for semi-automated border detection gives markedly reproducible data as previously described.21 Images of at least 1cm length of the distal common carotid arteries were obtained. The optimal angle of incidence was used, defined as the longitudinal angle of approach where both branches of the internal and external carotid artery were visualized simultaneously. The carotid intima-media thickness (c-IMT) was defined as the mean of the left and right common carotid artery thickness. Plaque in the extracranial carotid tree was defined according to the Mannheim consensus criteria.25 Carotid ultrasound measurements were made by the same observer that performed the arterial function and echocardiographic evaluations (CR). The intra-observer variability of ultrasound measurements is low in our setting.24
Central hemodynamic characteristics were determined by applanation tonometry and SphygmoCor software as previously reported24 and provided in the online Supplementary Material. Aortic pulse wave velocity, augmentation index, reflected wave pressure and reflection magnitude, central systolic and pulse pressure, peripheral pulse pressure, pulse pressure amplification and forward wave pressure were measured.
NT-proBNP was determined by an electrochemiluminescence immunoassay on Cobas (Roche Diagnostics, Mannheim, Germany). The measurement range is 10–35,000 pg/mL. The intra-assay and inter-assay coefficients of variation are 3.0% and 4.8%, respectively.
Echocardiography was performed in accordance with the American Society of Echocardiography convention26 and employing a Philips CX50 POC Compact CompactXtreme Ultrasound System (Philips Medical Systems (Pty) Ltd, USA) equipped with a 1.8–4.2 MHz probe that allowed for M-mode, 2-D, pulsed and tissue Doppler measurements. We evaluated geometric characteristics, left ventricular systolic function and diastolic function variables including the early (E)/late (atrial) diastolic wave (A) ratio, the peak mitral annulus motion during early diastole (e’) and E/e’ ratio. E/A and e’ are markers of active left ventricular relaxation, and E/e’ is an index of left ventricular filling pressure.4,27 Our methodology was previously described27 and is provided in the online Supplementary Material.
Systemic vascular resistance was calculated from mean arterial pressure, right atrial pressure and cardiac output according to the equation (mean arterial pressure – right atrial pressure)=systemic vascular resistance × cardiac output assuming that right atrial pressure=0 mmHg.
Data Analysis
Results are given as mean (SD), median (interquartile range) or number (percentages) as appropriate. Non-normally distributed variables were logarithmically transformed prior to entering them in multivariate linear regression models.
The associations of cardiovascular risk factors with E/e’, e’ and E/A were first assessed in age, sex and race adjusted models. This was followed by established confounder (age, sex, race, weight, height, mean blood pressure and heart rate)24 adjusted analysis; when anthropometric characteristic-diastolic function relationships were evaluated, weight and height were not included as potential established confounders in the models. Associations were then reassessed in multivariate models in which potential determinants as identified in the previous models were entered simultaneously.
Subsequently, we assessed whether cardiovascular risk factor-diastolic function relationships were explained by left ventricular mass, cardiac preload (left ventricular end-diastolic volume)28 and/or cardiac afterload (central systolic blood pressure29 and systemic vascular resistance30) by adding the respective variables to the models.
Statistical analysis was performed on IBM SPSS statistics program (version 23.0 IBM, USA). Significance was set at p<0.05.
Results
Patient Characteristics
The main recorded patient characteristics are given in Table 1. Mean (SD) age was 53.5 (10.8) years, approximately one-third were women and black patients comprised the largest proportion (46.52%) of enrolled study subjects. Mean body mass index (BMI) was in the overweight range (26.9%); overweight (BMI=25–29.9 kg/m2) and obesity (BMI >30 kg/m2) were present in 19 (44.2%) and 10 (23.3%) patients, respectively. Hypertension, dyslipidemia and diabetes were present in 86.0%, 83.7% and 25.6% of the participants, respectively. Antihypertensive agents, lipid lowering drugs (statins with or without ezetimibe), insulin therapy and oral glucose lowering medications were employed in 37 (86.0%), 33 (76.7%), 6 (14.0%) and 5 (11.6%) of patients, respectively.
 |
Table 1 Main Recorded Patient Characteristics in 43 Kidney Transplant Recipients |
Mean (SD) estimated glomerular filtration rate22 was 63 (22) mL/min/1.73m2; glomerular filtration rate ranged from 22 to 104 mL/min/1.73m2. Median (IQR) intact parathyroid hormone levels were 68.0 (49.0–155.6) pg/mL; 21 (48.8%) patients had raised intact parathyroid concentrations (>65 pg/mL), and among those with a time since transplantation of more than 1 year (n=39), the respective levels were >130 pg/mL in 12 (30.8%), which is in keeping with persistent secondary hyperparathyroidism.15 Vitamin D, calcium and phosphate supplementation was used in 15 (34.8%), 3 (7%) and 1 (2.3%) of the participants.
While mean haemoglobin concentrations (13.9 g/dl) were in the normal range, 12 (27.9%) patients had anaemia (haemoglobin <12 g/dl in women and <13 g/dl in men),14 which was severe (haemoglobin <11g/dl)14 in 4 (9.4%) of study participants. None of the patients were treated with intravenous or oral iron, or erythropoietin-stimulating agents.
Mean (IQR) carotid intima-media thickness was 0.583 (0.495–0.706) and plaque was found in less than one-third of the participants (n=13 (30.2%)).
Mean (SD) pulse wave velocity was 9.9 (3.0) m/sec; mean (SD) augmentation index, reflection magnitude and pulse pressure amplification were 62.1 (17.0)%, 63.0 (17.4)%, 135 (19), respectively; median (IQR) central pulse pressure was 40 (33–45) mmHg. Median (IQR) NT-proBNP concentrations were 188 (33–400) pg/mL.
Mean (SD) E/e’ was 9.4 (3.9) whereas an E/e’ of >14 that is in keeping with diastolic dysfunction,4,9 was present in 3 (7%) of participants. Ejection fraction was <50% in 4 (9.3) patients of which only one (2.3%) had an ejection fraction of <40%.
Employed immunosuppressive therapy comprised glucocorticoids (n=41 (95.3%)), mycophenolate mofetil (n=31 (72.1%)), calcineurin (n=30 (69.8%)) and mTOR (n=14 (32.6%)) inhibitors and azathioprine (n=8 (18.6%)).
Associations of Cardiovascular Risk Factors with E/e’, e’ and E/A
Table 2 shows the associations of cardiovascular risk factors with E/e’. In age, sex and race adjusted analysis, estimated GFR and haemoglobin and intact parathyroid hormone concentrations were associated with E/e’ (model 1). Upon further adjustment for established confounders in the present context, only haemoglobin and intact parathyroid hormone levels remained related to E/e’ (model 2). Subsequently, EGFR and haemoglobin concentrations were entered together with intact parathyroid hormone levels in separate multivariate models (models 3 and 4) as the former two variables were strongly collinear (Spearman’s rho=0.525). Only haemoglobin and intact parathyroid hormone concentrations were significantly or with borderline significance (p=0.08) associated with E/e’. When all three cardiovascular risk factors were entered simultaneously in a stepwise regression model (model 5), haemoglobin and intact parathyroid hormone levels were associated with E/e’ whereas estimated glomerular filtration rate was excluded from the model. Sex was also retained with a P value of 0.02 by SPSS in Model 5.
Table 3 gives the associations of cardiovascular risk factors with e’ and E/A. Three anthropometric characteristics including waist–height ratio, waist circumference and body mass index were associated with both e’ and E/A in age, sex and race (model 1) as well as in established confounder (model 2) adjusted analysis. Waist–hip ratio was not associated with e’ and E/A (partial R=−0.178, P=0.3 and partial R=−0.197, P=0.2, respectively, in age, sex and race adjusted models).
Measures of atherosclerosis and arterial function, NT-proBNP concentrations and cardiovascular drug use, vitamin D, calcium and phosphate supplementation as well as immunosuppressive agent use were not related to E/e’, e’ and E/A (data not shown).
Associations of Haemoglobin and Intact Parathyroid Levels and Anthropometric Measures with Diastolic Function Parameters After Additional Adjustment for Left Ventricular Mass Index and Preload and Afterload Measures
Table 4 shows the associations of haemoglobin and intact parathyroid hormone concentrations with E/e’ as well as those of anthropometric measures with e’ and E/A after additional adjustment (to established confounders) for left ventricular mass index (Model 1), cardiac preload as estimated by left ventricular end-diastolic volume (Model 2), cardiac afterload as estimated by central systolic blood pressure and systemic vascular resistance (Model 3) and left ventricular mass index together with left ventricular end-diastolic volume, central systolic blood pressure and systemic vascular resistance (Model 4). The cardiovascular risk factor-diastolic function relationships were consistent in each of the respective models.
Impact of Time Since Transplantation on the Associations of Haemoglobin and Intact Parathyroid Levels and Anthropometric Measures with Diastolic Function Parameters
In this study, there was heterogeneity in terms of transplant duration as it ranged from 0.5 to as much as 33.8 years. The median transplant duration was 13.28 years. To determine whether this can affect the generalizability of our results, we first compared the relevant recorded characteristics in patients with a transplant duration of <13.3 years compared to ≥13.3 years. We then assessed the impact of transplant duration on the associations of haemoglobin and intact parathyroid levels and anthropometric measures with diastolic function parameters by adding interaction terms (together with their individual components) to the age, sex and race adjusted models in Tables 2 and 3 and performing stratified analysis.
 |
Table 2 Associations of Cardiovascular Risk Factors with E/E’ in 43 Kidney Transplant Recipients |
 |
Table 3 Associations of Cardiovascular Risk Factors with e’ and E/A in 43 Kidney Transplant Recipients |
As given in Table 5, in age, sex and race adjusted analysis, compared to patients with a transplant duration of <13.3 years, those with a transplant duration of ≥13.3 years were more frequently female and less often Asian, and had less abdominal adiposity, lower heart rates and larger haemoglobin levels and central systolic blood pressures. Intact parathyroid hormone concentrations also tended to be lower (p=0.07) in patients with a transplant duration of ≥13.3 years compared to <13.3 years. The estimated glomerular filtration rate was 61.1 mL/min/1.73m2 and 65.1 mL/min/1.73m2 (p=0.5) in patients with a transplant duration of ≥13.3 years compared to <13.3 years.
 |
Table 5 Recorded Characteristics in 43 Kidney Transplant Recipients Upon Stratification by Median Transplant Duration |
Transplant duration did not impact the estimated glomerular filtration rate-E/e’ (interaction p=0.6), haemoglobin-E/e’ (interaction p=0.2) and intact parathyroid hormone-E/e’ (interaction p=0.5) relationships. However, transplantation duration did impact the waist–height-e’ association (interaction p=0.03). Transplant duration did not significantly impact other anthropometric measure-e’ and anthropometric-E/A relationships (interaction p=0.1 to 0.8).
As shown in Supplementary Table 1, in line with the interaction analysis results and in age, sex and race adjusted models, the associations of estimated glomerular filtration rate and haemoglobin and intact parathyroid hormone levels with E/e’ appeared similar in patients with a transplant duration of <13.3 years compared to ≥13.3 years. None of these associations were significant, this presumably because of the small number of patients in both groups. Further adjustment for other potential confounders (see Table 2) did not alter these results (data not shown).
As given in Tables 6 and 7 and also in keeping with the interaction analysis results, significant anthropometric measure-e’ and, to a lesser extent, anthropometric–E/A relationships were found in patients with a transplant duration of ≥13.3 years but not <13.3 years.
 |
Table 6 Associations of Cardiovascular Risk Factors with e' in 43 Kidney Transplant Recipients Upon Stratification by Median Transplant Duration |
 |
Table 7 Associations of Cardiovascular Risk Factors with E/A in 43 Kidney Transplant Recipients Upon Stratification by Median Transplantation Duration |
Discussion
To our knowledge, this is the first study that evaluated potential determinants of diastolic function in stable kidney transplant participants. Our main findings were as follows: (1) haemoglobin concentrations were inversely associated with E/e’ whereas intact parathyroid hormone levels were directly related to E/e’; (2) anthropometric measures including waist–height ratio, waist circumference and body mass index were each associated with both e’ and E/A; (3) the haemoglobin-E/e’, intact parathyroid hormone-E/e’, anthropometric measure-e’ and anthropometric measure–E/A relationships were each independent of left ventricular mass index and cardiac preload and afterload measures and (4) estimated glomerular filtration rate was associated with E/e’, but this relationship was no longer significant after adjustment for established confounders as well as haemoglobin and intact parathyroid hormone levels.
Post transplantation anaemia was previously reported to be associated with all-cause mortality, graft loss and heart failure.14,31 Our current findings suggest that post transplantation anaemia may contribute to heart failure through increasing left ventricular stiffness and pressures due to impaired left ventricular passive relaxation.3,4,27 Anaemia is associated with left ventricular hypertrophy, which is a strong independent predictor of cardiovascular mortality in CKD patients.3 However, impaired diastolic function predates left ventricular hypertrophy in CKD.3,32 This argues against an important role of left ventricular hypertrophy in impaired diastolic function. Our finding that post transplantation anaemia was associated with E/e’ independent of left ventricular mass supports this notion. Moreover, abnormal left ventricular geometry as represented by hypertrophy and concentric remodelling was present in only 15 (34.9%) patients. This may have been consequential to the selection of patients with favourable cardiovascular disease risk profiles for transplantation, the consistent use of antihypertensive agents with overall adequate blood pressure control and post transplantation regression of left ventricular hypertrophy.5 Anaemia also remained significantly associated with E/e’ when we adjusted for cardiac preload and afterload measures. Our results therefore indicate that post transplantation anaemia may mediate impaired diastolic function through cardiomyocyte hypoxia and consequent cardiac fibrosis.16–20
Our finding that 27.9% of our patients had post transplantation anaemia is in keeping with previously reported findings.14 The causes of anaemia differ somewhat in kidney transplant recipients compared to dialysis patients and include graft rejection and immunosuppressant therapy.14 Based on a randomized trial and a large observational study, Gafter-Gvili et al14 recently recommended targeting a haemoglobin of 12.5 to 13g/dl in kidney transplant recipients with appropriate erythropoietin stimulating agent and iron therapy. None of our patients received iron or erythropoietin stimulating agent therapy. In this regard, in the STRESAM study,33 only 5% of patients received treatment with an erythropoietin stimulating agent. Taken together, post transplantation anaemia comprises a comorbidity that may be in need of more systematic management14 than is currently practised. Whether this can improve cardiovascular outcomes in kidney transplant recipients requires further study.
Increased parathyroid hormone concentrations are associated with cardiovascular events and mortality in CKD and non-CKD patients.21 However, the role of high parathyroid hormone levels as a cardiovascular risk factor in stable kidney transplant recipients has not been reported thus far. In this study, 30.8% of our patients had persistent secondary hyperparathyroidism, which is also in line with previous reports.15 We found that, as applied to haemoglobin concentrations, parathyroid levels were associated with E/e’ independent of established confounders, left ventricular hypertrophy and cardiac preload and afterload measures in kidney transplant recipients. In this regard, experimental studies using rat cardiomyocytes have shown that parathyroid hormone can cause mitochondrial Ca2+ excess that results in oxidative stress, necrotic cell death and consequent fibrosis.21 In another study, parathyroidectomy prevented the development of myocardial hypertrophy, fibrosis and apoptosis in 5/6 nephrectomised rats.34 Also, the use of the calcimimetic agent cinacalcet in haemodialysis patients for 20 weeks decreased not only parathyroid hormone, calcium and phosphate levels but also the left ventricular mass index and E/e’ ratio.35 These reported data together with our current findings indicate that the impact of persistent hyperparathyroidism and its adequate management15 on cardiovascular outcomes in kidney transplant recipients also deserves further study.
Even a fully functional kidney allograft does not entirely restore the glomerular filtration rate.1 A reduction in glomerular filtration rate is reportedly an independent risk factor for cardiovascular disease and mortality in kidney transplant recipients.1 In the present study, an age, sex and race adjusted association was found between the estimated glomerular filtration rate and E/e’ but this relationship was explained by established confounders and parathyroid hormone and haemoglobin concentrations.
Excess adiposity is prevalent and its association with heart failure was previously identified in kidney transplant recipients.1,31 Overweight or obesity was present in 67.5% of our patients. Except for waist–hip ratio, adiposity measures were independently associated with e’ and E/A. Excess adiposity is also associated with impaired left ventricular active relaxation in non-CKD persons4,36,37 and can be reversed by weight loss.38 Dialysis duration prior to kidney transplantation comprises another reported risk factor for heart failure in kidney transplant recipients.31 In this study, dialysis duration prior to kidney transplantation was not associated with E/e’, e’ or E/A (data not shown).
In the present investigation, the heterogeneity in relation to transplant duration called for performing a stratified analysis. In patients with a prolonged transplant duration (≥13.3 years), haemoglobin concentrations were larger, intact parathyroid hormone concentrations tended to be smaller and abdominal adiposity was reduced compared to those with a shorter transplant duration. Conceptually, this may represent an improvement of the respective cardiovascular risk factors over time after transplantation. Alternatively, our respective results may originate in a selection bias due to an overall better survival among patients with a prolonged compared to shorter transplant duration. Indeed, anaemia, bone mineral disorder and excess adiposity can increase cardiovascular disease and mortality in kidney transplant patients.39,40 More importantly, our interaction analysis results indicated that transplant duration did not influence the potential impact of haemoglobin and parathyroid hormone levels on E/e’. By contrast, abdominal adiposity was inversely associated with measures of left ventricular relaxation in patients with a prolonged but not shorter transplant duration. This may suggest that exposure to excess adiposity may need to be prolonged for it to reduce left ventricular relaxation in kidney transplant recipients. Taken together, the role of kidney transplant duration on cardiovascular risk requires further study in future larger and longitudinal investigations.
Our study has limitations. Firstly, the study design was cross-sectional, which precludes determining cause–effect relationships. Secondly, as applied to previously reported investigations by us,37,41,42 we assessed the associations of cardiovascular risk factors with E/e’, e’ and E/A as continuous variables, which requires inclusion of both high and low values. As only 3 (7%) patients had an E/e’ of >14 that is in keeping with diastolic dysfunction,4,9 the number of included patients was too small to reliably assess associations of cardiovascular risk factors with an increased E/e’ in adequately adjusted logistic regression models. Thirdly, as we did not determine atrial volume index and tricuspid regurgitation velocity, we could not apply the currently suggested algorithm for the detection of diastolic dysfunction4 in this patient population. However, given that at a mean age of 53.5 years, 86% of our patients were hypertensive and 25.6% had diabetes, it is highly likely that a substantial proportion of them had diastolic dysfunction. Fourthly, haemoglobin concentrations were assessed on one occasion only, ie on the same day that other measurements were made. Anaemia may need to be present for prolonged time periods in order for myocardial fibrosis to develop. We may therefore have underestimated the effect of low haemoglobin concentrations on diastolic function. Fifthly, all participants were enrolled at a single centre. Sixthly, the number of patients included was relatively small, particularly in the analysis stratified by transplantation duration. A strength of this investigation is that our conclusions originate in multivariable regression models in which we consistently adjusted for potential established confounders.
In conclusion, haemoglobin and parathyroid hormone concentrations as well as adiposity measures are independently associated with diastolic function in kidney transplant recipients. Whether adequate management of post transplantation anaemia,14 persistent secondary hyperparathyroidism15 and excess adiposity1 can prevent the development of heart failure with preserved ejection fraction in kidney transplant recipients merits further investigation.
Ethics Statement
This study was performed according to the Helsinki Declaration of 1975 as revised in 2013. All organs were donated voluntarily with written informed consent, which was conducted in accordance with the Declaration of Istanbul. The study was approved by the University of the Witwatersrand Human (Medical) Research Ethics Committee (protocol number: M15-08-43) in Johannesburg, South Africa. Written informed consent was obtained in each patient prior to participation.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
The study was supported by the South African National Research Foundation.
Disclosure
The authors declare no conflict of interest.
References
1. Devine PA, Courtney AE, Maxwell AP. Cardiovascular risk in renal transplant recipients. J Nephrol. 2019;32(3):389–399. doi:10.1007/s40620-018-0549-4
2. Sarier M, Ozen NS, Guler H, et al. Prevalence of sexually transmitted diseases in asymptomatic renal transplant recipients. Exp Clin Transplant. 2018. doi:10.6002/ect.2017.0232
3. Wang X, Shapiro JI. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat Rev Nephrol. 2019;15(3):159–175.
4. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–310.
5. Hawwa N, Shrestha K, Hammadah M, Yeo PSD, Fatica R, Tang WHW. Reverse remodelling and prognosis in contemporary patients with cardiac dysfunction. J Am Coll Cardiol. 2015;66(16):1779–1787. doi:10.1016/j.jacc.2015.08.023
6. Patel RK, Mark PB, Johnston N, McGregor E, Dargie HJ, Jardine AG. Renal transplantation is not associated with regression of left ventricular hypertrophy: a magnetic resonance study. Clin J Am Soc Nephrol. 2008;3(6):1807–1811. doi:10.2215/CJN.01400308
7. Lentine KL, Schnitzler MA, Abbott KC, et al. De novo congestive heart failure after kidney transplantation: a common condition with poor prognostic implications. Am J Kidney Dis. 2005;46(4):720–733. doi:10.1053/j.ajkd.2005.06.019
8. Rigatto C, Parfrey P, Foley R, Negrijn C, Tribula C, Jeffrey J. Congestive heart failure in renal transplant recipients: risk factors, outcomes, and relationships with ischemic heart disease. J Am Soc Nephrol. 2002;13(4):1084–1090. doi:10.1681/ASN.V1341084
9. Kim MK, Kim B, Lee JY, et al. Tissue Doppler-derived E/e’ ratio as a parameter for assessing diastolic heart failure and as a predictor of mortality in patients with chronic kidney disease. Korean J Intern Med. 2013;28(1):35–44. doi:10.3904/kjim.2013.28.1.35
10. Ahmed A, Rich MW, Sanders PW, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99(3):393–398. doi:10.1016/j.amjcard.2006.08.042
11. Escoli R, Carvalho MJ, Cabrita A, Rodrigues A. Diastolic dysfunction, an underestimated new challenge in dialysis. Ther Apher Dial. 2019;23(2):108–117. doi:10.1111/1744-9987.12756
12. Himelman RB, Landzberg JS, Simonson JS, et al. Cardiovascular consequences of renal transplantation: changes in left ventricular morphology and function. J Am Coll Cardiol. 1988;12(4):915–923. doi:10.1016/0735-1097(88)90454-8
13. Ducloux D, Motte G, Challier R, Gibey R, Chalopin J-M. Serum homocysteine total and cardiovascular disease occurrence in chronic, stable renal transplant recipients: a prospective study. J Am Soc Nephrol. 2000;11:134–137. doi:10.1681/ASN.V111134
14. Gafter-Gvili A, Gafter U. Posttransplantation anemia in kidney transplant recipients. Acta Haematol. 2019;142(1):37–43. doi:10.1159/000496140
15. Santos RD, Rossi A, Coyne D, Maw TT. Management of post-transplant hyperparathyroidism and bone disease. Drugs. 2019;79(5):501–513. doi:10.1007/s40265-019-01074-4
16. Mistry N, Mazer CD, Sled JG, et al. Red blood cell antibody-induced anemia causes differential degrees of tissue hypoxia in kidney and brain. Am J Physiol Reg Integr Comp Physiol. 2018;314:R611–R622. doi:10.1152/ajpregu.00182.2017
17. Caramelo C, Justo S, Gil P. Anemia in heart failure: pathophysiology, pathogenesis, treatment, and incognitae. Rev Esp Cardiol. 2007;60:848–860. doi:10.1157/13108999
18. D’Amario D, Migliaro S, Borovac JA, et al. Microvascular dysfunction in heart failure with preserved ejection fraction. Front Physiol. 2019;10:1347. doi:10.3389/fphys.2019.01347
19. Lopez B, Gonzalez A, Hermida N, Laviades C, Diez J. Myocardial fibrosis in chronic kidney disease: potential benefits of torasemide. Kidney Int. 2008;Suppl 111:S19–S23. doi:10.1038/ki.2008.512
20. Losi MA, Memoli B, Contaldi C, et al. Myocardial fibrosis and diastolic dysfunction in patients on chronic haemodialysis. Nephrol Dial Transplant. 2010;25:1950–1954. doi:10.1093/ndt/gfp747
21. Fujii H. Association between parathyroid hormone and cardiovascular disease. Ther Apher Dial. 2018;22(3):236–241. doi:10.1111/1744-9987.12679
22. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
23. International Summit on Transplant Tourism and Organ Trafficking. The declaration of Istanbul on organ trafficking and transplant tourism. Clin J Am Soc Nephrol. 2008;3:1227–1231. doi:10.2215/CJN.03320708
24. Gunter S, Robinson C, Woodiwiss AJ, et al. Arterial wave reflection and subclinical atherosclerosis in rheumatoid arthritis. Clin Exp Rheumatol. 2018;36(3):412–420.
25. Touboul PJ, Hennerici MJ, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the Advisory Board of the 3rd and 4th watching the risk symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23(1):75–80. doi:10.1159/000097034
26. Sahn DJ, De Maria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58(6):1072–1083. doi:10.1161/01.CIR.58.6.1072
27. Mokotedi L, Gunter S, Robinson C, et al. The impact of different criteria sets on the estimated prevalence and associated risk factors of diastolic dysfunction in rheumatoid arthritis. Int J Rheumatol. 2017;2017:2323410. doi:10.1155/2017/2323410
28. Kapoor KPM, Bhardwaj V, Sharma A, Kiran U. Global end-diastolic volume an emerging preload marker vis-à-vis other markers – have we reached our goal? Ann Card Anaesth. 2016;19:699–704. doi:10.4103/0971-9784.191554
29. Agabiti-Rosei E, Mancia G, O’Rourke MF, et al. Central blood pressure measurements and antihypertensive therapy. Hypertension. 2007;50:154–160. doi:10.1161/HYPERTENSIONAHA.107.090068
30. Collins S, Martindale J. Optimizing hypertensive acute heart failure management with afterload reduction. Curr Hypertens Rep. 2018;20:9. doi:10.1007/s11906-018-0809-7
31. House AA, Wanner C, Sarnak MJ, et al. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2019;95(6):1304–1317. doi:10.1016/j.kint.2019.02.022
32. Winterberg PD, Jiang R, Maxwell JT, Wang B, Wagner MB. Myocardial dysfunction occurs prior to changes in ventricular geometry in mice with chronic kidney disease (CKD). Physiol Rep. 2016;4(5):e12732. doi:10.14814/phy2.12732
33. Vanrenterghem Y, Ponticelli C, Morales JM, et al. Prevalence and management of anemia in renal transplant recipients: a European survey. Am J Transplant. 2003;3(7):835–845. doi:10.1034/j.1600-6143.2003.00133.x
34. Rodriguez-Ayala E, Avila-Diaz M, Foyo-Niembro E, Amato D, Ramirez-San-Juan E, Paniagua R. Effect of parathyroidectomy on cardiac fibrosis and apoptosis: possible role of aldosterone. Nephron Physiol. 2006;103(3):112–118. doi:10.1159/000092244
35. Choi SR, Lim JH, Kim MY, et al. Cinacalcet improves endothelial dysfunction and cardiac hypertrophy in patients on hemodialysis with secondary hyperparathyroidism. Nephron Clin Pract. 2012;122(1–2):1–8. doi:10.1159/000347145
36. Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57(12):1368–1374. doi:10.1016/j.jacc.2010.10.042
37. Libhaber CD, Norton GR, Majane OHI, et al. Contribution of central and general adiposity to abnormal left ventricular diastolic function in a community sample with high prevalence of obesity. Am J Cardiol. 2009;104(11):1527. doi:10.1016/j.amjcard.2009.07.020
38. Leichman J, Wilson EB, Scarborough T, et al. Dramatic reversal of derangements in muscle metabolism and diastolic left ventricular function after bariatric surgery. Am J Med. 2008;121(11):966–973. doi:10.1016/j.amjmed.2008.06.033
39. Carminatti M, Tedesco-Silva H, Fernandes NMS, Sanders-Pinheiro H. Chronic kidney disease progression in kidney transplant recipients: a focus on traditional risk factors. Nephrology. 2019;24:141–147. doi:10.1111/nep.13483
40. Erturk T, Berber I, Cakir U. Effect of obesity on clinical outcomes of kidney transplant patients. Transplant Proc. 2019;51:1093–1095. doi:10.1016/j.transproceed.2019.02.012
41. Bamaiyi AJ, Norton GR, Peterson V, Libhaber CD, Sarelli P, Woodiwiss AJ. Limited contribution of left ventricular mass and remodelling to the impact of blood pressure on diastolic function in a community sample. J Hypertens. 2019;37(6):1191–1199. doi:10.1097/HJH.0000000000002051
42. Bello H, Norton GR, Peterson VR, et al. Hemodynamic determinants of age versus left ventricular diastolic function relations across the full adult age range. Hypertension. 2020;75:1574–1583. doi:10.1161/HYPERTENSIONAHA.119.14622
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.