Back to Journals » Journal of Inflammation Research » Volume 16
Association of Neutrophil-to-Lymphocyte Ratio with Nutrition in Patients with Various Types of Malignant Tumors: A Multicenter Cross-Sectional Study
Authors Kang L, Liu X, Ji W, Zheng K , Li Y, Song Y , He H , Wang X, Yang T, Guan M, Zhu G, Gao Y, Guan Y, Wang L, Li W
Received 25 December 2022
Accepted for publication 17 March 2023
Published 27 March 2023 Volume 2023:16 Pages 1419—1429
DOI https://doi.org/10.2147/JIR.S401189
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Lihua Kang,1 Xiangliang Liu,1 Wei Ji,1 Kaiwen Zheng,1 Yuguang Li,2 Yanqiu Song,1 Hua He,1 Xiaomeng Wang,1 Tingting Yang,1 Meng Guan,1 Ge Zhu,1 Yangyang Gao,1 Yanjie Guan,1 Lei Wang,1 Wei Li1
1Cancer Center, The First Hospital of Jilin University, Changchun, Jilin Province, People’s Republic of China; 2College of Instrumentation and Electrical Engineering, Jilin University, Changchun, Jilin Province, People’s Republic of China
Correspondence: Wei Li, Cancer Center, The First Hospital of Jilin University, No. 71 Xinmin Street, Changchun, 130021, People’s Republic of China, Tel +86-13756661267, Email [email protected]
Aim: Neutrophil-to-lymphocyte ratio (NLR) is an index of systemic inflammation. This study is to clarify the role of NLR in body functional status, nutritional risk and nutritional status in the course of tumor.
Methods: A multi-center cross-sectional study of patients with various types of malignant tumors was accrued from the whole country. There were 21,457 patients with completed clinical data, biochemical indicators, physical examination, the Patient-Generated Subjective Global Assessment (PG-SGA) and Nutrition Risk Screening 2002 (NRS2002) survey. Logistic regression analysis was used to figure out the influencing factors of NLR, and four models were established to evaluate the influence of NLR on body functions, nutritional risks and nutritional status.
Results: Male patients, TNM stage IV, total bilirubin, hypertension and coronary atherosclerotic heart disease (CAHD) were independent predictors of NLR > 2.5. BMI, digestive systemic tumors and triglyceride negatively affect NLR in multivariable logistic regression. NLR was an independent predictor of Karnofsky Performance Scale (KPS), fat store deficit in all degrees, moderate and severe muscle deficit, mild fluid retention and PG-SGA grade.
Conclusion: Male patients and those with hypertension and CAHD are prone to systemic inflammation. Systemic inflammation significantly degrades body function status and nutritional status, increases nutritional risk and influences fat and muscle metabolism in patients with malignant tumor. Improving the intervenable indicators such as elevating albumin and pre-albumin, decreasing total bilirubin and enhancing nutrition support are imperative. Obesity and triglyceride behave like anti-systemic inflammation, which is misleading due to reverse causation in the course of malignancy.
Keywords: neutrophil-to-lymphocyte ratio, inflammation, tumor, nutrition, body function
Introduction
Currently, it is widely accepted that systemic inflammatory response has been shown to play an important role in the development and metastases of various types of malignant tumors. The inflammation in tumor microenvironment is characterized by the presence of host leucocytes both in stroma and tumor site.1,2 It has been associated with unsatisfactory clinical outcomes, affecting tumor proliferation, angiogenesis, metastasis, and response to treatment.3 Common systematic inflammatory markers used in clinical practice include neutrophil, C-reactive protein (CRP), the Glasgow Prognostic Score (GPS) or its modified version.4 Besides, lymphocyte is regarded as the surrogate of cell-mediated immunity. The neutrophil-to-lymphocyte ratio (NLR) is derived from the absolute neutrophil count divided by the absolute lymphocyte count, which represents unique immune status under cancer circumstance. Thus, NLR should be a reliable, inexpensive and convenient marker.5 In recent years, emerging evidence has demonstrated the role of NLR in evaluating treatment response and predicting prognosis in almost all kinds of cancer.6–10
Nutrition-related status also plays an important role in malignant tumors. Malignant tumor patients, especially those of the digestive system tumors often suffer from an absence of appetite, nausea, diarrhea and abdominal discomfort, which cause tissue wasting and malnutrition, even cachexia.11,12 The decreased body function and malnutrition may result in increased susceptibility to infection, an increased risk of complications, poor response and unplanned interruptions of treatments, a lower quality of life, reduced survival and higher health-care costs.13–15 It can also contribute to tumor development through the suppression of anti-tumor immunity.16
Systemic inflammatory response is clearly implicated in the progressive nutritional and functional decline in malignant tumor patients. The underlying metabolic alterations associated with systemic inflammation may contribute to the development of loss of body function and malnutrition in these patients.17,18 In the current study, we investigate the association between NLR and body functional status, nutritional risk and nutritional status in a large number of patients with various types of malignant tumor, in order to further clarify the role of systemic inflammation in nutrition-related status in the course of malignant tumor.
Patients and Methods
Patients
The study was part of the Investigation on Nutritional Status and its Clinical Outcomes of Common Cancers (INSCOC), a multi-center cross-sectional observational study of patients with various types of cancer, which was initiated and implemented by the Chinese Cancer Society Nutrition and Support Committee which complied with the Helsinki Declaration and was approved by the Ethical Committee of all participating hospitals (Supplementary 1). According to the INSCOC project, all information was collected within 48 hours at admission. There were 21,457 patients and their completed clinical data, biochemical indicators, physical examination and the Nutrition Risk Screening 2002 (NRS2002) and the Patient-Generated Subjective Global Assessment (PG-SGA) questionnaire. The process of data selection and analysis are listed briefly in Figure 1.
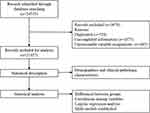 |
Figure 1 Flowchart of data selection and analysis. |
Methods
NLR
NLR was calculated by neutrophils/lymphocytes using data from a complete blood count that was routinely performed at the time of admission. Our study defined 2.5 as the cutoff value of NLR according to a meta-analysis based on the data from 13 studies with 3729 patients, which suggested that cutoff value of 2.5 showed a significant prognostic effect on disease-free survival and overall survival.19
Body Functional Status
Karnofsky Performance Scale (KPS) was committed by professional physicians at the time of admission. And the KPS was classified into three levels by 0–50 score, 60–80 score and 90–100 score, which were used to represent the body functional status of individuals as disability, partial disability and ability.
Nutritional Risk
NRS2002 questionnaire was also done when admission to evaluate the risk of malnutrition by professional physicians. NRS2002 <3 score meant no malnutrition risk, and re-evaluation was needed regularly, but when the score ≥3, the patients were regarded as in risk of malnutrition and future evaluation of nutritional status should be performed.
Nutritional Status
PG-SGA,20 a validated tool, was used to assess patients’ nutritional status and completed at the time of admission. All assessment personnel were trained by INSCOC to minimize the bias. Patients with PG-SGA score 0–1 were considered well nourished, PG-SGA score 2–3 were mildly malnourished, PG-SGA score 4–8 were moderately malnourished and PG-SGA score ≥9 were severely malnourished. The worksheet 4 of PG-SGA evaluated the overall condition of fat stores, muscle status and fluid status. In fat stores and muscle status, score 0 meant in normal condition, score 1 meant mild deficit, score 2 meant moderate deficit and score 3 meant severe deficit. In fluid status, ascites and edema in ankle and sacrum are evaluated to normal condition (score 0), mild retention (score 1), moderate retention (score 2) and severe retention (score 3).
Statistical Analysis
All the statistical analyses were performed using the SPSS statistical package (version 26.0; SPSS, Chicago, IL) and R project for statistical computing (version 4.2.2). All categorical variables are described by frequency and percentage. Chi-square test was used to assess the significance of differences between categorical variable groups. Spearman correlation analysis was done to test the correlations between categorical variables. Univariate and multivariate (Forward: Wald) logistic regression analysis were used to figure out the influencing factors of NLR. Four models were established to evaluate the influence of NLR on body functional status, nutritional risk, metabolic status and nutritional status. The P ≤ 0.05 stands for statistically significant differences.
Results
Demographics and Clinical-Pathologic Characteristics
Among the 21,457 involved patients, 47.8% suffered digestive system tumors and 29.0% were in TNM stage IV. The non-digestive system tumors mainly including lung cancer (16.6%), breast cancer (13.2%), nasopharyngeal cancer (6.2%) and cervical cancer (4.6%). BMI of 11.6% patients was below 18.5kg/m2. Twenty percent of patients were overweight (BMI 25.0 kg/m2–29.9 kg/m2) and 2.5% patients were obese (BMI ≥30.0 kg/m2). Thirty-two percent of patients combined with underlying disease. Hypertension and coronary atherosclerotic heart disease (CAHD) were most frequent in the prevalence of 13.4% and 3.8%, and diabetes was in the prevalence of 3.6%. While 81.7% patients received antineoplastic therapy, mere 26.4% patients received nutrition support therapy before. According to PG-SGA classification, the proportions of well nutrition, mild malnutrition, moderate malnutrition and severe malnutrition were 20.5%, 21.7%, 31.5% and 26.3%, respectively. Other clinical-pathologic characteristics in detail are shown in Table 1.
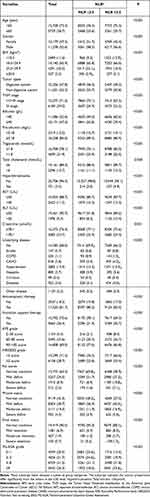 |
Table 1 Demographics and Clinical-Pathologic Characteristics [n (%)] |
Differences of Clinical-Pathologic Characteristics Between the NLR Subgroups
In order to further understand the relationship between NLR and clinical-pathologic characteristics, all of the enrolled patients were divided according to their NLR and NLR in subgroups were analyzed. NLR >2.5 was more frequently observed in patients suffered digestive system tumors and with TNM stage IV (P < 0.001). Likewise, NLR >2.5 was also more frequently observed in patients ≥65 years of age, male patients, patients with BMI <18.5, patients with underlying disease of chronic obstructive pulmonary disease (COPD), CAHD, hypertension and diabetes, patients with KPS 0–80 score, patients with NRS2002 ≥3 score and patients with PG-SGA ≥9 score (P < 0.001). NLR >2.5 was more frequently observed in patients with mild to severe deficit of fat stores and muscle status, and in all subgroups of fluid status (P < 0.001). NLR did differ by hyperbilirubinemia, albumin, pre-albumin, AST, ALT, triglyceride, and creatinine levels (P < 0.05). In contrast, NLRs did not differ by total cholesterol (P = 0.058). Besides, NLR is also related to antineoplastic therapy history (P < 0.001) and nutrition support therapy history (P < 0.001) (Table 1).
Univariate and Multivariate Logistic Regression Analysis of NLR>2.5
After examination of correlations between clinical-pathologic characteristics and NLR in Supplementary 2, logistic regression analysis was done to figure out the influencing factors of NLR. In the univariate analysis as detailed in Table 2, all included factors were significant predictors of NLR >2.5 (P < 0.05) except total cholesterol (P = 0.082), hepatitis (P = 0.650), cirrhosis (P=0.948), and others in underlying disease (P = 0.218). However, variables such as age, AST, ALT, creatinine and antineoplastic therapy were not significant predictors in the multivariate analysis. Indeed, all underlying disease except hypertension (OR = 1.409, 95% CI 1.066–1.861, P = 0.016) and CAHD (OR = 1.219, 95% CI 1.045–1.422, P = 0.012) were not significant predictors of NLR >2.5. Male patients (OR = 1.308, 95% CI 1.169–1.463), non-digestive system cancer (OR = 1.145, 95% CI 1.021–1.284), TNM stage IV (OR = 1.526, 95% CI 1.359–1.713), hyperbilirubinemia (OR = 2.238, 95% CI 1.609–3.113), hypertension (OR = 1.409, 95% CI 1.066–1.861), CAHD (OR = 1.219, 95% CI 1.045–1.422) and nutrition support therapy (OR = 1.548, 95% CI 1.359–1.764) remained strong independent predictors of NLR >2.5 (P < 0.05). Besides albumin (OR = 0.744, 95% CI 0.666–0.830), pre-albumin (OR = 0.722, 95% CI 0.662–0.900) and triglyceride (OR = 0.812, 95% CI 0.711–0.926) was unfavorable predictors of NLR >2.5. Taking BMI 18.5kg/m2−24.9 kg/m2 as reference, the OR (95% CI) for BMI <18.5 kg/m2 and 25.0 kg/m2–29.9 kg/m2 were 1.282 (1.078–1.524) and 0.847 (0.739–0.972), respectively (P < 0.05). The BMI ≥30 kg/m2 category was not defined as an independent indicator of NLR >2.5 (P = 0.306).
 |
Table 2 Univariate and Multivariate Regression Analysis of NLR |
The Role of NLR in Body Functional Status, Nutritional Risk, Nutritional Status and Metabolic Status
Four models were established adjusted for different factors in order to figure out the role of NLR in individuals, as shown in Table 3. NLR <2.5 was favorable predictor for higher KPS grade (OR = 2.303, 95% CI 1.559–3.403, for 60–80 score; OR = 3.712, 95% CI 2.528–5.450, for 90–100 score) and NRS2002 grade (OR = 1.207, 95% CI 1.025–1.420), even in Model 4 which adjusted all enrolled parameters like age, gender, BMI, serological indexes, underlying disease, tumor types, TNM stage, antineoplastic therapy and nutrition support therapy. Likewise, NLR <2.5 was unfavorable predictor of fat store deficit in all degrees, the ORs were 0.792, 0.622 and 0.453 for mild, moderate and severe deficit in Model 4, respectively (P < 0.05). When the dependent variable was muscle status, NLR was only predictor of moderate and severe deficit with OR (95% CI) = 0.636 (0.492–0.823) (P = 0.001) and 0.533 (0.374–0.760) (P = 0.001). And when the dependent variable was fluid status, NLR merely predicted mild retention (OR = 1.600, 95% CI 1.096–2.334, P = 0.015). At last, NLR <2.5 was unfavorable predictor of higher PG-SGA grade in Model 4, and the ORs were 0.835, 0.705 and 0.512 for 2–3 score subgroup, 4–8 score subgroup and ≥9 score subgroup, respectively (P < 0.05).
 |
Table 3 Multi-Models to Figure Out the Role of NLR in Body Functional Status, Nutritional Risk, Metabolic Status and Nutritional Status |
Discussion
As a cross-sectional study, the large sample size of 21,457 cases ensured that it reflected the reality of malignant tumor patients. In this study, NLR >2.5 was found significantly damages body function status and nutritional status, increases nutritional risk and influences fat and muscle metabolism. Elevating albumin and pre-albumin, decreasing total bilirubin and enhancing nutrition support may be helpful in improving systematic inflammation status. In addition, reverse causation was detected, which remind physicians cautiously interpret the statistics in the course of malignancy.
Up to 20% of all human cancers result from chronic inflammation and persistent infections, such as Helicobacter pylori induced gastric cancer, hepatitis B/C induced hepatocellular carcinoma and inflammatory bowel diseases associated colorectal cancer.21,22 Also, cancer-elicited inflammation triggered by mutations contributes to malignant progression through the recruitment and activation of inflammatory cells.23 In cancer patients, lymphocyte is the surrogate of impaired cell-mediated immunity, whereas neutrophilia is a response to systemic inflammation.24 Another explanation is neutrophil may play both the role of promotion of cancer cell growth and metastasis and/or suppression of lymphocyte.25 Besides immune response, the inflammation-based status also induces changes in adipose and protein metabolism and alterations in energy consumption in order to ameliorate malignant cells’ growth, proliferation and metastasis. From the host perspective, the metabolic dysfunction results in the physical function impairment, malnutrition, declined tolerance of therapy and even higher mortality. Increasing evidence has indicated that the NLR, a useful marker of systemic inflammation with the advantage of wide availability, reproducible and inexpensive, would also be an effective indicator of nutrition-related status and prognosis in various types of tumors.26,27 This study also revealed that higher NLR significantly degrades body function status and nutritional status, increases nutritional risk and harms fat and muscle metabolism in patients with malignant tumor.
Aggressive systemic inflammation is more frequent in digestive system malignant tumor patients due to complications like obstruction and fistula, symptoms like nausea and vomit, absorption dysfunction and treatment-related gastrointestinal abnormalities.28 However, digestive system malignant tumors inverted to be a situation of less likely developing higher NLR after considering the overall features (non-digestive system vs digestive system, OR = 1.145, 95% CI 1.021–1.284), which highlights the importance of improving the intervenable indicators such as elevating albumin, pre-albumin and decreasing total bilirubin. Surprisingly, nutrition support therapy was revealed as a risk factor of systemic inflammation (OR = 1.548, 95% CI 1.359–1.764) that was due to the extremely scarcity of nutrition support in China. According to the investigation published by 2020 Chinese Conference on Oncology, the nutrition support rate of malignant tumor patients is mere 31.2% and 55% patients in urgent need of nutrition support (PG-SGA ≥ 9) have never received nutrition support.29 In our study, 79.5% patients had malnutrition with almost three-fourths of these patients being moderate and severe malnutrition and the nutrition support rate is only 26.4%. Thus, the reality is that a large percent of patients who have received nutrition support combined with great disease burden and cruel consumption, which implies the imperative of improving nutrition support in cancer patients.
Another thing should be stated is the relationship between obesity and inflammation in cancer patients. Obesity is considered as a risk factor of the incidence of 13 types of cancers.30 However, researches have reported that cancer patients with higher BMI obtain better prognosis, which is known as the “obesity paradox”.31 Chronic inflammation plays a key role in obesity,32 but hosts with higher BMI were identified as less likely to be combined with increasing NLR in our research. The obesity paradox is elaborated as methodological limitations frequently.33 Likewise, the reason of inversed relationship between BMI and NLR may also be due to reverse causation. Cancer-related weight loss, such as sarcopenia and cachexia, is the result of metabolic dysfunction and manifests as catabolism of muscle and fat mediated mainly by IL-6 and TNF through STAT3 and NF-κB pathway.34 Thus, higher BMI at diagnosis implies less disturbance by the abnormal metabolism, so monitoring weight changes is critical to reveal the precise relationship between obesity and systemic inflammation and its role in cancer prognosis.
The same situation also occurs in the relationship between TG and NLR. Increased TG is often associated with smoking, insulin resistance and obesity, which is pro-inflammatory.35 However, TG seems an anti-inflammatory factor in our search (OR = 0.812, 95% CI 0.711–0.926), which was in contrast with the discovery of hypertension and CAHD as risk factors of higher NLR. This should also be explained by the inverse causation. Wasting-related metabolism induces transformation from white to brown adiposity tissue, upregulates β-oxidation of free fatty acids and lipolysis in cancer patients,36,37 and thus, higher serologic TG level indicates less consumption. Zhang et al38 also reported that higher ratio of TG and high-density lipoprotein cholesterol related to greater muscle mass.
Besides, muscles and adiposity are two body compartments and behave differently in cancer. High-quality muscles represent well tolerance of chemotherapy39 and body function.40 Subcutaneous adiposity, the main producer of leptin, associates with survival benefit.41 Likewise, NLR is always associated with muscle and fat deficit in our research, which refers to subcutaneous adiposity including orbital fat pad, triceps skinfold thickness and 12th-rib fat thickness. Unlike subcutaneous adiposity, visceral fat and ectopic lipid, such as muscle fat infiltration, are pro-inflammatory by secreting IL-6, TNF, IFN-γ and IL-1β and promoting insulin resistance.42 Shu et al43 reported that TG was positively related with CRP in those patients whose BMI <24kg/m2 but not in those whose BMI≥24kg/m2, which indicated that obesity may manipulate more than increased level of TG in inflammation. However, in the course of malignant tumors, both obesity and TG should be interpreted carefully.
Given the huge sample size of the study on NLR in malignant tumor patients, the reversed causations existed imply that the interpretation of statistical results must be cautious and take enough consideration of realities. Still, there are several limitations. First, considering the feasibility of implementation in multi-centers, muscles and fat are evaluated by physicians according to the practical conscience of PG-SGA but not precise tools like computer tomography or magnetic resonance. Second, according to the results, weight loss should be analyzed which may offer an interpretation of obesity paradox. Third, data on visceral fat were not involved in the study. Due to the different roles of subcutaneous fat and visceral fat in inflammation, we are going to figure out the correlation between systemic inflammation and adiposity in different locations.
Conclusion
NLR is a systematic inflammation marker. Male patients and those with hypertension and CAHD have generally higher levels of NLR. In the course of malignancy, NLR manifests as a biomarker of nutritional status, nutritional risk and fat and muscle metabolism. These results indicate the usefulness of NLR in routine clinical practice to improve patient assessments and interventions. Future studies need longitudinal assessment of NLR and dynamic body composition and nutrition assessment to explain the causations, as well as the identification of NLR with therapeutic benefits.
Abbreviations
NLR, neutrophil-to-lymphocyte ratio; CAHD, coronary atherosclerotic heart disease; TG, triglyceride; CRP, C-reactive protein; GPS, the Glasgow Prognostic Score; INSCOC, the Investigation on Nutritional Status and its Clinical Outcomes of Common Cancers; KPS, Karnofsky Performance Scale; BMI, body mass index; COPD, chronic obstructive pulmonary disease; PG-SGA, Patient-Generated Subjective Global Assessment; NRS2002, Nutrition Risk Screening 2002; TNM stage, the Tumor Node Metastasis classification by the American Joint Committee on Cancer (AJCC) 7th Edition.
Ethics Approval and Consent to Participate
The study complied with the Helsinki Declaration and was approved by the Ethics Committee of the first affiliated hospital of Jilin University (2017-362).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Goh BK, Chok AY, Allen JC
2. Goh BK, Tan DM, Chan CY, et al. Are preoperative blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios useful in predicting malignancy in surgically-treated mucin-producing pancreatic cystic neoplasms? J Surg Oncol. 2015;112(4):366–371. doi:10.1002/jso.23997
3. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
4. Hsieh MC, Wang SH, Chuah SK, Lin YH, Lan J, Rau KM. A prognostic model using inflammation- and nutrition-based scores in patients with metastatic gastric adenocarcinoma treated with chemotherapy. Medicine. 2016;95(17):e3504. doi:10.1097/MD.0000000000003504
5. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. doi:10.1016/j.critrevonc.2013.03.010
6. Luo G, Guo M, Liu Z, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22(2):670–676. doi:10.1245/s10434-014-4021-y
7. Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200(2):197–203. doi:10.1016/j.amjsurg.2009.08.041
8. Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109(2):416–421. doi:10.1038/bjc.2013.332
9. Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol. 2015;21(9):2807–2815. doi:10.3748/wjg.v21.i9.2807
10. Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23(1):31–39. doi:10.1016/j.suronc.2013.12.001
11. Cohen SJ, Pinover WH, Watson JC, Meropol NJ. Pancreatic cancer. Curr Treat Options Oncol. 2000;1(5):375–386. doi:10.1007/s11864-000-0065-2
12. Ronga I, Gallucci F, Riccardi F, Uomo G. Anorexia-cachexia syndrome in pancreatic cancer: recent advances and new pharmacological approach. Adv Med Sci. 2014;59(1):1–6. doi:10.1016/j.advms.2013.11.001
13. Pacelli F, Bossola M, Rosa F, Tortorelli AP, Papa V, Doglietto GB. Is malnutrition still a risk factor of postoperative complications in gastric cancer surgery? Clin Nutr. 2008;27(3):398–407. doi:10.1016/j.clnu.2008.03.002
14. Sierzega M, Niekowal B, Kulig J, Popiela T. Nutritional status affects the rate of pancreatic fistula after distal pancreatectomy: a multivariate analysis of 132 patients. J Am Coll Surg. 2007;205(1):52–59. doi:10.1016/j.jamcollsurg.2007.02.077
15. Schwegler I, von Holzen A, Gutzwiller JP, Schlumpf R, Mühlebach S, Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. 2010;97(1):92–97. doi:10.1002/bjs.6805
16. Gao Y, Zhou S, Jiang W, Huang M, Dai X. Effects of ganopoly (a ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Invest. 2003;32(3):201–215. doi:10.1081/IMM-120022979
17. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–226. doi:10.1097/MCO.0b013e32832a7902
18. Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care. 2005;8(3):265–269. doi:10.1097/01.mco.0000165004.93707.88
19. Wu J, Chen M, Liang C, Su W. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio in cervical cancer: a meta-analysis and systematic review. Oncotarget. 2017;8(8):13400–13412. doi:10.18632/oncotarget.14541
20. Song C, Cao J, Zhang F, et al. Nutritional risk assessment by scored patient-generated subjective global assessment associated with demographic characteristics in 23,904 common malignant tumors patients. Nutr Cancer. 2019;71(1):50–60. doi:10.1080/01635581.2019.1566478
21. Wang K, Karin M. Tumor-elicited inflammation and colorectal cancer. Adv Cancer Res. 2015;128:173–196.
22. Yu JM, Yang M, Xu HX, et al. association between serum C-reactive protein concentration and nutritional status of malignant tumor patients. Nutr Cancer. 2019;71(2):240–245. doi:10.1080/01635581.2018.1524019
23. Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18(3):121–126. doi:10.4103/aam.aam_56_18
24. Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955–965. doi:10.2147/OTT.S153290
25. Lecot P, Sarabi M, Pereira Abrantes M, et al. Neutrophil heterogeneity in cancer: from biology to therapies. Front Immunol. 2019;10:2155. doi:10.3389/fimmu.2019.02155
26. McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. 2008;67(3):257–262. doi:10.1017/S0029665108007131
27. Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18(1):360. doi:10.1186/s12916-020-01817-1
28. Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi:10.1093/jnci/dju124
29. Song C, Wang K, Guo Z, et al. Investigation of nutritional status in Chinese patients with common cancer. Sci Sin Vitae. 2020;50(12):1437–1452. doi:10.1360/SSV-2020-0297
30. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer--viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–798. doi:10.1056/NEJMsr1606602
31. Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18(9):56. doi:10.1007/s11912-016-0539-4
32. Stienstra R, van Diepen JA, Tack CJ, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108(37):15324–15329. doi:10.1073/pnas.1100255108
33. Lee DH, Giovannucci EL. The obesity paradox in cancer: epidemiologic insights and perspectives. Curr Nutr Rep. 2019;8(3):175–181. doi:10.1007/s13668-019-00280-6
34. Fonseca G, Farkas J, Dora E, von Haehling S, Lainscak M. Cancer cachexia and related metabolic dysfunction. Int J Mol Sci. 2020;21(7):2321. doi:10.3390/ijms21072321
35. Bala C, Gheorghe-Fronea O, Pop D, et al. The association between six surrogate insulin resistance indexes and hypertension: a population-based study. Metab Syndr Relat Disord. 2019;17(6):328–333.
36. Dahlman I, Mejhert N, Linder K, et al. Adipose tissue pathways involved in weight loss of cancer cachexia. Br J Cancer. 2010;102(10):1541–1548. doi:10.1038/sj.bjc.6605665
37. Das SK, Eder S, Schauer S, et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333(6039):233–238. doi:10.1126/science.1198973
38. Zhang C, Yang Q, Zhang Y, et al. The relationship between the ratio of triglyceride to high-density lipoprotein cholesterol and muscle loss in patients with type 2 diabetes. Chin J Diabetes Mellitus. 2020;12(09):721–725.
39. Huiskamp LFJ, Chargi N, Devriese LA, May AM, Huitema ADR, de Bree R. The predictive value of low skeletal muscle mass assessed on cross-sectional imaging for anti-cancer drug toxicity: a systematic review and meta-analysis. J Clin Med. 2020;9(11):3780. doi:10.3390/jcm9113780
40. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O, Wright JM. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017;12(1):e0169548. doi:10.1371/journal.pone.0169548
41. Ebadi M, Baracos VE, Bathe OF, Robinson LE, Mazurak VC. Loss of visceral adipose tissue precedes subcutaneous adipose tissue and associates with n-6 fatty acid content. Clin Nutr. 2016;35(6):1347–1353. doi:10.1016/j.clnu.2016.02.014
42. Ebadi M, Martin L, Ghosh S, et al. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer. 2017;117(1):148–155. doi:10.1038/bjc.2017.149
43. Shu Y, He S, Chen XP, et al. 甘油三酯和高密度脂蛋白胆固醇与炎症的关系 [Relations between fasting serum lipids and high-sensitivity C-reactive protein level in Chengdu residents]. Zhonghua Xin Xue Guan Bing Za Zhi. 2012;40(2):125–130. Chinese. doi:10.3760/cma.j.issn.0253-3758.2012.02.010.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
