Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 13
Association of IL-17 and IL-23 Gene Variants with Plasma Levels and Risk of Vulvovaginal Candidiasis in a Chinese Han Population
Authors Li W, Shi W, Yin Y, Chen J, Luo L
Received 1 August 2020
Accepted for publication 16 November 2020
Published 15 December 2020 Volume 2020:13 Pages 725—733
DOI https://doi.org/10.2147/PGPM.S275073
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Wei Li,1 Wenyin Shi,1 Yujun Yin,2 Juan Chen,1 Lanlan Luo3
1Department of Gynecology, The Fourth Affiliated Hospital of Jiangsu University, Zhenjiang, Jiangsu, People’s Republic of China; 2Department of Gynaecology and Obstetrics, Dantu District People’s Hospital, Zhenjiang, Jiangsu, People’s Republic of China; 3Department of Obstetrics and Gynecology, Taizhou People’s Hospital, Taizhou, People’s Republic of China
Correspondence: Lanlan Luo Tel/Fax +86-523-86606267
Email [email protected]
Background: Vulvovaginal candidiasis (VVC) is a common vaginal inflammatory disease in females. The interleukin (IL)-23/IL-17 axis was involved in vaginal inflammation. Nevertheless, the relationship between gene polymorphisms in the IL-23/IL-17 axis and VVC risk is still unexplored.
Methods: We enrolled 217 VCC cases and 326 controls in this study. The genotyping of all polymorphisms was implemented by PCR-RFLP methods.
Results: Data indicated that IL-17F gene rs763780, IL-17A gene rs2275913, and IL-23R rs11209026 polymorphisms were linked with an elevated risk of VVC in Chinese ethnicity. Subgroup analyses uncovered that IL-23R rs11209026, IL-17A rs10484879 and IL-17F rs763780 polymorphisms increased the risk of VVC among smokers or individuals with BMI ≥ 25 kg/m2. Additionally, IL-17F rs763780 polymorphism was shown to increase the risk of recurrent VVC (RVVC). Furthermore, IL-23 and IL-17 serum levels were higher among VVC cases than controls. We also observed that IL-23 and IL-17 gene polymorphisms were related to their serum levels. Receiver operating characteristics (ROC) curve analysis found that IL-17 and IL-23 serum levels were associated with the relapse of VVC.
Conclusion: In conclusion, this study indicates that polymorphisms in the IL-23/IL-17 axis increase the risk of VVC.
Keywords: IL-17, IL-23, case–control study, vulvovaginal candidiasis, polymorphism
Introduction
Vulvovaginal candidiasis (VVC) is a common vaginal infection caused mainly by the opportunistic Candida albicans, which is second only to bacterial vaginosis.1 VVC is the most prevalent human candidal infection, and more than 75% childbearing women are affected by overgrowth of opportunistic Candida species at least once in their life.2 In addition, recurrent VVC (RVVC) affects approximately 8% of the women globally, which is defined as 4 or more episodes of this disorder per year.3 Vaginal itching, pain, burning and redness are the primary disease symptoms of VVC.4 Up to date, the underlying pathogenic mechanisms of VVC are still poorly understood. Predisposing factors including genetic factors, the use of antibacterial agents, hormones, sexual activity, age, and some pathologies is reported to contribute to the development of VVC.5 Thus, different variants of relevant genes may exert effects on the vaginal mucosal defense mechanisms against Candida species, thereby affecting susceptibility to VVC.6,7
VVC, a primary disease associated with insufficient clearance and persistent fungal infections, has been regarded as the outcome of inadequate host defenses against colonization.3 The vaginal immune response includes innate immunity and acquired immune response. The vaginal mucosa consists of different lymphoid tissues and lymphocytes, which have important immune functions and exert anti-infective effects.8 Candida components are mainly processed by phagocytic cells; and T helper (Th) cells are differentiated in cellular subsets based on antigen specificity, which leads to pathogen clearance ultimately.9 Th17 cells, a subtype of T cells, secrete interleukin-17 (IL-17) and IL-22.10 Studies have indicated that pro-inflammatory cytokines including IL-17A and IL-17F stimulated the chronic vaginal inflammation, which produced by activated Th17 lymphocytes.3 Peters et al indicated that Th17/IL-17 axis signaling was indispensable for the immunopathogenesis of VVC.11 An animal study suggested that IL-17 played a key role in the immune reaction of vaginal candidiasis.12 Th17 phenotype is stabilized by IL-23, belonging to the IL-12 cytokine family, which could stimulate IL-17 production.13 IL-23/IL-17 axis was reported to alter the patterns and the potential role of Th1 cells in some autoimmune and infectious diseases such as VVC.14
The IL23 receptor (IL23R) gene, containing at least 11 exons, which is located on chromosome 1p31. IL-17A and IL-17F genes are shown to locate on chromosome 6p12. The link of IL-17 and IL-23 gene variants with VVC risk was not investigated before. We assumed that IL-17 and IL-23 gene variants may associate with the pathogenesis of VVC. Thus, we conducted this study to assess the link between variants of IL-17 and IL-23 gene variants and VVC risk in Chinese Han individuals. Additionally, we evaluated the predicting effects of the serum levels of IL-17 and IL-23 on the relapse of VVC.
Patients and Methods
Subjects
This study enrolled 217 women who were diagnosed with VVC according to their clinical examination and symptoms, and the diagnosis was confirmed by culture of the vaginal discharge collected from the posterior fornix. Healthy controls were 326 women with no history of vaginal Candida infection, and the culture of vaginal pathogens is currently negative. Controls who had vaginal diseases, gynecological diseases, or infectious diseases were excluded. To the population, annual VVC attack frequency was questioned. Forty-six women who had four or more symptomatic VVC attacks were diagnosed with RVVC. Family history of VVC was regarded as at least one first-degree relatives or at least two second-degree relatives having VVC. All patients and control subjects were enrolled from the Fourth Affiliated Hospital of Jiangsu University (Zhenjiang, China).
Written informed consent of all participants was got from all the participants. This study was completely approved by the Ethics committees of Fourth Affiliated Hospital of Jiangsu University and in accordance with the standards of Declaration of Helsinki.
Genotyping
The DNA samples of peripheral blood leukocytes were extracted from the study participants using a DNA Purification Kit (Tiangen Biotech). Genotyping was done by PCR-RFLP methods. The primers were as follows: 5ʹ-CTTTTCTGGCAGGGTCATTTTG-3ʹ (rs11209026F), 5ʹ-CAGAAGACCTACATGTTACT-3ʹ (rs2275913F) and forward: 5ʹ-GTGTAGGAACTTGGGCTGCATCAAT-3ʹ (rs763780F). IL-17 and IL-23 serum levels were assessed by use of human IL-17 and IL-23 ELISA kits (Sino Biological, Beijing, China).
Statistical Analysis
All relevant statistical analyses were performed on SPSS 22.0 for Windows (SPSS Inc., Chicago, USA). Student’s t-test and Chi-square (χ2) test were used for analyzing continuous variables and categorical variables, respectively. χ2-test was utilized to evaluate Hardy–Weinberg equilibrium (HWE). Using logistic regression analysis, the comparison of allele and genotype distributions in two groups was estimated. ORs and 95% CIs were presented. Subgroup analyses and cross-over analysis were analyzed. Regression models were adjusted for age, smoking, and drinking. Using receiver operating characteristics (ROC) curves, we assessed the area under ROC curves (AUC), specificity and sensitivity of the serum levels of IL-17 and IL-23 for predicting the relapse of VVC. All data were regarded as statistically significant with a P value lower than 0.05.
Results
Estimation of the Sample Size
We used the APP Quanto to estimate the sample sizes of this study before conduction. We set the following conditions: α = 0.05, β = 0.1, power value = 0.8, and the estimated OR=2.0. The allele frequency of rs763780 polymorphism was 0.19, and the estimated sample sizes of case and control groups were 140 and 140, respectively; For rs2275913 polymorphism, the allele frequency was 0.29, and the sample sizes of case and control groups were 133 and 133, respectively; For rs11209026 polymorphism, the allele frequency was 0.09, and the sample sizes of case and control groups were 197 and 197, respectively. In this study, we enrolled 217 VCC cases and 326 controls, indicating that the sample size of this study had enough power value (>80%) to evaluate the association between those polymorphisms and VCC risk.
Characteristics of the Study Population
We summarize the clinical features of all individuals in Table 1. The numbers of children and miscarriages were higher in VVC cases than those of controls (P < 0.05). As for age, smoking, BMI, and drinking, no significant differences between the VVC cases and controls were obtained. Among all VVC cases, 46 (21.2%) were RVVC, and 171 (78.8%) were VVC, respectively.
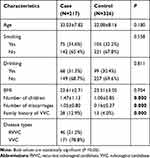 |
Table 1 Patient Demographics and Risk Factors in Vulvovaginal Candidiasis |
Association Between IL-17 and IL-23 Gene Polymorphisms and VVC Risk
The genotype and allele distributions for IL-17 and IL-23 gene polymorphisms are shown in Table 2. We found that IL-23R rs11209026 polymorphism was connected to the risk of VVC. AA+GA or GA genotype or A allele increased the risk of VVC (GA vs AA: adjusted OR, 1.58; 95% CI, 1.02–2.43; P = 0.043; A vs G: OR, 1.50; 95% CI, 1.02–2.19; P = 0.038). In addition, IL-17F rs763780 and IL-17A rs10484879 polymorphisms were also found to increase the risk of VVC. These results were still positive in previous genetic models even after adjusting for age, smoking and drinking. Next, we assessed the subgroup analyses of BMI, age, drinking and smoking. A significantly increased risk of VVC was shown in VVC patients with BMI ≥25 kg/m2 or smoking for IL-17F rs763780, IL-23R rs11209026, and IL-17A rs10484879 polymorphisms (Table 3). Additionally, we evaluated the associations between IL-23R and IL-17 gene variants and types of VVC, and found that rs763780 polymorphism of the IL-17F gene increased the risk of RVVC (Supplemental Table 1). No significant relationship was indicated in the analyses of IL-17A rs10484879, and IL-23R rs11209026 polymorphisms and status of VVC.
 |
Table 2 Genotype Frequencies of IL-23R and IL-17 Gene Polymorphisms in Cases and Controls |
 |
Table 3 Stratified Analyses Between IL-23R and IL-17 Gene Polymorphisms and the Risk of Vulvovaginal Candidiasis |
Cross-Over Analysis
Due to the potential interaction between genetic factors and environmental factors, we next analyzed the combined effects of the IL-23R and IL-17 gene variants and either alcohol consumption or smoking on VVC risk. For IL-23R gene rs11209026 polymorphism, data indicated that smokers carrying the GA genotype increased the risk of VVC when compared with non-smokers who carried GG genotype (OR = 2.32, 95% CI = 1.17–4.57; P = 0.014) (Table 4). Regarding IL-17A gene rs2275913 polymorphism, comparing with non-smokers carrying GG genotype, smokers carrying the AA genotype elevated the risk of VVC (OR = 2.82, 95% CI = 1.09–7,29; P = 0.028). As for IL-17F gene rs763780 polymorphism, smokers with the CC genotype also showed an increased susceptibility to VVC when comparing with non-smokers carrying TT genotype. However, no positive association was observed between VVC risk and drinking. The data suggested significant interactions between IL-23 and IL-17 gene variants and smoking.
 |
Table 4 Genetic (G) and Environmental (E) Factors 2 × 4 Fork Analysis |
Association of IL-23 and IL-17 Serum Levels with the Relapse of VVC
Next, we measured the serum levels of IL-17 and IL-23. Data revealed that serum levels of IL-17 and IL-23 among VVC patients were markedly higher than controls (Supplemental Figure 1 and Supplemental Figure 2). In addition, we assessed whether IL-23 and IL-17 gene polymorphisms were linked with their serum levels. We found that GA genotype carriers of IL-23R gene rs11209026 polymorphism showed higher IL-23 serum levels compared with GG genotype carriers among VVC patients (Supplemental Figure 1). Besides, this study revealed that rs2275913 and rs763780 polymorphisms were related to IL-17 serum levels in VVC patients (Supplemental Figure 2).
Last, a ROC curve was utilized to evaluate the predictive values of IL-23 and IL-17 serum levels in detecting RVVC in this Chinese Han population (Table 5). The cutoff values of IL-23 and IL-17 were 28.67 with a sensitivity of 73.91% and a specificity of 56.73%, and 5.98 with a sensitivity of 69.57% and a specificity of 71.35%, respectively. The AUC values of IL-17 and IL-23 serum levels for predicting RVVC were 0.755 (95% CI: 0.69–0.81), and 0.653 (95% CI: 0.58–0.71, Figure 1), respectively. The Youden index of IL-17 and IL-23 was 0.409 and 0.306, respectively.
 |
Table 5 Predictive Values of IL-17 and IL-23 Serum Levels in Detecting Recurrent Vulvovaginal Candidiasis |
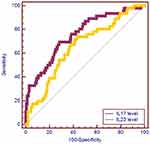 |
Figure 1 ROC curve for IL-23 and IL-17 serum levels to predict the occurrence of RVVC. |
Discussion
In this study, we found that IL-17A gene rs2275913, IL-17F gene rs763780, and IL-23R rs11209026 polymorphisms increased the risk of VVC in Chinese Han population. Subgroup analyses showed IL-17A rs10484879, IL-23R rs11209026, and IL-17F rs763780 polymorphisms increased the risk for VVC among smokers or individuals with BMI ≥25 kg/m2. In addition, rs763780 polymorphism of the IL-17F gene was shown to elevate the risk of RVVC. And then, we observed that IL-17 and IL-23 serum levels among VVC patients were higher among than controls. Last, we found that increased IL-23 and IL-17 serum levels were associated with the relapse of VVC.
The IL-23/IL-17 axis was shown to mediate vaginal immunity and inflammation.12,15,16 The IL-23R is mainly expressed on activated T cells, and it is indispensable for maintaining and activating the effects of Th17 cells on secreting IL-17.17,18 Transcriptomic analysis of vaginal tissue showed an elevated expression of host genes including Th17 cytokine secretion in a murine model of VVC.3 The IL-17 gene polymorphisms were reported to be associated with several immunopathologies related to inflammation.19,20 As for other infectious and immune diseases, a host of studies have explored the relationship between IL-23/IL-17 pathway gene polymorphisms and corresponding disease risk. McGovern et al indicated that variants of IL-23/IL-17 pathway genes were markedly associated with the susceptibility to Crohn’s disease.21 Omrane et al observed that IL-17F and IL-23R gene polymorphisms were not related to susceptibility to colorectal cancer.22 However, they obtained an association between these SNPs and clinical features of colorectal cancer.22 Regarding necrotizing enterocolitis in premature infants, IL-23R rs10889677 and IL-17A rs2275913 polymorphisms were not related to the susceptibility to necrotizing enterocolitis; however, IL-17F rs763780 polymorphism elevated the risk of necrotizing enterocolitis.23 In addition, Louahchi et al from Algeria found no association between IL-23R and IL-17 gene variants and rheumatoid arthritis risk.24 A study by Catanoso et al revealed that IL-23R and IL-17 gene variants showed no association with psoriatic arthritis susceptibility.25 A Turkish study suggested IL-23R and IL-17 polymorphisms had no link with alopecia areata risk.26 On the whole, IL-23R and IL-17 polymorphisms were related to the risk of some specific disorders. Types of disease, various sample sizes, clinical heterogeneity, and different races may account for conflicting findings of these studies, which need further studies to validate it.
Given the crucial role of the IL-23R and IL-17 genes in the development of infectious and immune diseases, we assumed that IL-23R and IL-17 gene variants may be associated with the susceptibility to VVC. Firstly, we searched the SNPinfo Web Server and found the potential functional effects of these chosen variants: IL-23R rs11209026 and IL-17F rs763780 polymorphisms are nsSNPs, which could cause amino acid variation and lead to the alteration of protein after translation; IL-17A gene rs2275913 is a transcription factor binding site (TFBS), which helps the transcription factors regulate relevant gene expressions. Although functional effects of those variants are important, the potential link of these single nucleotide polymorphisms (SNPs) in the IL23/IL17 axis with the risk of VVC remains unexplored. In this study, we uncovered a link between IL-17A gene rs2275913, IL-23R rs11209026, and IL-17F gene rs763780 polymorphisms and VVC risk. Data indicated that all these SNPs increased the risk of VVC. Subgroup analyses indicated that these SNPs elevated the risk of VVC among smokers and individuals with BMI ≥25 kg/m2, indicating that individuals with genotypes of these SNPs exposing to these factors are more prone to suffering VVC. In addition, we evaluated the association of VVC types with IL-23R and IL-17 polymorphisms, and found that IL-17F rs763780 polymorphism elevated the risk of RVVC, inferring that this SNP was related to the relapse of VVC.
Next, we evaluated the serum levels of IL-23 and IL-17 among VVC patients and healthy controls, and found that VVC patients had higher serum levels of IL-17 and IL-23 when comparing with healthy controls. It is of note that IL-23 expression in murine vaginal candidiasis was associated with its immune status and infection.27 Kolben et al revealed that levels of IL-23 were markedly lower in the VVC group,28 which indicated a compromised local immune response in vaginal mucosa. Obviously, the findings uncovered by Kolben et al were inconsistent with those of this study. Potential factors including different ethnicities, distinct sample sizes, and various stage severity of VVC may explain these disaccords. Additionally, we found that IL-17 and IL-23 gene polymorphisms were related to the serum levels of IL-17 and IL-23. Maybe IL-17 and IL-23 gene polymorphisms increased the risk of VVC via affecting their serum levels. Last, we used the ROC curve to evaluate the predictive values of IL-23 and IL-17 serum levels in detecting relapse of VVC, and found the AUC values of IL-23 and IL-17 serum levels for predicting RVVC were 0.653, and 0.755, respectively, indicating good ability of IL-23 and IL-17 to predict recurrence of VVC.
Several limitations were shown in this study. One, the sample size was small, which may present untrustworthy results. Two, we only investigated limited SNPs of IL-17 and IL-23 genes. Three, the interaction between genetic factors and environmental factors was not explored. Four, whether IL-23 and IL-17 affected the therapeutic effects of clinical medications should be addressed. Fifth, the possible gene–gene interactions were not investigated. We utilized the String online tool (http://string-db.org/) to observe these effects. Several genes including IL-2, IL-6, IL-13, IL-10, STAT3, CTLA4, and STAT6 participated in the interaction of IL-17 (Supplemental Figure 3). RELA, TYK2, JAK2, STAT3, IL12B, STAT4, STAT6, STAT1, and TYK2 showed interactions with IL-23 (Supplemental Figure 4). Last, cell and animal experiments should be performed to explore the role of IL-23 and IL-17 in the development of VVC.
In conclusion, IL-17 and IL-23 gene polymorphisms increase the risk of VVC. IL-17 and IL-23 serum levels are related with the relapse of VVC. Further studies are urgently warranted to verify these results.
Funding
This study was funded by Key Technology R&D Program of Gansu Province (grant no.144FKCA089), Woman & Child Health Research Program of Jiangsu Province (grant no.F201739) and Zhenjiang Social Development Project (grant no.SH2018045).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Rodriguez-Cerdeira C, Gregorio MC, Molares-Vila A, et al. Biofilms and vulvovaginal candidiasis. Colloids Surf B Biointerfaces. 2019;174:110–125. doi:10.1016/j.colsurfb.2018.11.011
2. Willems HME, Ahmed SS, Liu J, Xu Z, Peters BM. Vulvovaginal candidiasis: a current understanding and burning questions. J Fungi (Basel). 2020;6(1). doi:10.3390/jof6010027
3. Rosati D, Bruno M, Jaeger M, Ten OJ, Netea MG. Recurrent vulvovaginal candidiasis: an immunological perspective. Microorganisms. 2020;8(2):144. doi:10.3390/microorganisms8020144
4. Yano J, Peters BM, Noverr MC, Fidel PL
5. Goncalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42(6):905–927. doi:10.3109/1040841X.2015.1091805
6. Jaeger M, Pinelli M, Borghi M, et al. A systems genomics approach identifies SIGLEC15 as a susceptibility factor in recurrent vulvovaginal candidiasis. Sci Transl Med. 2019;11(496):496. doi:10.1126/scitranslmed.aar3558
7. Kalia N, Singh J, Sharma S, Kaur M. SNPs in 3ʹ-UTR region of MBL2 increases susceptibility to recurrent vulvovaginal infections by altering sMBL levels. Immunobiology. 2019;224(1):42–49. doi:10.1016/j.imbio.2018.10.009
8. De Bernardis F, Graziani S, Tirelli F, Antonopoulou S. Candida vaginitis: virulence, host response and vaccine prospects. Med Mycol. 2018;56(suppl_1):26–31. doi:10.1093/mmy/myx139
9. Hofs S, Mogavero S, Hube B. Interaction of Candida albicans with host cells: virulence factors, host defense, escape strategies, and the microbiota. J Microbiol. 2016;54(3):149–169. doi:10.1007/s12275-016-5514-0
10. Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi:10.1038/ni1254
11. Peters BM, Coleman BM, Willems HME, et al. The interleukin (IL) 17R/IL-22R signaling axis is dispensable for vulvovaginal candidiasis regardless of estrogen status. J Infect Dis. 2020;221(9):1554–1563. doi:10.1093/infdis/jiz649
12. Pietrella D, Rachini A, Pines M, et al. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS One. 2011;6(7):e22770. doi:10.1371/journal.pone.0022770
13. Chen Y, Wood KJ. Interleukin-23 and TH17 cells in transplantation immunity: does 23+17 equal rejection? Transplantation. 2007;84(9):1071–1074. doi:10.1097/01.tp.0000287126.12083.48
14. Bunte K, Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. 2019;20(14):3394. doi:10.3390/ijms20143394
15. Liu J, Wang HZ, Sun Y. Inhibition of CXCR4 by MicroRNA-1192 reduces the activation of Th17 cells and expression of inflammation factors in a mouse model of vulvovaginal candidiasis. Cell Physiol Biochem. 2018;50(3):893–910. doi:10.1159/000494475
16. Wang L, Wang C, Mei H, et al. Combination of estrogen and immunosuppressive agents to establish a mouse model of candidiasis with concurrent oral and vaginal mucosal infection. Mycopathologia. 2016;181(1–2):29–39. doi:10.1007/s11046-015-9947-5
17. Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116(5):1310–1316. doi:10.1172/JCI21404
18. Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120(2):247–254. doi:10.1016/j.jaci.2007.06.039
19. Arisawa T, Tahara T, Shibata T, et al. The influence of polymorphisms of interleukin-17A and interleukin-17F genes on the susceptibility to ulcerative colitis. J Clin Immunol. 2008;28(1):44–49. doi:10.1007/s10875-007-9125-8
20. Hammad A, Mosaad YM, Hammad EM, et al. Interleukin-17A rs2275913, interleukin-17F rs763780 and rs2397084 gene polymorphisms as possible risk factors in Juvenile lupus and lupus related nephritis. Autoimmunity. 2016;49(1):31–40. doi:10.3109/08916934.2015.1101071
21. McGovern DP, Rotter JI, Mei L, et al. Genetic epistasis of IL23/IL17 pathway genes in Crohn’s disease. Inflamm Bowel Dis. 2009;15(6):883–889. doi:10.1002/ibd.20855
22. Omrane I, Baroudi O, Bougatef K, et al. Significant association between IL23R and IL17F polymorphisms and clinical features of colorectal cancer. Immunol Lett. 2014;158(1–2):189–194. doi:10.1016/j.imlet.2014.01.002
23. Tian J, Liu Y, Jiang Y, et al. Association of single nucleotide polymorphisms of IL23R and IL17 with necrotizing enterocolitis in premature infants. Mol Cell Biochem. 2017;430(1–2):201–209. doi:10.1007/s11010-017-2972-6
24. Louahchi S, Allam I, Berkani L, et al. Association study of single nucleotide polymorphisms of IL23R and IL17 in rheumatoid arthritis in the Algerian population. Acta Reumatol Port. 2016;41(2):151–157.
25. Catanoso MG, Boiardi L, Macchioni P, et al. IL-23A, IL-23R, IL-17A and IL-17R polymorphisms in different psoriatic arthritis clinical manifestations in the northern Italian population. Rheumatol Int. 2013;33(5):1165–1176. doi:10.1007/s00296-012-2501-6
26. Aytekin N, Akcali C, Pehlivan S, Kirtak N, Inaloz S. Investigation of interleukin-12, interleukin-17 and interleukin-23 receptor gene polymorphisms in alopecia areata. J Int Med Res. 2015;43(4):526–534. doi:10.1177/0300060514549784
27. Yan W, Zhijian T, Zhixiang L, Dechao X, Jiawen J. Local IL-23 expression in murine vaginal candidiasis and its relationship with infection and immune status. J Huazhong Univ Sci Technol Med Sci. 2006;26(2):245–247. doi:10.1007/BF02895828
28. Kolben T, Pieper K, Goess C, et al. IL-23, IFN-alpha, and IFN-beta in the vaginal fluid of patients suffering from vulvovaginal candidosis. Clin Exp Obstet Gynecol. 2017;44(1):7–10.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
