Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 14
Association of HMGA2 Polymorphisms with Glioma Susceptibility in Chinese Children
Authors Zhou J, Wang P, Zhang R, Huang X, Dai H , Yuan L, Ruan J
Received 12 March 2021
Accepted for publication 5 May 2021
Published 25 May 2021 Volume 2021:14 Pages 601—607
DOI https://doi.org/10.2147/PGPM.S310780
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Jingying Zhou,1 Pan Wang,1 Ran Zhang,2 Xiaokai Huang,1 Hanqi Dai,1 Li Yuan,3 Jichen Ruan1
1Department of Hematology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, 325027, Zhejiang, People’s Republic of China; 2Sydney School of Public Health, The University of Sydney, Camperdown, Sydney, NSW, 2006, Australia; 3Department of Pathology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, 510623, Guangdong, People’s Republic of China
Correspondence: Jichen Ruan
Department of Hematology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, 109 West Xueyuan Road, Wenzhou, 325027, Zhejiang, People’s Republic of China
Email [email protected]
Li Yuan
Department of Pathology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, 9 Jinsui Road, Guangzhou, 510623, Guangdong, People’s Republic of China
Email [email protected]
Background: Glioma is a malignant central nervous system tumor in children, with poor outcomes and prognosis. HMGA2 is a proto-oncogene with increased expression in various malignancies.
Methods: We explored the association of HMGA2 polymorphisms with glioma susceptibility in Chinese children using a case-control study (191 cases, 248 controls). HMGA2 single nucleotide polymorphisms (rs6581658 A>G; rs8756 A>C; rs968697 T>C) were genotyped using PCR-based TaqMan.
Results: Increased glioma susceptibility was associated with rs6581658 A>G; AG (adjusted odds ratio (OR) = 1.71, 95% confidence interval (CI) = 1.13– 2.58, P = 0.010) or GG (adjusted OR = 3.12, 95% CI = 1.26– 7.74, P = 0.014) genotype carriers had significantly raised glioma risk compared with AA genotype carriers. The rs6581658 AG/GG (adjusted OR = 1.85, 95% CI = 1.25– 2.73, P = 0.002) and AA/GG (adjusted OR = 2.58, 95% CI = 1.05– 6.33, P = 0.038) genotypes were associated with an increased risk of glioma relative to the AA genotype. Subjects with 2– 3 risk genotypes had a significantly elevated risk (adjusted OR = 1.93, 95% CI = 1.31– 2.84, P = 0.001) relative to those with 0– 1 risk genotype.
Conclusion: HMGA2 rs6581658 A>G is associated with glioma susceptibility in Chinese children.
Keywords: HMGA2, polymorphism, susceptibility, glioma
Introduction
Glioma is an intracranial tumor, that can be categorized into subtypes, as follows: diffuse astrocytic and oligodendroglial, other astrocytic, ependymal, or other glioma.1 Brain cancer is the leading cause of cancer deaths in children.2 From 2001 to 2010, the incidence of intracranial and intraspinal tumors in British children under the age of 15 was 1/1678, among which astrocytoma accounted for 40%.3 The most common type of glioma in children is pilocytic astrocytoma. The 5-year survival rates for patients with many glioma subtypes are relatively high; however, the 5-year survival rate for specific types of glioma, such as glioblastoma (GBM), is only 14%.3 Glioma occurrence can be influenced by ionizing radiation, allergic disease, and gene mutation. Although glioma can currently be treated using surgery, radiotherapy, and chemotherapy, its heterogeneity and invasiveness make it prone to drug resistance and recurrence. Hence, future prospects for treatment of pediatric brain tumors tend more towards molecularly targeted therapy.4,5 Some genetic loci have been identified as associated with increased susceptibility to adult glioma, including RTEL1,6 CDKN2A/B,6 PHLDB1,6 CCDC267 and TERT;7 however, these risk loci cannot fully explain the molecular genetic contribution to glioma. Further, pediatric glioma has molecular and genetic differences from adult glioma.8,9 Therefore, identification of suitable gene markers for application in pediatric glioma is increasingly important, and the influence of other genes on susceptibility to pediatric glioma warrants further study.
HMGA2 is a 160 kb gene located on chromosome 12. As an architectural transcription factor, HMGA2 has three AT-hook domains that interact with AT-rich sequences in DNA minor grooves, leading to alteration of the chromatin architecture and modulation of the maintenance and assembly of enhancer complexes.10 Although it is widely expressed in embryos, HMGA2 is not present in adults, except in stem cells. Further, it is expressed in tumor cells, including colon cancer,11 lung cancer,12 liver cancer,13 thyroid tumor14 and prostate cancer.15 Therefore, HMGA2 is considered to be a proto-oncogene that promotes the occurrence and development of tumors.16
HMGA2 expression is also increased in glioma.17 Genomic single nucleotide polymorphisms (SNPs) are closely related to disease susceptibility and prognosis;18,19 however, few studies have focused on the relationship between HMGA2 SNPs and glioma. Our research explored the association of HMGA2 polymorphisms with glioma susceptibility in Chinese children.
Materials and Methods
Patients and Controls
We selected participants from Guangzhou and Wenzhou, including 191 cases histopathologically diagnosed with glioma and a control group of 248 healthy children with no family history of cancer, who were matched for sex and age with those in the experimental group. The Institutional Review Board of two hospitals approved the study protocol (the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Guangzhou Women and Children’s Medical Center). In accordance with the Declaration of Helsinki, all subjects or their guardians signed informed consent forms.
Polymorphism Selection and Genotyping
We investigated the potentially functional HMGA2 polymorphisms and selected three HMGA2 polymorphisms (rs6581658 A>G, rs8756 A>C, and rs968697 T>C) based on data obtained in the SNPinfo (https://snpinfo.niehs.nih.gov/snpinfo/snpfunc.html) and dbSNP database (https://www.ncbi.nlm.nih.gov/snp/). The rs8756 A>C was located in 3ʹ untranslated region (UTR) of the HMGA2 gene. It may affect microRNA binding affinity and subsequently affects expression and stabilization of HMGA2 gene. The rs6581658 A>G and rs968697 T>C were located in the 5ʹ near gene region. Binding of transcription factors may be affected, which may influence the transcription of HMGA2. There was no significant linkage disequilibrium among the selected SNPs (r2 < 0.8). All SNPs had minor allele frequencies > 5% and potential biological function. Genomic DNA was extracted from venous blood samples and genotyped by TaqMan real-time PCR. The principle of tagging SNP selection and the genotyping methods used were described in our previous publications.20–22
Statistical Analysis
Distributions of demographic characteristics and genotype frequencies in both groups and appropriate intergroup comparisons were assessed by chi-square analysis. Hardy-Weinberg equilibrium (HWE) was evaluated in the control group using the goodness-of-fit chi-square test, whereas the association between the HMGA2 SNPs and glioma susceptibility was assessed by univariate and multivariate unconditional logistic regression analysis, to generate odds ratio (OR) and 95% confidence interval (CI) values. We performed stratified analysis, according to age, sex, glioma subtype, and clinical grade. The criterion for statistical significance was P < 0.05. All two-sided statistical analyses were performed using SAS version 9·1 (SAS Institute, Cary, NC, USA).
Results
Participant Characteristics
The general characteristics of subjects are presented in Table 1. All participants were < 168 months old, with mean ages of patients (n = 191) of 62.74 ± 47.28 months and of controls (n = 248) of 53.90 ± 33.47 months. The majority of tumors were of the astrocytic subtype. In the case group, 57.59%, 19.90%, 8.90%, and 13.09% of patients were at clinical grades I–IV, respectively; clinical grade data were unavailable for 0.52% of patients.
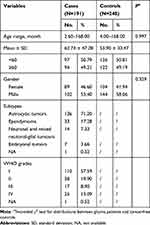 |
Table 1 Frequency Distribution of Selected Variables in Glioma Patients and Cancer-Free Controls |
Relationship Between HMGA2 SNPs and Glioma Risk
The detailed results are shown in Table 2. In analysis of the entire cohort, carriers of the rs6581658 AG genotype (adjusted OR = 1.71, 95% CI = 1.13–2.58, P = 0.010) or GG genotype (adjusted OR = 3.12, 95% CI = 1.26–7.74, P = 0.014) were found to have a significantly elevated risk of developing glioma compared with those with the AA genotype. Further investigation indicated that subjects with rs6581658 AG/GG (adjusted OR = 1.85, 95% CI = 1.25–2.73, P = 0.002) or AA/GG (adjusted OR = 2.58, 95% CI = 1.05–6.33, P = 0.038) genotypes had an increased risk of glioma relative to those with AA genotype. Moreover, we observed that individuals with 2–3 risk genotypes had a significantly elevated risk relative to those with 0–1 risk genotype (adjusted OR = 1.93, 95% CI = 1.31–2.84, P = 0.001).
 |
Table 2 HMGA2 Gene Polymorphisms and Glioma Susceptibility in Chinese Children |
Stratification Analysis
We next explored the effects of rs6581658 genotype and joint risk genotypes on glioma susceptibility following further stratification, according to age, sex, tumor subtype, and clinical grade. The results are presented in Table 3. The rs6581658 AG/GG genotypes significantly increased glioma susceptibility in children > 60 months old (adjusted OR = 2.07, 95% CI = 1.19–3.59, P = 0.010), males (adjusted OR = 2.07, 95% CI = 1.23–3.50, P = 0.007), patients with astrocytic tumor subtype (adjusted OR = 1.70, 95% CI = 1.10–2.64, P = 0.017), and those with ependymoma subtype (adjusted OR = 2.47, 95% CI = 1.17–5.21, P = 0.018), clinical grade I (adjusted OR = 1.77, 95% CI = 1.11–2.81, P = 0.016), grade IV (adjusted OR = 3.16, 95% CI = 1.29–7.69, P = 0.012), grade I+II (adjusted OR = 1.67, 95% CI = 1.10–2.55, P = 0.017) and grade III+IV (adjusted OR = 2.72, 95% CI = 1.38–5.33, P = 0.004).
 |
Table 3 Stratification Analysis of Risk Genotypes with Glioma Susceptibility |
On analysis of combination risk genotypes, we found that subjects with 2–3 risk genotypes had an increased glioma risk relative to those with 0–1 risk genotype, among the following subgroups: age < 60 months (adjusted OR = 1.97, 95% CI = 1.15–3.38, P = 0.014), age ≥ 60 months (adjusted OR = 2.01, 95% CI = 1.15–3.51, P = 0.014), males (adjusted OR = 1.70, 95% CI = 1.01–2.86, P = 0.046), patients with astrocytic tumors (adjusted OR = 1.89, 95% CI = 1.22–2.92, P = 0.004), patients with ependymoma (adjusted OR = 2.18, 95% CI = 1.01–4.68, P = 0.046) and children at clinical grade I (adjusted OR = 1.76, 95% CI = 1.10–2.79, P = 0.017), grade III (adjusted OR = 4.11, 95% CI = 1.29–13.15, P = 0.017), grade I+II (adjusted OR = 1.74, 95% CI = 1.15–2.64, P = 0.009), and grade III+IV (adjusted OR = 3.00, 95% CI = 1.43–6.29, P = 0.004).
Discussion
Here, we studied the association of HMGA2 polymorphisms with glioma susceptibility in Chinese children. The relationship between these three gene polymorphisms and glioma susceptibility has not been studied previously. We found that rs6581658 AG/GG was associated with a significantly increased risk of susceptibility to glioma.
HMGA2 promotes cancer progression through several functions: promoting cell proliferation and metastasis, influencing the cell cycle, inhibiting apoptosis, and conferring stem cell characteristics.23 HMGA2 overexpression can promote the migration and invasion of pancreatic cancer cells,24 and HMGA2 can inhibit apoptosis and promote cell proliferation in breast cancer, as well as conferring stem cell-like features, whereas knocking out HMGA2 led to cell cycle arrest at G2/M, reducing tumor invasiveness.25 Further, HMGA2 overexpression reduced the sensitivity of pancreatic cancer cells to the standard first-line drug, gemcitabine.26 HMGA2 is regulated as a downstream target of many miRNAs and is involved in progression of various tumors. HMGA2 can also promote epithelial-mesenchymal transition of esophageal squamous cell carcinoma, which can be targeted by binding of miR490-3p to its 3ʹ untranslated region.27 In addition, HMGA2 can be regulated via the miR-503-5p/HMGA2 and miR-150-5p/HMGA2 axes, to promote the progression of gastric and breast cancers, respectively.28,29 HMGA2 can also be targeted by miR-493 to inhibit tongue squamous cell carcinoma.30
In addition to the cancers mentioned above, HMGA2 is also associated with glioma. HMGA2 expression is up-regulated in glioma,31 which is associated with tumorigenicity, since increased HMGA2 in vivo can promote the glioma growth, and the increased clonogenicity in vitro also supports the role of HMGA2 in tumor initiation.32 Further, HMGA2 expression, a target gene of miR-107, could be enhanced by the long non-coding RNA LINC00152 through regulation of miR-107 expression and promotion of glioma occurrence.33 Moreover, HMGA2 expression level is associated with glioma grade. Compared with diffuse astrocytoma, HMGA2 expression is higher in glioblastoma multiforme and anaplastic astrocytoma34 and increased HMGA2 expression suggests worse prognosis. HMGA2 is also associated with glioma malignant degree. In GBM cell lines, HMGA2 increased GBM cell invasion, clonogenicity, and tumorigenicity.32 HMGA2 may also activate MMP2, which can increase glioma invasion.35 In contrast, HMGA2 suppression inhibits glioma growth. HMGA2 is suppressed by let-7g-5p and down-regulation of HMGA2 expression can promote GBM tumor cell apoptosis and inhibit their invasion, indicating that HMGA2 is a potential novel target gene in GBM.36 In addition, HMGA2 can be targeted by miR-370-3p, which inhibits glioma cell growth and invasion37 and is, therefore, a potential target for glioma therapy.
Many researchers have focused on the relationship between HMGA2 gene SNPs and susceptibility to different conditions. For example, HMGA2 SNPs are related to height. HMGA2 rs1042725 influences height variability in European populations, and the association is more pronounced in individuals with small size for gestational age (SGA).38 Further, HMGA2 rs7968902 is significantly correlated with the height of the Japanese population.39 We previously explored the effect of HMGA2 SNPs on susceptibility to different diseases and found that HMGA2 polymorphisms weakly influence Wilms tumor.40 In addition, the HMGA2 SNPs, rs8756A>C and rs968697T>C, are associated with lower susceptibility to neuroblastoma41 and hepatoblastoma,42 respectively.
This is the first study to investigate the relationship between HMGA2 SNPs and glioma susceptibility. We found that the relationship is highly significant. Nevertheless, this study has limitations. First, the prevalence of glioma is low, and the sample size was relatively moderate, which may affect the statistical power of the analysis. Second, we only selected three HMGA2 SNPs, while other HMGA2 SNPs may also be related to glioma susceptibility. Third, the sample was from Chinese children alone, therefore, the results may not be relevant to other populations. Fourth, this study was retrospective; therefore, selection bias cannot be avoided. Finally, environmental factors, which can also influence glioma susceptibility, were not taken into consideration.
Conclusion
We analyzed HMGA2 SNPs and the risk of gliomas in the Chinese Han population. Our study is the first to identify a role for HMGA2 gene polymorphisms in glioma susceptibility. The results contribute to understanding of glioma etiology; however, further studies with a larger sample size, increased representation of other ethnic groups, and that consider interactions between the environment, genetics, and other factors, are required.
Abbreviations
OR, odds ratio; CI, confidence interval; GBM, glioblastoma; SNP, genomic single nucleotide polymorphism; HWE, Hardy-Weinberg equilibrium.
Ethics Approval and Informed Consent
The Institutional Review Board of two hospitals approved the study protocol (the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Guangzhou Women and Children’s Medical Center). All subjects or their guardians signed informed consent forms.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Acknowledgment
This study was supported by a grant from the National Natural Science Foundation of China (No: 81802346). The funder had no contribution to the research design, data acquisition and analysis, approval for publishing, or drafting the manuscript.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Malzkorn B, Reifenberger G. Practical implications of integrated glioma classification according to the World Health Organization classification of tumors of the central nervous system 2016. Curr Opin Oncol. 2016;28(6):494–501. doi:10.1097/CCO.0000000000000327
2. Hanz SZ, Adeuyan O, Lieberman G, Hennika T. Clinical trials using molecular stratification of pediatric brain tumors. Transl Pediatr. 2020;9(2):144–156. doi:10.21037/tp.2020.03.04
3. Stiller CA, Bayne AM, Chakrabarty A, Kenny T, Chumas P. Incidence of childhood CNS tumours in Britain and variation in rates by definition of malignant behaviour: population-based study. BMC Cancer. 2019;19(1):139. doi:10.1186/s12885-019-5344-7
4. Patel RR, Ramkissoon SH, Ross J, Weintraub L. Tumor mutational burden and driver mutations: characterizing the genomic landscape of pediatric brain tumors. Pediatr Blood Cancer. 2020;67(7):e28338. doi:10.1002/pbc.28338
5. Cacciotti C, Fleming A, Ramaswamy V. Advances in the molecular classification of pediatric brain tumors: a guide to the galaxy. J Pathol. 2020;251(3):249–261. doi:10.1002/path.5457
6. Viana-Pereira M, Moreno DA, Linhares P, et al. Replication of GWAS identifies RTEL1, CDKN2A/B, and PHLDB1 SNPs as risk factors in Portuguese gliomas patients. Mol Biol Rep. 2020;47(2):877–886. doi:10.1007/s11033-019-05178-8
7. Walsh KM, Rice T, Decker PA, et al. Genetic variants in telomerase-related genes are associated with an older age at diagnosis in glioma patients: evidence for distinct pathways of gliomagenesis. Neuro Oncol. 2013;15(8):1041–1047. doi:10.1093/neuonc/not051
8. Collins KL, Pollack IF. Pediatric low-grade gliomas. Cancers. 2020;12(5):1152. doi:10.3390/cancers12051152
9. Guerreiro Stucklin AS, Ryall S, Fukuoka K, et al. Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat Commun. 2019;10(1):4343. doi:10.1038/s41467-019-12187-5
10. Huang YM, Cheng CH, Pan SL, Yang PM, Lin DY, Lee KH. Gene expression signature-based approach identifies antifungal drug ciclopirox as a novel inhibitor of HMGA2 in colorectal cancer. Biomolecules. 2019;9(11):688. doi:10.3390/biom9110688
11. Chen RX, Chen X, Xia LP, et al. N(6)-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat Commun. 2019;10(1):4695. doi:10.1038/s41467-019-12651-2
12. Zeng Z, Zhao G, Rao C, et al. Knockdown of lncRNA ZFAS1-suppressed non-small cell lung cancer progression via targeting the miR-150-5p/HMGA2 signaling. J Cell Biochem. 2019. doi:10.1002/jcb.2954
13. Han S, Han B, Li Z, Sun D. Downregulation of long noncoding RNA CRNDE suppresses drug resistance of liver cancer cells by increasing microRNA-33a expression and decreasing HMGA2 expression. Cell Cycle. 2019;18(19):2524–2537. doi:10.1080/15384101.2019.1652035
14. Titov SE, Ivanov MK, Demenkov PS, et al. Combined quantitation of HMGA2 mRNA, microRNAs, and mitochondrial-DNA content enables the identification and typing of thyroid tumors in fine-needle aspiration smears. BMC Cancer. 2019;19(1):1010. doi:10.1186/s12885-019-6154-7
15. Guo Z, He C, Yang F, Qin L, Lu X, Wu J. Long non-coding RNA-NEAT1, a sponge for miR-98-5p, promotes expression of oncogene HMGA2 in prostate cancer. Biosci Rep. 2019;39(9):BSR20190635. doi:10.1042/BSR20190635
16. Zhang S, Mo Q, Wang X. Oncological role of HMGA2 (Review). Int J Oncol. 2019;55(4):775–788. doi:10.3892/ijo.2019.4856
17. Li Q, Wu Q, Li Z, et al. LncRNA LINC00319 is associated with tumorigenesis and poor prognosis in glioma. Eur J Pharmacol. 2019;861:172556. doi:10.1016/j.ejphar.2019.172556
18. Kong J, Chen X, Wang J, et al. Genetic polymorphisms in the vitamin D pathway and non-small cell lung cancer survival. Pathol Oncol Res. 2020;26(3):1709–1715. doi:10.1007/s12253-019-00702-4
19. Wang Z, Dai J, Hu N, et al. Identification of new susceptibility loci for gastric non-cardia adenocarcinoma: pooled results from two Chinese genome-wide association studies. GUT. 2017;66(4):581–587. doi:10.1136/gutjnl-2015-310612
20. Yang Z, Deng Y, Zhang K, et al. LIN28B gene polymorphisms modify hepatoblastoma susceptibility in Chinese children. J Cancer. 2020;11(12):3512–3518. doi:10.7150/jca.42798
21. Pan J, Zhu J, Wang M, et al. Association of MYC gene polymorphisms with neuroblastoma risk in Chinese children: a four-center case-control study. J Gene Med. 2020;22(8):e3190. doi:10.1002/jgm.3190
22. Lu T, Li L, Zhu J, et al. AURKA rs8173 G>C polymorphism decreases wilms tumor risk in Chinese children. J Oncol. 2019;2019:9074908. doi:10.1155/2019/9074908
23. De Martino M, Fusco A, Esposito F. HMGA and cancer: a review on patent literatures. Recent Pat Anticancer Drug Discov. 2019;14(3):258–267. doi:10.2174/1574892814666190919152001
24. Gong J, Wang Y, Jiang B, Xu B, Hu C. Impact of high-mobility-group A2 overexpression on epithelial-mesenchymal transition in pancreatic cancer. Cancer Manag Res. 2019;11:4075–4084. doi:10.2147/CMAR.S199289
25. Mansoori B, Duijf PHG, Mohammadi A, et al. Overexpression of HMGA2 in breast cancer promotes cell proliferation, migration, invasion and stemness. Expert Opin Ther Targets. 2020:1–11. doi:10.1080/14728222.2020.1736559
26. Xiao G, Wang X, Yu Y. CXCR4/Let-7a axis regulates metastasis and chemoresistance of pancreatic cancer cells through targeting HMGA2. Cell Physiol Biochem. 2017;43(2):840–851. doi:10.1159/000481610
27. Pan Z, Lin J, Wu D, et al. Hsa_circ_0006948 enhances cancer progression and epithelial-mesenchymal transition through the miR-490-3p/HMGA2 axis in esophageal squamous cell carcinoma. Aging. 2019;11(24):11937–11954. doi:10.18632/aging.102519
28. Cai X, Nie J, Chen L, Yu F. Circ_0000267 promotes gastric cancer progression via sponging MiR-503-5p and regulating HMGA2 expression. Mol Genet Genomic Med. 2020;8(2):e1093. doi:10.1002/mgg3.1093
29. Wang Z, Wang P, Cao L, et al. Long intergenic non-coding RNA 01121 promotes breast cancer cell proliferation, migration, and invasion via the miR-150-5p/HMGA2 axis. Cancer Manag Res. 2019;11:10859–10870. doi:10.2147/CMAR.S230367
30. Jiao D, Liu Y, Tian Z. microRNA-493 inhibits tongue squamous cell carcinoma oncogenicity via directly targeting HMGA2. Onco Targets Ther. 2019;12:6947–6959. doi:10.2147/OTT.S210567
31. Ma S, Fu T, Zhao S, Gao M. MicroRNA-34a-5p suppresses tumorigenesis and progression of glioma and potentiates Temozolomide-induced cytotoxicity for glioma cells by targeting HMGA2. Eur J Pharmacol. 2019;852:42–50. doi:10.1016/j.ejphar.2019.03.005
32. Kaur H, Ali SZ, Huey L, et al. The transcriptional modulator HMGA2 promotes stemness and tumorigenicity in glioblastoma. Cancer Lett. 2016;377(1):55–64. doi:10.1016/j.canlet.2016.04.020
33. Liu X, Yidayitula Y, Zhao H, Luo Y, Ma X, Xu M. LncRNA LINC00152 promoted glioblastoma progression through targeting the miR-107 expression. Environ Sci Pollut Res Int. 2018;25(18):17674–17681. doi:10.1007/s11356-018-1784-x
34. Liu B, Pang B, Hou X, et al. Expression of high-mobility group AT-hook protein 2 and its prognostic significance in malignant gliomas. Hum Pathol. 2014;45(8):1752–1758. doi:10.1016/j.humpath.2014.02.028
35. Zhang S, Zhang H, Yu L. HMGA2 promotes glioma invasion and poor prognosis via a long-range chromatin interaction. Cancer Med. 2018;7(7):3226–3239. doi:10.1002/cam4.1534
36. Jia WQ, Zhu JW, Yang CY, et al. Verbascoside inhibits progression of glioblastoma cells by promoting Let-7g-5p and down-regulating HMGA2 via Wnt/beta-catenin signalling blockade. J Cell Mol Med. 2020;24(5):2901–2916. doi:10.1111/jcmm.14884
37. Lulli V, Buccarelli M, Ilari R, et al. Mir-370-3p impairs glioblastoma stem-like cell malignancy regulating a complex interplay between HMGA2/HIF1A and the oncogenic long non-coding RNA (lncRNA) NEAT1. Int J Mol Sci. 2020;21(10):3610. doi:10.3390/ijms21103610
38. Bouatia-Naji N, Marchand M, Cavalcanti-Proenca C, et al. Smallness for gestational age interacts with high mobility group A2 gene genetic variation to modulate height. Eur J Endocrinol. 2009;160(4):557–560. doi:10.1530/EJE-08-0794
39. Takeshita H, Fujihara J, Soejima M, et al. Confirmation that SNPs in the high mobility group-A2 gene (HMGA2) are associated with adult height in the Japanese population; wide-ranging population survey of height-related SNPs in HMGA2. Electrophoresis. 2011;32(14):1844–1851. doi:10.1002/elps.201100128
40. Cheng J, Zhuo Z, Yang L, et al. HMGA2 gene polymorphisms and Wilms tumor susceptibility in Chinese children: a four-center case-control study. Biotechnol Appl Biochem. 2020;67(6):939–945. doi:10.1002/bab.1857
41. Liu J, Hua RX, Cheng Y, et al. HMGA2 gene rs8756 A>C polymorphism reduces neuroblastoma risk in Chinese Children: a four-center case-control study. Onco Targets Ther. 2020;13:465–472. doi:10.2147/OTT.S229975
42. Li L, Zhuo Z, Yang Z, et al. HMGA2 polymorphisms and hepatoblastoma susceptibility: a five-center case-control study. Pharmgenomics Pers Med. 2020;13:51–57. doi:10.2147/PGPM.S241100
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
