Back to Journals » Nature and Science of Sleep » Volume 13
Association of Craniofacial and Upper Airway Morphology with Cardiovascular Risk in Adults with OSA
Authors Zhang L, Zhang X, Li YM , Xiang BY, Han T, Wang Y, Wang C
Received 5 August 2021
Accepted for publication 20 September 2021
Published 1 October 2021 Volume 2021:13 Pages 1689—1700
DOI https://doi.org/10.2147/NSS.S332117
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Li Zhang,1– 3,* Xiaolei Zhang,1,2,*,3– 5 Yi Ming Li,1,2 Bo Yun Xiang,1,2 Teng Han,1,2 Yan Wang,1,2 Chen Wang1– 5
1Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, China-Japan Friendship Hospital, Beijing, People’s Republic of China; 2National Clinical Research Center for Respiratory Diseases, Beijing, People’s Republic of China; 3Peking University Health Science Center, Beijing, People’s Republic of China; 4Capital Medical University, Beijing, People’s Republic of China; 5The Graduate School of Peking Union Medical College, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaolei Zhang; Chen Wang
Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, China-Japan Friendship Hospital, 2 Yinghua Dongjie, Chaoyang District, Beijing, 100029, People’s Republic of China
Tel/Fax +86-10-8420-6380
Email [email protected]; [email protected]
Background and Objective: Clinical and population-based studies have demonstrated a strong association between obstructive sleep apnea (OSA) and cardiovascular disease (CVD). Anatomical abnormalities of the craniofacial region and upper airway are important risk factors for OSA. The objective of this study was to investigate the association of craniofacial and upper airway morphology with CVD risk biomarkers.
Methods: One hundred and sixty-nine male patients with OSA underwent in-laboratory polysomnography (PSG) and upper airway computed tomography (CT) scanning. Ten-year Framingham CVD risk score (FRS) was calculated and categorized into low- and moderate-to-high-risk groups. N-terminal pro B-type natriuretic peptide (NT-proBNP) was measured as a biomarker of increased myocardial wall stress.
Results: Compared to the low-risk group, total sleep time (TST), the proportion of N3 (N3%) and mean oxygen saturation (SpO2mean) were lower, while the arousal index of non-rapid eye movement (NREM) sleep, apnea index (AI) of NREM sleep, apnea hypopnea index (AHI) of NREM sleep, oxygen desaturation index (ODI) and percentage of total sleep time spent with oxyhemoglobin saturation below 90% (TST90) were higher in the moderate-to-high risk group. The corrected upper airway length (UAL), ANB angle and gonion-gnathion-hyoid angle were larger for subjects in the moderate-to-high risk group than those in the low-risk group. In multiple regression analysis, TST, AINREM and adjusted UAL were independently associated with moderate-to-high CVD risk. Plasma NT-proBNP levels were higher in patients in the moderate- to high-risk group, and among the PSG and CT scan parameters, only SPO2mean was marginally associated with NT-proBNP (r=0.183, P=0.054).
Conclusion: Craniofacial and upper airway features may contain valid cues about CVD risk, and sleep duration, obstructive event type and occurrence phase may be closely related to CVD risk for patients with OSA.
Keywords: craniofacial and upper airway morphology, obstructive sleep apnea, Framingham cardiovascular risk, N-terminal pro B-type natriuretic peptide
Introduction
Obstructive sleep apnea (OSA) is a nocturnal physiological stressor with the pathophysiological characteristics of intermittent hypoxia, sleep fragmentation, autonomic nervous system disturbances and wide intrathoracic pressure swings.1 The alterations in sympathetic activation that occur during sleep persist during wakefulness. Studies have shown that OSA increases the upregulation of markers of systemic inflammation and prothrombotic markers, the same markers associated with an increased cardiovascular or atherogenic risk.2,3 An increasing body of evidence has demonstrated that OSA is associated with increased cardiovascular risk. OSA is highly prevalent among patients with cardiovascular disease (CVD), and several large population-based cohort studies have found that patients with both conditions have worse clinical outcomes, including an increased risk of cardiovascular and cerebrovascular morbidity and mortality; however, the precise mechanisms are not fully established.4–7
OSA is a complex and heterogeneous disease with different subtypes and a multifactorial background, among which anatomical limitations are one of the most important risk factors, although no specific craniofacial phenotype for patients with OSA has been identified.8,9 Craniofacial skeletal dimensions interact with upper airway soft-tissue sizes to contribute to the patency of the upper airway. Studies have found that the craniofacial and upper airway features are correlated with the severity of OSA.10,11 In addition, several recent studies reported that some facial features, including perceived age, facial adiposity and shapes, were associated with human cardiovascular health among the general population and among subjects suspected of having coronary artery disease (CAD).12–14 Several small sample size studies reported that some craniofacial features of OSA patients with myocardial infarction or heart failure were different from those without these CVDs.15,16 Goldner et al found that children with congenital heart disease showed different craniofacial growth compared with patients without heart disease.17 In light of these findings, it is plausible that some craniofacial anatomic features may be associated with OSA-related CVD risk.
The Framingham risk score (FRS), which was originally developed based on data from the Framingham Heart Study, is a widely used algorithm to estimate the 10-year cardiovascular risk of an individual. Recent studies have used FRS for CVD risk evaluation for patients with OSA.18 N-terminal pro B-type natriuretic peptide (NT-proBNP) has been identified as a biomarker of increased myocardial wall stress in the context of OSA in the general population.19 There is growing evidence that primary prevention and early intervention will reduce the risk for new-onset CVD in otherwise asymptomatic persons. Current clinical guidelines stress risk assessment as the key to the selection of individuals for primary prevention. In the current study, we aimed to investigate the association of craniofacial and upper airway morphology with these CVD risk biomarkers (FRS and NT-proBNP) in adult patients with OSA, which may be useful for patient selection for CVD primary prevention and early intervention.
Materials and Methods
Study Population
One hundred and sixty-nine eligible Chinese male patients who demonstrated an apnea-hypopnea index (AHI) >5 events per hour overnight in laboratory polysomnography (PSG) from January 2018 to November 2020 were prospectively enrolled. Subjects with the following characteristics were excluded from the study: (1) age <18 years or >70 years; (2) pre-existing CVD, including coronary insufficiency, myocardial infarction, strokes, peripheral artery disease, and heart failure; (3) other significant comorbidities or diseases such as severe pulmonary disease, chronic kidney or liver disease, and cancer; (4) the use of lipid-lowering medication; (5) acceptance of noninvasive positive airway pressure treatment during the past 6 months; and (6) a history of craniofacial or upper airway surgery. This study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent, and the Institutional Review Board of China-Japan Friendship Hospital approved the protocol.
Polysomnography (PSG) Study and Sleep Questionnaire
Full overnight PSG (Alice 6, Philips Respironics, Murrysville, Pennsylvania, United States) was performed in a sleep center. All data were analyzed by experienced technologists according to the 2012 American Academy of Sleep Medicine criteria.20 The Epworth Sleepiness Scale (ESS) was measured based on previous publications, with a score greater than 10 indicating excessive daytime sleepiness.
Upper Airway Computed Tomography (CT) Scanning
All subjects underwent a multidetector computed tomography (MDCT) scan from the level of the calvaria to the top of the sternum using a 16-slice scanner (Light speed, GE Medical Systems). Scanning was performed at the end of the quiet tidal inspiration with the subject awake and supine, with a neutral head position. Because flexion or extension of the neck may influence the measurements of the upper airway and there is no validated head position for upper airway evaluation in the supine position, this neutral position was defined by aligning the Frankfurt plane, a plane from the inferior margin of the orbit to the superior portion of the external auditory meatus, perpendicular to the scanning table as described in previous studies.21,22 Patients were instructed to refrain from movement or swallowing. The scans were acquired at a 0.5 mm collimation/interval and were reconstructed at a 1 mm thickness/interval, with 120 kV, 100 mA, and a rotation time of 0.5 s. Axial and sagittal image reconstructions were performed to allow for linear and area measurements using an advantage workstation, version 4.5 (GE Health care). All measurements were performed by a single investigator who was blinded to the clinical status of the patients and was under the guidance of a specialist in orthodontics.
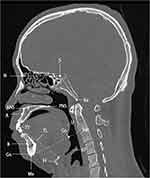
The following landmarks were identified on the midline sagittal reconstruction as described in previous studies21,23 (Figure 1): point A, point B, anterior nasal spine (ANS), posterior nasal spine (PNS), nasion (N), basion (Ba), gonion (Go), menton (Me), gnathion (Gn), center of the sella turcica (S), hyoid bone (H), the tip of the tongue (TT), epiglottis (Ep), uvula (U). Based on these landmarks, the following measurements were made: cranial base length (NS, distance between N and S), cranial base angle (NSBa, angle formed by the sella-nasion-basion), lower anterior face height (LAFH, distance between ANS and Me), total anterior face height (TAFH, distance between N and Me), face width (distance between the left tragion and right tragion), SNA (angle formed by the sella-nasion-point A), SNB (angle formed by sella-nasion-point B), ANB (angle formed by point A-nasion-point B), hard palate length (HPL, distance between ANS and PNS), MPH (vertical distance from the hyoid bone to the mandibular plane), Go-Gn-H (angle formed by the gonion-gnathion-hyoid bone), tongue length (TL, the length from the anterosuperior point of the hyoid to TT), soft palate length (SPL, the length from PNS to the tip of U), maximum thickness of the soft palate (SPT-max, the maximum thickness of the soft palate on the midsagittal plane), upper airway length (UAL, length from the hard palate to the epiglottis), and corrected airway length (UAL/height). The tongue area (TA) was obtained by tracing the contours of the tongue on the axial plane using the area measurement method. The measurements of the cross-sectional area (CSA) of the region of interest were made as follows: CSA at the level of the hard palate (HP), the minimum CSA of the retropalatal region (RP, from the inferior border of the hard palate to the terminal of the uvula), the minimum CSA of the retroglossal region (RG, from the terminal of the uvula to the superior border of the epiglottis), and CSA at the level of the free margin of the epiglottis.
FRS Calculation and Biochemistry Tests
For estimation of the specific atherosclerotic CVD risk within the next 10 years, we utilized a point system based on the Framingham Heart Study and updated the NCEP guidelines. FRS was categorized as low (<10%), moderate (10–20%), or high (20%), and the CVD FRS risk was defined as dichotomous variables: low risk and moderate-to-high risk in this study. The NT-proBNP and lipid profiles were determined on the morning of fasting after the PSG study.
Statistical Analysis
Comparisons of continuous variables between groups were determined by the unpaired t-test, and the chi-square test or Fisher exact test was used for categorical data. Univariate logistic regression analysis was used to evaluate the association between demographic, PSG, craniofacial and upper airway CT scan parameters and the CVD FRS risk. Variables with P < 0.10 according to the bivariate analysis were selected for the multivariate regression analysis. Pearson correlation coefficients were used to determine the association of the potentially relevant factors with the level of NT-proBNP. The mediating effect of the severity of OSA between the craniofacial features and the CVD risk was examined by mediation analysis. A value of P < 0.05 was considered statistically significant. All analyses were conducted with SPSS 22.0 (SPSS Inc., Chicago, Illinois, United States).
Results
Basic Demographic and Clinical Characteristics
One hundred sixty-nine subjects were enrolled in the study, with 101 subjects in the low and 68 in the moderate-to-high CVD risk group according to FRS. The basic demographic features, including age, body mass index (BMI), neck circumference (NC), waist circumference (WC), hip circumference (HC), ratio of waist-to-hip (WHR), and ESS, were similar between the two groups. Compared with the low CVD risk group, subjects in the moderate-to-high CVD risk group were more hypertensive in systolic blood pressure. Plasma levels of total cholesterol, low-density lipoprotein cholesterol (LDL-c) and NT-proBNP were higher in patients in the moderate- to high-risk group (Table 1).
 |
Table 1 Basic Demographic and Clinical Characteristics |
PSG Characteristics
Total sleep time (TST), the proportion of N3 (N3%) and mean oxygen saturation (SpO2mean) were lower, while the arousal index of NREM sleep, apnea index (AI) of NREM sleep, apnea hypopnea index (AHI) of NREM sleep, oxygen desaturation index (ODI) and percentage of total sleep time spent with oxyhemoglobin saturation below 90% (TST90) were higher in the moderate-to-high CVD risk group than in the low-risk group (Table 2).
 |
Table 2 Polysomnography Parameters |
Upper Airway and Craniofacial CT Measurements
The corrected upper airway length, ANB angle and Go-Gn-H angle were larger for subjects in the moderate-to-high CVD risk group than for those in the low-risk group. Other CT measurement parameters were similar between the two groups (Table 3).
 |
Table 3 Upper Airway and Craniofacial CT Measurements |
Correlates of Moderate-to-High CVD Risk and NT-proBNP
Among the basic demographic and PSG parameters, TST, N3%, arousal index in NREM sleep, AHINREM, AINREM, ODI, SpO2mean and TST90 were associated with moderate-to-high CVD risk according to the unadjusted analysis (Table 4). Among the craniofacial and upper airway CT scan parameters, ANB angle, adjusted upper airway length and Go-Gn-H angle were associated with moderate-to-high CVD risk according to the unadjusted analysis (Table 5). In multiple regression analysis, TST, AINREM and adjusted upper airway length were found to be independently associated with moderate-to-high CVD risk (Tables 4 and 5). Mediation analyses were performed to investigate the potential interrelationship among adjusted upper airway length, AINREM and FRS, and a partial mediating effect of AINREM was observed in the association between adjusted upper airway length and FRS for patients with OSA (Table 6).
 |
Table 4 Possible Demographic and Polysomnographic Predictors of Moderate-to-High CVD Risk |
 |
Table 5 Possible Craniofacial and Upper Airway Morphologic Predictors of Moderate-to-High CVD Risk |
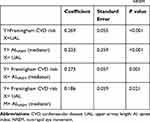 |
Table 6 Mediation Analysis of Associations with Upper Airway Length and Framingham CVD Risk Score Mediated by AINREM |
Among the PSG and CT scan parameters, only SPO2mean was marginally associated with plasma NT-proBNP levels (r=0.183, P=0.054).
Discussion
Little is known about the association of craniofacial and upper airway features and CVD risk among patients with OSA. In this study, computed tomography (CT) scanning was used to measure the craniofacial skeletal and soft tissue structures of the upper airway, and FRS and NT-proBNP were used to assess CVD risk. The main findings of the current study indicate the following. First, despite a similar age, BMI and body fat distribution, patients with moderate to high Framingham CVD risk had more retrusion of the mandible, lower placement of the hyoid bone and increased length of the upper airway compared with patients with low CVD risk. Upper airway length was associated with the Framingham CVD risk, irrespective of the severity of OSA, highlighting the independent contribution of upper airway length to CVD risk among male patients with OSA. Second, among the PSG parameters, TST and AINREM were associated with future CVD risk. Third, among the craniofacial and upper airway features and PSG parameters, only SpO2mean was marginally associated with plasma NT-proBNP levels.
The anatomical relationship of the craniofacial skeletal and soft tissue structures of the upper airway determines its patency. Craniofacial disharmony is an important predisposing factor in the development of OSA. A previous study found that the human face contains valid cues for physiological measures relevant to cardiovascular health, including BMI, percentage body fat, and blood pressure.13 Inoshita et al studied the craniofacial anatomical features of 17 OSA patients with heart failure (HF) and 34 OSA patients without HF matched for age, BMI, and obstructive AHI and found that OSA patients with HF have a greater tongue area than those without HF.16 Davoudmanesh et al compared the cephalometric variables between 6 OSA patients with myocardial infarction (MI) and 56 OSA patients without MI who were similar in terms of age, gender, and neck and abdominal circumferences, and BMI, and found that OSA patients with MI showed a significantly larger tongue length.15 In the current study, we found that compared with patients with low CVD risk, patients with moderate to high Framingham CVD risk had more retrusion of the mandible (larger ANB angle), lower placement of the hyoid bone (larger Go-Gn-H angle) and increased length of the upper airway, and upper airway length was independently associated with moderate to high CVD risk, which suggests that the cephalometric features may contain some valid information for identifying CVD risk and poor cardiovascular health among patients with OSA.
There is a close interrelationship between chronic upper airway function and craniofacial development and structure. Craniofacial anatomy disorders are one of the most important causes of obstruction of airflow. Chronic mouth breathing during childhood, which is associated with upper airway obstruction, can adversely affect craniofacial development, which can further worsen upper airway obstruction. The development of pulmonary hypertension and right heart dysfunction from chronic upper airway obstruction is complex. Hypoxemia and hypercarbia-induced respiratory acidosis are potent mediators of pulmonary vasoconstriction that can lead to reversible and irreversible chronic changes in the pulmonary vasculature and promote pulmonary hypertension and right ventricular dysfunction, with consequent impairment of systemic cardiac output. Studies have found that surgical treatment of chronic airway obstruction in most patients results in an improvement in at least one cardiovascular or cerebrovascular outcome measure, including pulmonary and systemic blood pressure, blood lipids, cardiac ejection fractions and biochemical markers associated with adverse cardiovascular and/or cerebrovascular outcomes.24 In addition, lineage studies found that pharyngeal mesoderm (PM) progenitors contribute to both pharyngeal muscle and heart progenitors (the second heart field, SHF). SHF plays critical roles in the development of the arterial pole of the heart and craniofacial morphogenesis.25 An important clinical correlate is that perturbation of the cardio craniofacial developmental field results in linked cardiac and craniofacial defects, as seen in human genetic syndromes such as DiGeorge syndrome.26 The crosstalk between craniofacial development and cardiac health deserves further investigation.
The normal sleep-wake cycle is characterized by diurnal variations in blood pressure, heart rate, and cardiac events due to the fluctuation of autonomic nervous system tone. OSA disrupts this normal sleep-heart interaction and has been associated with multiple adverse cardiovascular effects, including hypertension, ischemic heart disease, stroke, and arrhythmias. However, this link has not been proven by randomized controlled trials.27 One important explanation may be that the widely used AHI is not sufficiently accurate to describe the full picture of OSA. In the present study, we found that the AI of NREM sleep (but not the mean AHI of total sleep time) was independently associates with the FRS for CVD risk. Previous studies examining the effect of the sleep state on cardiovascular changes during obstructive respiratory events have yielded inconclusive results. Jelic et al reported no differences in the BP and HR profiles over the course of an obstructive event between different sleep states.28 Garpestad and Okabe reported a greater post-event BP elevation during REM in adult OSA patients,29,30 and Pirzada reported that patients with REM-OSA are at an increased risk of cardiometabolic complications.31 However, O’Driscoll et al found that the changes in HR and BP during obstructive events were more pronounced during NREM sleep and speculated that the large surge in cardiovascular activity post-event was related to the increased ventilatory response to hypoxia and the resultant large inspiratory effort and a greater fluctuation of intrathoracic pressure in NREM.32 In addition, similar to a previous study among nonobese Asian patients with OSA,33 we found that only the more severe form of obstructive events (apnea rather than hypopnea) was associated with the occurrence of cardiovascular-related diseases. Calcaianu et al found that a higher frequency of apneic events (AI/AHI) is associated with increased levels of troponin-I and NT-proBNP in patients with acute coronary syndrome (ACS).34 The association between apneic events and future CVD risk may be due to apnea leading to more severe SpO2 desaturation than hypopnea. Additionally, compared with hypopnea, increased inspiratory efforts against a completely closed airway during apnea create greater negative intrathoracic pressures and elevated venous return, which in turn overloads the right ventricle and increases the afterload on the left ventricle. Therefore, the event type and occurrence phase should be considered when estimating the severity of OSA and the associated long-term cardiovascular effect.
OSA can be complicated by a decreased sleep duration, which may be a consequence of OSA or an associated primary insomnia, and these patients are often more severely affected than those with a normal sleep duration. However, few data have addressed OSA, objective sleep duration, and the prevalent cardiovascular risk factors, and the findings are not consistent. The results from the ELSA-Brasil Study found that short sleep duration was not associated with prevalent cardiometabolic risk factors.35 It is worth noting that this investigation comprises a large cohort of adult participants who were not referred to sleep studies. In our study, the moderate to high CVD risk group showed worse polysomnographic markers of sleep quality (decreased TST, N3 sleep, and increased arousal index), and TST was independently associated CVD risk in patients with OSA. Consistent with our findings, André et al found that lower TST was independently associated with cardiovascular comorbidities among patients suffering from moderate/severe OSA.36 Another study among referral patients with OSA also demonstrated that objective short sleep duration was associated with hypertension.37 Several intermediate pathophysiological mechanisms have been reported to be involved in short sleep times, such as changes in the autonomic nervous system (ANS) with global sympathetic overactivity, inflammatory responses, oxidative stress, endothelial dysfunction, atherosclerosis, insulin resistance, and a prothrombotic state. Of note, currently, most of the clinical evidence comes from cross-sectional studies, so a causal inference between decreased sleep duration and the risk of cardiovascular disease has not been confirmed. Further studies with longitudinal designs are warranted to delineate the temporal association between objective sleep duration and CVD risk and to determine whether interventions to optimize sleep time may also contribute to a lower CVD risk among patients with OSA.
N-terminal pro B-type natriuretic peptide (NT-proBNP) is a cardiac hormone with vasodilatory and diuretic cardiac properties secreted in response to increased ventricular preload and myocardial wall stress. Ljunggren et al found an association between higher BNP levels and the presence and increased severity of OSA among normal weight women.38 However, similar to most studies, we did not find an association between NT-proBNP and AHI. One possible reason is that NT-proBNP concentrations are generally low in patients with OSA, and the effect of cardiac stress may be diluted by the BNP-lowering effect of obesity for most overweight OSA patients. In the current study, only SpO2mean was marginally associated with the level of NT-proBNP. In line with our study, Sanchez et al did not find an association between NT-proBNP and AHI. However, there was a significant correlation between a higher NT-proBNP level and a higher percentage of total sleeping time spent with an oxygen saturation of less than 90%.39 Whether NT-proBNP is more closely related to hypoxia-induced oxidative stress among patients with OSA warrants further study.
Several limitations of this study should be mentioned. First, upper airway imaging assessment is still challenging despite advances in newer imaging technologies. MDCT provides a host of reliable information on both craniofacial skeletal and soft tissue and is considered to be more cost-effective and less demanding. Unlike cephalometry, supine MDCT does not have a validated head position, and the neck position may influence the upper airway dimensions;22 therefore, the patients in the current study were positioned in a relatively fixed head position to standardize the scanning procedure. Additionally, the scan interpretation errors were reduced by the main investigator reviewing the study and communicating with the orthodontist. However, procedure errors and measurement errors could still occur during the imaging and interpretation process, which may be further minimized by validation of the operating specifications and their combination with new image analysis technology. In addition, compared with new dynamic imaging techniques, static upper airway CT cannot assess the dynamic structural changes and movement of the airway, which may provide more accurate information about the obstruction sites. Second, ethnic differences exist in craniofacial characteristics and the anatomic predisposition to the presence and severities of OSA. Compared to Caucasians with OSA, craniofacial and local soft-tissue restriction may play a more important role than general obesity among Asian patients.40,41 In addition, the CVD risk is also influenced by local cultural and environmental factors, which are closely associated with ethnicity. Our study population was comprised of male OSA patients from a tertiary referral hospital in China, and these patients may be in many ways different from the general patient population; thus, our findings could not be generalized to patients with OSA of different ethnicities and phenotypes. Third, the FRS is based on findings from the Framingham Heart Study and it has been validated in European Americans and African Americans. Despite its widespread popularity, its validity needs to be further verified in populations other than the US population, eg, the Chinese population. Plasma NT-proBNP levels are generally low and varied in a small range in OSA patients without overt cardiovascular disease. In addition, the current study was cross-sectional, and the results need to be verified by follow-up data. Therefore, prospective longitudinal studies are required to establish the clinical role of these biomarkers for characterization of the cardiovascular risk profile of OSA among subjects from large multiethnic populations. Last, one night PSG in a sleep center cannot preclude the first night effect; however, it provides more objective information on sleep structure and duration compared with the more commonly used questionnaires.
Conclusions
This study was the first to reveal that some craniofacial and upper airway morphology features, such as upper airway length, may be associated with the general 10-year CVD risk independent of the severity of OSA. Decreased sleep duration and AI during NREM sleep might also contribute to the CVD risk for patients with OSA. According to our current findings, it may be reasonable to pay attention to craniofacial features, sleep duration, and the type and occurrence stage of obstructive events during CVD risk assessment and patient selection for primary prevention among patients with OSA.
Abbreviations
AASM, American Academy of Sleep Medicine; AI, apnea index; AHI, apnea hypopnea index; ANB, angle measurement from point A to the nasion to point B; BMI, body mass index; CSA, cross sectional area; DBP, diastolic blood pressure; ESS, Epworth Sleepiness Scale; FRS, Framingham risk score; Go-Gn-H, angle formed by gonion-gnathion-hyoid bone; HC, hip circumference; HI, hypopnea index; HP, hard palate; HPL, hard palate length; IHD, ischemic heart disease; LAFH, lower anterior face height; MPH, distance from the hyoid to the mandibular plane; NC, neck circumference; NREM, non-rapid eye movement; NT-proBNP, N-terminal pro B-type natriuretic peptide; ODI, oxygen desaturation index; REM, rapid eye movement; RG, retroglossal region; RP, retropalatal region; SBP, systolic blood pressure; SNA, angle measurement from the sella to the nasion to point A; SNB, angle measurement from the sella to the nasion to point B; SpO2, blood oxygen saturation; SPL, soft palate length; SPT-max, maximum thickness of the soft palate; TA, tongue area; TAFH, total anterior face height; TL, tongue length; TST, total sleep time; TST90, percentage of total sleep time spent with oxyhemoglobin saturation below 90%; UAL, upper airway length; WC, waist circumference; WHR, ratio of waist-to-hip.
Acknowledgments
This work was supported by research grants from the clinical and translational medicine research Project (2020-I2M-C&T-B-094) from the Chinese Academy of Medical Sciences. We would like to thank Dr. L.L. Ma in the Stomatology department, China Japan friendship hospital, Beijing, China, for her assistance and guidance in craniofacial measurement.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Ryan S, Cummins EP, Farre R, et al. Understanding the pathophysiological mechanisms of cardiometabolic complications in obstructive sleep apnoea: towards personalized treatment approaches. Eur Respir J. 2020;56(2):1902295. doi:10.1183/13993003.02295-2019
2. Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland family study. Am J Respir Crit Care Med. 2010;182(6):826–833. doi:10.1164/ajrccm.201001-0020OC
3. Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013;9(10):1003–1012. doi:10.5664/jcsm.3070
4. Tietjens JR, Claman D, Kezirian EJ, et al. Obstructive sleep apnea in cardiovascular disease: a review of the literature and proposed multidisciplinary clinical management strategy. J Am Heart Assoc. 2019;8(1):e010440. doi:10.1161/jaha.118.010440
5. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi:10.1056/nejm200005113421901
6. Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. doi:10.1161/circulation.109.901801
7. Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi:10.1164/ajrccm.200911-1746OC
8. Brennan LC, Kirkham FJ, Gavlak JC. Sleep-disordered breathing and comorbidities: role of the upper airway and craniofacial skeleton. Nat Sci Sleep. 2020;12:907–936. doi:10.2147/nss.S146608
9. Osman AM, Carter SG, Carberry JC, Eckert DJ. Obstructive sleep apnea: current perspectives. Nat Sci Sleep. 2018;10:21–34. doi:10.2147/nss.S124657
10. Ito E, Tsuiki S, Maeda K, Okajima I, Inoue Y. Oropharyngeal crowding closely relates to aggravation of OSA. Chest. 2016;150(2):346–352. doi:10.1016/j.chest.2016.03.005
11. Liu SY, Huon LK, Lo MT, et al. Static craniofacial measurements and dynamic airway collapse patterns associated with severe obstructive sleep apnoea: a sleep MRI study. Clin Otolaryngol. 2016;41(6):700–706. doi:10.1111/coa.12598
12. Lin S, Li Z, Fu B, et al. Feasibility of using deep learning to detect coronary artery disease based on facial photo. Eur Heart J. 2020;41(46):4400–4411. doi:10.1093/eurheartj/ehaa640
13. Stephen ID, Hiew V, Coetzee V, Tiddeman BP, Perrett DI. Facial shape analysis identifies valid cues to aspects of physiological health in Caucasian, Asian, and African populations. Front Psychol. 2017;8:1883. doi:10.3389/fpsyg.2017.01883
14. Christoffersen M, Tybjærg-Hansen A. Visible aging signs as risk markers for ischemic heart disease: epidemiology, pathogenesis and clinical implications. Ageing Res Rev. 2016;25:24–41. doi:10.1016/j.arr.2015.11.002
15. Davoudmanesh Z, Bayat M, Abbasi M, Rakhshan V, Shariati M. Cephalometric risk factors associated with myocardial infarction in patients suffering from obstructive sleep apnea: a pilot case-control study. Cranio. 2017;35(1):15–18. doi:10.1080/08869634.2016.1169615
16. Inoshita A, Kasai T, Takahashi M, et al. Craniofacial anatomical risk factors in men with obstructive sleep apnea and heart failure: a pilot study. Sleep Breath. 2014;18(2):439–445. doi:10.1007/s11325-013-0906-4
17. Goldner MT, Martins e Martins M, Quintão CC, Mendes AM. Craniofacial characteristics of patients with heart disease. Am J Orthod Dentofacial Orthop. 2009;136(4):554–558. doi:10.1016/j.ajodo.2007.10.056
18. D’Agostino RB
19. Maeder MT, Mueller C, Schoch OD, Ammann P, Rickli H. Biomarkers of cardiovascular stress in obstructive sleep apnea. Clin Chim Acta. 2016;460:152–163. doi:10.1016/j.cca.2016.06.046
20. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
21. Schorr F, Kayamori F, Hirata RP, et al. Different craniofacial characteristics predict upper airway collapsibility in Japanese-Brazilian and white men. Chest. 2016;149(3):737–746. doi:10.1378/chest.15-0638
22. Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1673–1689. doi:10.1164/ajrccm.152.5.7582313
23. Neelapu BC, Kharbanda OP, Sardana HK, et al. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: a systematic review and meta-analysis of cephalometric studies. Sleep Med Rev. 2017;31:79–90. doi:10.1016/j.smrv.2016.01.007
24. Halle TR, Oh MS, Collop NA, Quyyumi AA, Bliwise DL, Dedhia RC. Surgical treatment of OSA on cardiovascular outcomes: a systematic review. Chest. 2017;152(6):1214–1229. doi:10.1016/j.chest.2017.09.004
25. Tzahor E, Evans SM. Pharyngeal mesoderm development during embryogenesis: implications for both heart and head myogenesis. Cardiovasc Res. 2011;91(2):196–202. doi:10.1093/cvr/cvr116
26. Harel I, Maezawa Y, Avraham R, et al. Pharyngeal mesoderm regulatory network controls cardiac and head muscle morphogenesis. Proc Natl Acad Sci U S A. 2012;109(46):18839–18844. doi:10.1073/pnas.1208690109
27. McEvoy RD, Antic NA, Heeley E, et al.; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi:10.1056/nejm1606599
28. Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep. 2002;25(8):850–855.
29. Garpestad E, Ringler J, Parker JA, Remsburg S, Weiss JW. Sleep stage influences the hemodynamic response to obstructive apneas. Am J Respir Crit Care Med. 1995;152(1):199–203. doi:10.1164/ajrccm.152.1.7599824
30. Okabe S, Hida W, Kikuchi Y, et al. Role of hypoxia on increased blood pressure in patients with obstructive sleep apnoea. Thorax. 1995;50(1):28–34. doi:10.1136/thx.50.1.28
31. Pirzada AR, BaHammam AS. Rapid eye movement predominant obstructive sleep apnoea. Curr Opin Pulm Med. 2021. doi:10.1097/MCP.0000000000000817
32. O’Driscoll DM, Foster AM, Ng ML, et al. Acute cardiovascular changes with obstructive events in children with sleep disordered breathing. Sleep. 2009;32(10):1265–1271. doi:10.1093/sleep/32.10.1265
33. Chirakalwasan N, Teerapraipruk B, Simon R, et al. Comparison of polysomnographic and clinical presentations and predictors for cardiovascular-related diseases between non-obese and obese obstructive sleep apnea among Asians. J Clin Sleep Med. 2013;9(6):553–557. doi:10.5664/jcsm.2748
34. Calcaianu G, Bresson D, Calcaianu M, et al. The Importance of apneic events in obstructive sleep apnea associated with acute coronary syndrome. Sleep Disord. 2019;2019:6039147. doi:10.1155/2019/6039147
35. Drager LF, Santos RB, Silva WA, et al. Short sleep duration, and their interactions with sleepiness and cardiometabolic risk factors in adults: the ELSA-Brasil study. Chest. 2019;155(6):1190–1198. doi:10.1016/j.chest.2018.12.003
36. André S, Andreozzi F, Van Overstraeten C, et al. Cardiometabolic comorbidities in obstructive sleep apnea patients are related to disease severity, nocturnal hypoxemia, and decreased sleep quality. Respir Res. 2020;21(1):35. doi:10.1186/s12931-020-1284-7
37. Ren R, Covassin N, Yang L, et al. Objective but not subjective short sleep duration is associated with hypertension in obstructive sleep apnea. Hypertension. 2018;72(3):610–617. doi:10.1161/HYPERTENSIONAHA.118.11027
38. Ljunggren M, Lindahl B, Theorell-Haglöw J, Lindberg E. Association between obstructive sleep apnea and elevated levels of type B natriuretic peptide in a community-based sample of women. Sleep. 2012;35(11):1521–1527. doi:10.5665/sleep.2202
39. Sánchez A, Schwartz AR, Sánchez PL, et al. Hemodynamic and inflammatory markers of sleep apnea-hypopnea syndrome and nocturnal hypoxemia: effects of treatment with nasal continuous positive airway pressure. Arch Bronconeumol. 2008;44(10):531–539. Spanish. doi:10.1157/13126833
40. Sutherland K, Lee RW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology. 2012;17(2):213–222. doi:10.1111/j.1440-1843.2011.02082.x
41. Hnin K, Mukherjee S, Antic NA, et al. The impact of ethnicity on the prevalence and severity of obstructive sleep apnea. Sleep Med Rev. 2018;41:78–86. doi:10.1016/j.smrv.2018.01.003
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.