Back to Journals » OncoTargets and Therapy » Volume 11
Association of clinicopathologic characteristics and outcomes with EZH2 expression in patients with breast cancer in East Azerbaijan, Iran
Authors Boostani F, Dolatkhah R , Fakhrjou A , Farassati F , Sanaat Z
Received 15 August 2017
Accepted for publication 9 December 2017
Published 19 January 2018 Volume 2018:11 Pages 449—457
DOI https://doi.org/10.2147/OTT.S149210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yao Dai
Farnaz Boostani,1 Roya Dolatkhah,1 Ashraf Fakhrjou,2 Faris Farassati,3 Zohreh Sanaat1
1Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran; 2Tabriz University of Medical Sciences, Tabriz, Iran; 3Midwest Biomedical Research Foundation, Kansas City, MO, USA
Background: Recently, it was found that the overexpression and mutation status of EZH2 affect cancer progression and patient outcome in several human tumors. We aimed to evaluate the clinicopathologic significance of EZH2 in patients with breast cancer.
Methods: This was an analytical descriptive study of surgical specimens of primary breast tumors. Specimens were analyzed immunohistochemically for EZH2, estrogen receptor, progesterone receptor, Ki-67, P53, and human epidermal growth factor receptor 2 (HER2) expressions. Regression analysis was performed to calculate hazard ratios (HRs) and 95% CIs. Kaplan–Meier and Cox regression models were used to estimate the overall survival (OS) and disease-free survival (DFS).
Results: We included 100 patients with breast cancer (mean age 51.05±9.54 years). The multivariate regression analysis showed that HER2-positive patients had approximately twice the levels of EZH2 expression compared with HER2-negative patients (HR 2.16, 95% CI 0.48–11.49). The likelihood of EZH2 expression was significantly higher in patients with lymph node involvement than in those without (HR 8.44, 95% CI 3.06–23.33; P≤0.05). EZH2 expression did not have any significant effect on the OS, although the mean OS in high EZH2 expression was shorter than for those with low EZH2 expression (655 vs 787 days; log-rank P=0.336). The mean DFS was 487 days for patients with high EZH2 expression compared with 908 days for those with low EZH2 expression (log-rank P=0.188).
Conclusion: There was no association found between EZH2 expression and OS and DFS in our patients. Further studies involving larger sample sizes, and conducted in different populations, are needed to validate this hypothesis.
Keywords: breast cancer, tumor markers, enhancer of zeste homolog 2 protein, survival analysis
Background
Cancer is among the leading causes of death in both developed and developing countries,1 with early detection and treatment remaining the best strategy to improve prognosis and quality of life. Although many studies have tried to identify biomarkers for these purposes in various human cancers, their success has been limited. Thus, effective novel biomarkers remain a topic of great clinical interest.2
Breast cancer is the most common malignancy and the leading cause of death from cancer among women worldwide.3 Although several prognostic markers exist for breast cancer, only a few are clinically useful in humans. Therefore, additional models and therapeutic targets are being developed to improve our understanding of the biology of human breast cancer and help with the development of novel treatment options.4 Recent advances in our knowledge of apoptosis control, cell proliferation, tumor progression, and differentiation pathways have improved our understanding of the mechanisms underlying breast cancer progression.
EZH2 is a catalytic subunit of the epigenetic regulator polycomb repressive complex 2 (PRC2) located on chromosome 7q35.5–8 PRC2, which includes EZH2 suppressor of zeste 12 (SUZ12), and embryonic ectoderm development, is responsible for trimethylating histone 3 lysine residue 27 (H3K27).4,9 Several articles have implicated EZH2 in cell proliferation, invasion, apoptosis, angiogenesis, metastasis, stem cell maintenance, drug resistance, and disease progression in cancer.2,10–12 Moreover, EZH2 overexpression and mutations have been found in a wide range of human tumors, including breast, prostate, ovarian, lung, gastric, colon, urogenital tract (including uterine, urinary bladder, renal cell carcinoma, kidney, and renal cell cancer), brain, endometrial, hematological, head and neck (squamous cell carcinoma), liver, and pancreatic biliary cancers, as well as lymphomas, melanomas, and sarcomas.2,4,9,11–15
In breast cancer, EZH2 overexpression is often associated with more advanced disease, higher histologic grade, increased tumor cell proliferation, lymph node invasion, larger tumor size, metastasis, and inferior overall survival (OS) and disease-free survival (DFS).12–14 Some study results also showed that EZH2 overexpression was associated with larger tumor size, advanced disease, and significantly reduced DFS and OS, but added that it was associated with estrogen receptor (ER)-negative and progesterone receptor (PR)-negative status.6,10,12 Although Reijm et al reported that there was no significant association between EZH2 protein expression and menopausal status, tumor histology, or PR status, they found significant positive associations with the number of lymph nodes involved, the histologic grade, and the human epidermal growth factor receptor 2 (HER2) status.13 In patients with breast cancer, Bachmann et al reported that high EZH2 expression was associated with high histologic grade, locally advanced cancer, and distant metastasis.16
The association of EZH2 expression with clinicopathologic features such as PR status, HER2 expression, nuclear grade, and proliferative index is inconsistent in the existing literature. To evaluate the clinicopathologic significance of EZH2 expression in breast cancer, we therefore examined the correlations between changes in EZH2 expression and important clinical variables (ie, ER, PR, HER2, Ki-67, P53, DFS, and OS).
Materials and methods
We analyzed the surgical specimens of primary breast tumors from patients who underwent surgery at Tabriz University of Medical Sciences, Tabriz, Iran, between April 2009 and February 2017. The study protocol was approved by the ethics committee of Tabriz University of Medical Sciences (Permit no 5/d/988072). All the patients signed a consent form during hospitalization and referring to our clinic, to allow us review their medical records and tissue sample slides, for any future studies.
In all the cases, we retrieved archived slides of breast tumor tissue stained with hematoxylin and eosin and reviewed them to confirm pathological features based on the 2012 World Health Organization classification.17 Suitable tissue blocks were identified for immunohistochemical (IHC) analysis, and we constructed tissue microarrays using the collected tissue. We performed pathologic TNM classification and staging for all the cases, using the seventh edition of the American Joint Committee on Cancer criteria.18 The histologic grade of invasive breast carcinoma was graded according to the breast carcinoma classification of the World Health Organization.17
IHC analysis
IHC staining was performed in all the cases for ER, PR, HER2, Ki-67, P53, and EZH2 biomarkers. For histologic study, tumor samples were fixed in 10% buffered formalin and embedded as paraffin blocks. The sections were stained by hematoxylin and eosin, and histologic grading was done according to the Bloom and Richardson criteria, as modified in previous studies.17–19 This grading methodology is based on the sum of the degree of tubule formation, nuclear pleomorphism, and mitotic activity.
Sections for IHC analysis were deparaffinized through graded alcohols and xylene, placed in an EDTA buffer solution (pH 9.0), and then heated to 100°C in a microwave oven at 900 W for 2–5 minutes and 180 W for 5 minutes until boiling. Then, the slides were left in the solution to cool down at room temperature for ~15 minutes and were rinsed in tris-buffered saline (pH=7.6) for 5 minutes. Endogenous peroxidase with 3% hydrogen peroxidase in methanol was added for 10 minutes to block nonspecific binding, and then the slides were incubated with the primary antibodies overnight in 4°C. The following primary antibodies were used: ER (clone ID5; Dako Denmark A/S, Glostrup, Denmark), PR (clone PgR636; Dako Denmark A/S), Ki-67 (cloneMIB-1; Dako Denmark A/S), P53 (cloneD07; Dako Denmark A/S), HER/2neu (REF: A0485, 1/200; Dako Denmark A/S). Then, the slides were incubated in EnVision for 30 minutes and, after rinsing in tris-buffered tween for 5 minutes, were incubated with chromogen for a further 5 minutes. Finally, the slides were rinsed in tris-buffered saline, counterstained with hematoxylin, and dehydrated with alcohols (96% and 100%) and xylene before being sealed with a cover slip. For EZH2 antibody, we used 1.15 diluted phosphate-buffered saline solution.
The criteria of assessment of the proportion and intensity of staining in our study was as follows:19
Score of proportion:
0=no staining
1=<1% nuclei staining
2=1%–10% nuclei staining
3=11%–33% nuclei staining
4=34%–66% nuclei staining
5=67%–100% nuclei staining
Score of intensity:
0= no staining
1= weak staining
2= moderate staining
3= strong staining.
The total score of proportion and intensity was the final score of expression. The positive EZH2 staining was in brown and within the nuclei. Low scores (range 0–4) were classified as indicating low EZH2 expression, and high scores (range 5–8) were classified as indicating high EZH2 expression (Figure 1). ER, PR, Ki-67, P53, and HER2 statuses were determined by IHC analysis.
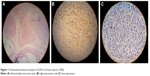  | Figure 1 Immunohistochemical analysis of EZH2 in breast cancer (×400). |
Clinical outcome assessment
After surgery, all patients were followed up every 4–6 months for the first 5 years and every 12 months thereafter. OS was defined as the time from the date of surgery to the date of death from breast cancer or the date of last follow-up if patients were still alive. DFS was measured from the date of surgery to local recurrence or distant metastasis, date of death, or the last recorded date.
Statistical analysis
Descriptive data were analyzed using IBM SPSS Version 17 (IBM Corporation, Armonk, NY, USA). Simple and multiple logistic regression analyses were performed to calculate the unadjusted and adjusted hazard ratios (HRs) with their 95% CIs. EZH2 expression was used as the dependent variable and P-values ≤0.05 were considered statistically significant in all analyses. For the survival analyses, the OS and DFS were defined as the time from date of surgery to the date of death and relapse, respectively. The Kaplan–Meier method, with the log-rank test, was used to assess statistical significance. Finally, the Cox proportional hazard method was used to evaluate the relationship between EZH2 expression and the independent variables (ie, ER, PR, Ki-67 P53, and HER2 status) on OS and DFS.
Results
We included 100 patients with breast cancer (mean age 51.05±9.54 years, range 34–75 years); their clinicopathologic characteristics are shown in Table 1. Patients received multimodal therapy, including adjuvant chemotherapy, surgery, and radiation therapy, and patients with HER2-positive tumors were also given adjuvant trastuzumab. All patients with hormone receptor-positive cancers received tamoxifen or an aromatase inhibitor.
Correlation coefficients for clinicopathologic characteristics and EZH2 expression (Spearman’s correlation analyses)
ER (r=−0.173, P=0.084), PR (r=−0.136, P=0.179), Ki-67 (r=−0.081, P=0.421), P53 (r=−0.135, P=0.181), and HER (r=0.134, P=0.183) statuses were not significantly correlated with EZH2 expression in the breast cancer tissue. Other clinicopathologic characteristics did not correlate with EZH2 expression.
Associations between EZH2 expression and clinicopathologic characteristics
The associations between EZH2 expression and clinicopathologic characteristics are shown in Table 2. High EZH2 expression was significantly correlated with lymph node status (P<0.05). Age, menopausal status, tumor size, metastatic status, histologic grade, lymphovascular invasion status, operation status, ER status, PR status, HER2 status, Ki-67 expression, and p53 expression did not correlate with EZH2 expression.
In the univariate analyses, EZH2 expression was significantly associated with lymph node status only (HR 8.44, 95% CI 3.056–23.3, P<0.05). After adjustment for prognostic factors, multivariate analysis revealed that no factors were associated with EZH2 expression (Table 3).
Survival analysis and association of EZH2 expression and patient outcome
The median follow-up period for all patients was 1,250 days. At the time of analysis, there were 8 and 13 deaths suitable for inclusion in the OS and DFS analyses, respectively. The mean OS rates of patients with high and low EZH2 expression were 655 days (95% CI 407.55–902.44) and 787.5 days (95% CI 0–1,615.6), respectively (log-rank Mantel–Cox P=0.336; Figure 2A). The mean DFS rates for high and low EZH2 expression were 487.27 days (95% CI 296.07–678.48) and 908.5 days (95% CI 362.64–1,454.36), respectively (log-rank Mantel–Cox P=0.18; Figure 2B). EZH2 expression was not associated with either the OS or the DFS (P=0.4). By contrast, Cox proportional hazard regression showed that lymphatic invasion was significantly associated with poor DFS (HR 0.007, 95% CI 0.00–0.43, P<0.05). Multivariate analysis confirmed that there was no significant association between EZH2 expression and either the DFS (HR 3.69, 95% CI 0.46–29.39, P=0.217) or the OS (HR 2.75, 95% CI 0.32–23.50, P=0.35) (Figure 2; Tables 4 and 5).
  | Figure 2 Kaplan–Meier curves showing association of EZH2 expression in patients with breast cancer. (A) Overall survival (P=0.336) and (B) disease-free survival (P=0.18). |
Discussion
In this study, we evaluated EZH2 expression in 100 breast cancer specimens, identifying that expression was high in 74 patients and low in 26 patients. Consistent with research by Reijm et al,13 we confirmed that there was no significant association between EZH2 expression and menopausal status, tumor histology, and ER, PR, Ki-67, or P53 status. In another study, however, Inari et al showed that metastatic lesions exhibited significantly higher levels of Ki-67 expression (75.0% vs 57.3%, P=0.010) and EZH2 (82.3% vs 56.3%, P<0.0001) compared with primary lesions.6 Therefore, the lack of a significant association between EZH2 and Ki-67 expression in our study may have been because most patients had nonmetastatic disease.
Consistent with the research by Reijm et al,13 the present study also showed that high EZH2 expression was associated with the lymph node status. However, this association was only significant in the univariate analysis (HR 8.44, 95% CI 3.056–23.3, P<0.05). By contrast, the associations between EZH2 and the other clinicopathologic characters did not reach statistical significance.
The potential association between EZH2 and HER2 remains controversial. Collett et al and Holm et al each found a high abundance of EZH2 in HER2-positive breast tumors.20,21 In contrast, no association was found in either this study or that by Kleer et al,22 which may be due to the small number of patients with HER2-positive tumors in our samples. This should be reviewed in a study with a larger sample size.
Recently, Bae et al and Jang et al reported that high EZH2 expression was a prognostic factor for shorter OS in patients with breast cancer.23,24 Therefore, we examined the association of EZH2 expression with OS and DFS. Although we showed that the mean OS and DFS were shorter in patients with high EZH2 expression than in patients with low EZH2 expression, there was no statistically significant association between the survival rates. This is probably due to the difference in the number of patients and the short follow-up period between our studies. Differences in the EZH2 scoring system can also contribute to this issue, indicating that there is a need to develop a uniform scoring system to help compare research in the future.
Breast cancer is the most common malignancy and the leading cause of cancer-related death among women worldwide.3,24 Moreover, based on reports published by the Iranian Cancer Registry in 2009, breast cancer was the most common cancer in females in Iran and accounted for a massive 24.6% of all cases of cancer.25 Between 2005 and 2010, there was an increase in the age-standardized incidence rate for breast cancer from 23.1 to 28.3 per 100,000 women.25,26 According to the most recent data of the Population Based Cancer Registry of East Azerbaijan, there were ~683 new breast cancer cases, with an age standardized incidence rate of 31 per 100,000 women in the East Azerbaijan province. Given the local prevalence of breast cancer, and the lack of research in Iran, our research might also illuminate the clinicopathologic characteristics of breast cancer specific to our region.
This study has some limitations. First, we analyzed a small number of cases and cannot exclude the possibility of selection bias. Second, the study had a short follow-up period for determining survival compared with other studies on this topic, making it difficult to assess the impact of EZH2 expression on disease progression and death. Third, EZH2 scoring was performed in-house, whereas previous studies have used different EZH2 scoring methods; it is therefore possible that the differences among the scoring methods accounts for the lack of comparability in some of the results. Despite these limitations, our study benefits from having evaluated a series of breast cancers from a single institution, as well as from having immunohistochemically analyzed EZH2 expression, Ki-67 proliferation, HER2 overexpression, ER status, and PR status.
Conclusion
We showed a significant association between EZH2 and lymph node status but failed to show any significant associations between EZH2 and other clinicopathologic characteristics. Also, increased EZH2 expression was not associated with poor OS or DFS in patients with breast cancer. However, to the best of our knowledge, this is the first study to have evaluated the association of EZH2 and clinicopathologic characteristics in the north west of Iran. Overall, our results indicate that EZH2 expression might be a useful marker of poor prognosis.
Acknowledgments
The authors would like to thank the Hematology and Oncology Research Center for supporting this research study (grant no 95/12). This study was conducted as part of a fellowship thesis for Dr Boostani (thesis no 94/5-9/30). Dr Robert Sykes (www.doctored.org.uk) provided technical editing services for the final drafts of this manuscript. We would like to thank Dr Morteza Ghojazadeh for his help in editing/formatting the manuscript.
Author contributions
FB provided substantial contributions to conception and design of manuscript, drafting the article, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to accuracy or integrity of any part of the work are appropriately investigated and resolved.
RD contributed to analysis and interpretation of results, revising the article critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
AF contributed to the analysis and interpretation of results, revising the article critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FF provided substantial contributions to conception and design of manuscript, revising the article critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ZS provided substantial contributions to conception and design of manuscript, drafting the article, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM, Parkin DM. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. Int J Cancer. 2013;133(3):721–729. | ||
Chen S, Huang L, Sun K, et al. Enhancer of zeste homolog 2 as an independent prognostic marker for cancer: a meta-analysis. PLoS One. 2015;10(5):e0125480. | ||
Roh S, Park SY, Ko HS, Sohn JS, Cha EJ. EZH2 expression in invasive lobular carcinoma of the breast. World J Surg Oncol. 2013;11:299. | ||
Choi HJ, Jang S, Ryu JE, et al. Significance of EZH2 expression in canine mammary tumors. BMC Vet Res. 2016;12(1):164. | ||
Noordhuis MG, Eijsink JJ, Roossink F, et al. Prognostic cell biological markers in cervical cancer patients primarily treated with (chemo)radiation: a systematic review. Int J Radiat Oncol Biol Phys. 2011;79(2):325–334. | ||
Inari H, Suganuma N, Kawachi K, et al. Expression of enhancer of zeste homolog 2 correlates with survival outcome in patients with metastatic breast cancer: exploratory study using primary and paired metastatic lesions. BMC Cancer. 2017;17(1):160. | ||
Panousis D, Patsouris E, Lagoudianakis E, et al. The value of TOP2A, EZH2 and paxillin expression as markers of aggressive breast cancer: relationship with other prognostic factors. Eur J Gynaecol Oncol. 2011;32(2):156–159. | ||
Wassef M, Michaud A, Margueron R. Association between EZH2 expression, silencing of tumor suppressors and disease outcome in solid tumors. Cell Cycle. 2016;15(17):2256–2262. | ||
Jiang T, Wang Y, Zhou F, Gao G, Ren S, Zhou C. Prognostic value of high EZH2 expression in patients with different types of cancer: a systematic review with meta-analysis. Oncotarget. 2016;7(4):4584–4597. | ||
Beca F, Kensler K, Glass B, Schnitt SJ, Tamimi RM, Beck AH. EZH2 protein expression in normal breast epithelium and risk of breast cancer: results from the Nurses’ Health Studies. Breast Cancer Res. 2017;19(1):21. | ||
Neusquen LP, Filassi JR, Fristachi CE, et al. EZH2 protein expression and tumor response to neoadjuvant chemotherapy in locally advanced breast cancer. Rev Bras Ginecol Obstet. 2016;38(6):280–286. | ||
Shen L, Cui J, Liang S, Pang Y, Liu P. Update of research on the role of EZH2 in cancer progression. Onco Targets Ther. 2013;6:321–324. | ||
Reijm EA, Timmermans AM, Look MP, et al. High protein expression of EZH2 is related to unfavorable outcome to tamoxifen in metastatic breast cancer. Ann Oncol. 2014;25(11):2185–2190. | ||
Jiang H, Gupta R, Somma J. EZH2, a unique marker of malignancy in effusion cytology. Diagn Cytopathol. 2014;42(2):111–116. | ||
Sashida G, Iwama A. Multifaceted role of the polycomb-group gene EZH2 in hematological malignancies. Int J Hematol. 2017;105(1):23–30. | ||
Bachmann IM, Halvorsen OJ, Collett K, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24(2):268–273. | ||
Tan PH, Ellis IO. Myoepithelial and epithelial-myoepithelial, mesenchymal and fibroepithelial breast lesions: updates from the WHO Classification of Tumours of the Breast 2012. J Clin Pathol. 2013;66(6):465–470. | ||
Guo S, Li X, Rohr J, et al. EZH2 overexpression in different immunophenotypes of breast carcinoma and association with clinicopathologic features. Diagn Pathol. 2016;11:41. | ||
Ellis IO, Lee AH, Pinder SE, Rakha EA. Tumors of the breast. In: Flecher CDM, editor. Diagnostic Histopathology of Tumors. Vol 2. Philadelphia: Elsevier; 2014:1057–1145. | ||
Collett K, Eide GE, Arnes J, et al. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res. 2006;12(4):1168–1174. | ||
Holm K, Grabau D, Lovgren K, et al. Global H3K27 trimethylation and EZH2 abundance in breast tumor subtypes. Mol Oncol. 2012;6(5):494–506. | ||
Kleer CG, Cao Q, Varambally S, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100(20):11606–11611. | ||
Bae WK, Yoo KH, Lee JS, et al. The methyltransferase EZH2 is not required for mammary cancer development, although high EZH2 and low H3K27me3 correlate with poor prognosis of ER-positive breast cancers. Mol Carcinog. 2015;54(10):1172–1180. | ||
Jang SH, Lee JE, Oh MH, et al. High EZH2 protein expression is associated with poor overall survival in patients with luminal a breast cancer. J Breast Cancer. 2016;19(1):53–60. | ||
Jazayeri SB, Saadat S, Ramezani R, Kaviani A. Incidence of primary breast cancer in Iran: ten-year national cancer registry data report. Cancer Epidemiol. 2015;39(4):519–527. | ||
Bray F, Ferlay J, Laversanne M, et al. Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer. 2015;137(9):2060–2071. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





