Back to Journals » Clinical Interventions in Aging » Volume 17
Association of Cardiovascular Health Metrics with Dementia in Rural Chinese Older Adults: A Population-Based Study
Authors Han X, Wang Y, Jiang Z, Li Y, Dong Y, Cong L, Hou T, Liang Y, Laukka EJ, Du Y , Qiu C
Received 3 January 2022
Accepted for publication 27 May 2022
Published 10 June 2022 Volume 2022:17 Pages 947—956
DOI https://doi.org/10.2147/CIA.S356910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Xiaolei Han,1,2,* Yongxiang Wang,2,3,* Ziying Jiang,1 Yuanjing Li,4 Yi Dong,1 Lin Cong,1– 3 Tingting Hou,1– 3 Yajun Liang,4,5 Erika J Laukka,4 Yifeng Du,1– 3 Chengxuan Qiu1,4
1Department of Neurology, Shandong Provincial Hospital, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Neurology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China; 3Shandong Provincial Clinical Research Center for Neurological Diseases, Jinan, Shandong Province, People’s Republic of China; 4Aging Research Center and Center for Alzheimer Research, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet-Stockholm University, Stockholm, Sweden; 5Department of Global Public Health, Karolinska Institutet, Stockholm, Sweden
*These authors contributed equally to this work
Correspondence: Yifeng Du, Department of Neurology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, No. 324 Jingwuweiqi Road, Jinan, Shandong, 250021, People’s Republic of China, Tel +86-531-68776354, Fax +86-531-68773274, Email [email protected]
Purpose: We explore the associations of individual and composite cardiovascular health metrics with all-cause dementia, Alzheimer’s disease, and vascular dementia among rural-dwelling older adults and the potential age variations in their associations.
Patients and Methods: This community-based cross-sectional study included 4980 older adults (age ≥ 65 years; 57.23% women) from the baseline examination of MIND-China. In March–September 2018, data were collected via face-to-face interviews, clinical examinations, and laboratory test. We defined six cardiovascular health metrics according to the modified American Heart Association’s recommendations. We diagnosed dementia and its subtypes following the international criteria. Data were analyzed using logistic regression models.
Results: Of all the participants, 250 were diagnosed with dementia, including 165 with Alzheimer’s disease and 75 with vascular dementia. Ideal composite global cardiovascular health metrics (vs poor composite metrics) were associated with a multi-adjusted odds ratio (95% confidence interval) of 0.62 (0.42– 0.93) for dementia, 0.88 (0.52– 1.48) for Alzheimer’s disease, and 0.31 (0.16– 0.60) for vascular dementia. Moreover, ideal biological cardiovascular health metrics were associated with multi-adjusted odds ratio of 0.52 (0.28– 0.95) for dementia and 0.21 (0.06– 0.77) for vascular dementia in young–old adults (65– 74 years), whereas ideal behavioral cardiovascular health metrics were associated with multi-adjusted odds ratio of 0.48 (0.26– 0.89) for dementia and 0.16 (0.06– 0.43) for vascular dementia in old–old adults (≥ 75 years).
Conclusion: Our results suggest that ideal cardiovascular health metrics are cross-sectionally associated with a low likelihood of dementia and vascular dementia among rural-dwelling older Chinese adults. The associations vary with age, components of cardiovascular health metrics, and dementia subtypes.
Keywords: cardiovascular health, dementia, Alzheimer’s disease, vascular dementia, population-based study
Introduction
Dementia has become a global epidemic driven primarily by population aging.1 Over 50 million people are living with dementia worldwide, and the number is projected to triple by 2050, unless effective preventive interventions are identified and implemented.2 In the past three decades, evidence has accumulated that modifiable cardiometabolic risk factors (eg, hypertension, dyslipidemia, diabetes, and obesity), especially occurring in midlife, and unhealthy lifestyles (eg, smoking and physical inactivity) are associated with an increased risk of dementia, especially vascular dementia (VaD).3,4 Cerebral micro- and macrovascular lesions are supposed to be the underlying mechanisms linking cardiovascular risk factors with cognitive impairment and dementia.3,5 In addition, there is a potential that dementia risk may be reduced or the onset of dementia may be delayed through maintaining cardiovascular health.6 Notably, most studies have so far investigated the association of individual cardiovascular risk factors with dementia. However, exploring the composite cardiovascular health (CVH) profiles in association with cognitive outcomes may be more meaningful than individual factors because lifestyle and cardiometabolic risk factors often coexist in older adults, and they may interact with each other to affect the risk of dementia.
In 2010, the American Heart Association (AHA) proposed composite CVH metrics that integrated seven lifestyle-related factors (Life’s Simple 7): four behavioral metrics (smoking, body mass index [BMI], diet, physical activity) and three biological metrics (blood pressure, blood glucose, and total cholesterol).7 Numerous population-based studies have consistently shown associations of ideal CVH metrics with reduced risk of coronary heart disease, stroke, and all-cause mortality,8–10 and a few studies from Europe and North America have also examined the associations of optimal CVH among middle-aged or young-old adults with dementia.11–14 The associations of composite CVH metrics with dementia and main subtypes of dementia amongolder Chinese adults have not yet been reported. This is important because CVH and cardiovascular risk profiles, as well as their association with health outcomes, may differ markedly across ethnic groups.15
In addition, evidence has shown that the associations of some cardiovascular risk factors with dementia may vary with age.16 For example, obesity and hypertension are reported to be risk factors for dementia when occurring during midlife or young-old adulthood but not necessarily in late life or old–old adults.17,18 This implies that age variations may be relevant to consider when examining the associations of composite CVH metrics with dementia.
Therefore, in this community-based cross-sectional study of rural-dwelling Chinese older adults, we sought to examine the associations of composite CVH metrics with all-cause dementia, Alzheimer’s disease (AD), and VaD while taking into account potential age variations.
Materials and Methods
Study Design and Participants
This community-based cross-sectional study used data from the baseline survey of the ongoing Multimodal Interventions to delay Dementia and disability in rural China (MIND-China), which is part of the World-Wide FINGERS Network.19,20 Baseline assessments of MIND-China targeted people who were aged ≥65 years by the end of 2017 and living in the 52 villages of Yanlou Town, Yanggu County, western Shandong Province, as previously reported.19,21 Briefly, in March–September 2018, 5246 participants (80.3% of all eligible people) were examined as the baseline survey for MIND-China. Of these, 266 were excluded due to missing data on CVH measurements (n = 220) or severe mental disease and major depressive disorders (n = 46), leaving 4980 persons for the current analysis (Supplemental Figure 1).
The MIND-China study was approved by the Ethics Committee of Shandong Provincial Hospital in Jinan, Shandong. Written informed consent was obtained from all the participants, or in the case of cognitively impaired persons, from a proxy (usually a family member). The personal identity information in the MIND-China database had been removed before the data were released for analysis to ensure complete anonymity of all data. Research within the MIND-China study has been conducted in accordance with the Declaration of Helsinki. MIND-China was registered in the Chinese Clinical Trial Registry (registration no.: ChiCTR1800017758).
Data Collection and Assessments
The trained medical staff collected data via face-to-face interviews, clinical examinations, and laboratory tests following a structured questionnaire. Data included demographics, lifestyle factors (eg, smoking, alcohol consumption, and physical activity), medical history (eg, diabetes, coronary heart disease, and stroke), and use of medications. All medications were classified and coded according to the Anatomical Therapeutic Chemical system.22 Weight and height were measured with participants wearing light clothes and without shoes. BMI was calculated as weight (kg) divided by height squared (m2). After a 5-min rest, arterial blood pressure was measured on the right upper arm in a seated position using an electronic blood pressure monitor (Omron HEM-7127J; Omron Corporation, Kyoto, Japan). We assessed physical activity via questions of frequency (eg, daily, weekly, and monthly) and time (minutes) of walking, sports activities, and recreational activities. Physical activity was quantified as time (minutes) spent per week by multiplying participation frequency with average minutes spent per time. Then, we estimated the metabolic-equivalent of each kind of activity according to the 2011 compendium of physical activities.23 Peripheral blood samples were taken after an overnight fast, and blood samples were analyzed at the certified clinical laboratory of the local town health center. Fasting blood glucose (FBG) and total cholesterol were measured using an automatic biochemical analyzer (DIRUI CS-600B; DIRUI Corporation, Changchun, China). Apolipoprotein E (APOE) genotype was analyzed using polymerase chain reaction and dichotomized as carriers versus non-carriers of the ε4 allele.
Definition of Ideal Cardiovascular Health Metrics
We defined and categorized CVH metrics according to the AHA’s recommendations,7 with some modifications as previously reported and detailed in Supplemental Table 1. Briefly, we categorized each of the 6 CVH metric components into poor (score = 0), intermediate (score = 1), and ideal (score = 2) level. The ideal levels of the 6 CVH metrics were defined as follows: (1) ideal smoking status: never or quit >5 years;24 (2) ideal BMI: <24 kg/m2, used for Chinese adults;25 (3) ideal physical activity: ≥150 min/week moderate intensity, ≥75 min/week vigorous intensity, or equivalent combination; (4) ideal total cholesterol: untreated and <5.26 mmol/L; (5) ideal blood pressure: untreated and <120/80 mm Hg; and (6) ideal fasting blood glucose: untreated and <5.60 mmol/L. The global CVH metric score was calculated as the sum of scores of the above six CVH metric components.26 In addition, smoking, physical activity, and BMI were classified as behavioral CVH metrics, and FBG, total cholesterol, and blood pressure as biological CVH metrics.26 The composite global CVH metric score was divided into 3 categories: poor (score ≤5, reference), intermediate (6–7), and ideal (≥8), and the composite behavioral CVH metric score and biological CVH metric score were grouped as poor (score ≤2), intermediate (3–4), and ideal (≥5) levels, respectively.26
Diagnosis of Dementia, AD, and VaD
Dementia was diagnosed following a 3-step procedure, as previously described.19,27 Briefly, in step 1, the trained staff performed the first interview, clinical examination, and testing, and recorded all information (eg, medical history, cognitive complaint, global cognitive function, and the Chinese version of activities of daily living). In step 2, the neurologists specialized in dementia treatment and care reviewed all the records to screen people who were suspected of having dementia or who had insufficient information (eg, lack of data on cognitive and physical functioning) for a judgement of dementia. Finally, the neurologists conducted the second interview with people who were selected in step 2 or with informants or both, and the diagnosis was made based on all the assessments. The Diagnostic and Statistical Manual of Mental Disorders, fourth edition, criteria were followed for the diagnosis of clinical dementia.28 AD was diagnosed according to the National Institute of Aging-Alzheimer’s Association criteria,29 for probable AD. The diagnosis of probable VaD was made according to the criteria of National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherch´e et l’Enseignement en Neurosciences,30 which was based essentially on a self-reported history of stroke and clinical or neuroimaging evidence of stroke identified via neurological examination, as well as the presence of a clear temporal relation between the history of stroke and the onset of dementia. Dementia cases who could not be classified as AD or VaD were considered to have other types of dementia.
Statistical Analysis
Characteristics of the study participants by age groups (65–74 and ≥75 years) were compared using the chi-square test for categorical variables and the t-test for normal distributed continuous variables. The individual CVH components and the composite CVH metric scores were considered as the primary independent variables of interest. We employed binary and multinomial logistic regression models to examine the odds ratio (OR) and 95% confidence interval (CI) of dementia, AD or VaD (as dependent variables) associated with individual and composite CVH metrics (as independent variables) in the total sample. To explore the possible variations in the CVH metrics–dementia association, we examined the statistical interaction of CVH metrics with age groups (65–74 and ≥75 years). If a statistical interaction (p for interaction <0.05) or a marginally statistical interaction (0.05<p for interaction <0.10) was detected, we further performed stratifying analysis by age groups to assess the magnitude and direction of the interaction. In all the association analyses, we controlled for age (in years), sex, education, alcohol consumption, and APOE ε4 allele. Stata 14.0 for Windows (StataCorp LLC., College Station, TX, USA) was used for all analyses. Two-tailed p<0.05 was considered to be statistically significant.
Results
Characteristics of Study Participants
The mean age of the 4980 participants was 71.56 years (SD, 5.29), 57.23% were women, and 39.60% had no formal schooling education. Compared with those in the young-old group (65–74 years, n = 3753), people in the old–old group (≥75 years, n = 1227) were less educated (p < 0.001), had higher proportions of ideal smoking status, ideal BMI, ideal physical activity, and ideal FBG, had lower proportions of ideal blood pressure and total cholesterol (all p < 0.01), and were more likely to drink alcohol (p < 0.001) (Table 1). As expected, people in the old–old group were more likely to have dementia than those in the young-old group (11.25% vs 2.98%, p < 0.001).
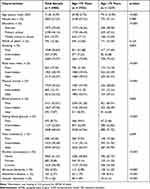 |
Table 1 Characteristics of Study Participants by Age Groups |
Associations of Individual CVH Metric Components with Dementia, AD, and VaD
Having ideal physical activity (OR = 0.74) or ideal FBG (OR = 0.38) was significantly associated with a reduced likelihood of all-cause dementia, but no smoking, ideal BMI, ideal blood pressure, and ideal total cholesterol were not significantly associated with dementia (Table 2). In addition, having ideal physical activity was significantly associated with a lower likelihood of VaD, but not with AD. Of note, having an ideal FBG was significantly associated with lower likelihoods of both AD and VaD (Table 2).
 |
Table 2 Associations of Individual Cardiovascular Health Metrics with Dementia, Alzheimer’s Disease, and Vascular Dementia (n = 4980) |
Associations of Composite CVH Metrics with Dementia, AD, and VaD
Compared with people with poor global CVH metrics, those with an ideal level of global CVH metrics had a significantly reduced likelihood of dementia (OR = 0.65) after controlling for demographic and other potential confounders (Table 3). Regarding subtypes of dementia, the ideal global CVH metrics were significantly associated with the decreased likelihood of VaD (OR = 0.31), but not for AD (OR = 0.88) (Table 3). In addition, compared with poor CVH metrics, having an intermediate or ideal level of behavioral and biological CVH metrics was not significantly associated with dementia and AD. The ideal level of behavioral CVH metrics, as compared with the poor level, was significantly associated with a lower likelihood of VaD (OR = 0.41), but not ideal biological CVH metrics.
 |
Table 3 Associations of Composite Cardiovascular Health Metrics with Dementia, Alzheimer’s Disease, and Vascular Dementia (n = 4980) |
Associations of Composite CVH Metrics with Dementia, AD, and VaD by Age Groups
There were no statistical or marginally statistical interactions of CVH metrics with age groups (65–74 versus ≥75 years) on the likelihood of dementia and the main subtypes of dementia. However, we detected a marginally statistical interaction between age strata and biological CVH metrics based on the likelihood of all-cause dementia and VaD (p for interactions < 0.10). Further analysis stratified by age strata showed that the association of the ideal biological CVH metrics with a lower likelihood of all-cause dementia (OR = 0.52, 95% CI: 0.28–0.95) (Figure 1A) and VaD (OR = 0.21, 95% CI: 0.06–0.77) (Figure 1C) was stronger in the young–old than old–old groups. However, biological CVH metrics were not significantly associated with AD in either age group (Figure 1B).
In addition, there were statistically significant interactive effects of age strata with ideal behavioral CVH metrics on the likelihood of all-cause dementia, AD, and VaD (p for all interactions <0.05). Further analysis stratified by age strata showed that the associations of ideal composite behavioral CVH metrics with a decreased likelihood of all-cause dementia (OR = 0.48, 95% CI: 0.26–0.89) and VaD (OR = 0.16, 95% CI: 0.06–0.43) were significant only among the old–old group (Figure 1D and F), whereas the ideal behavioral CVH metrics was significantly associated with a higher likelihood of AD in the young-old group (OR = 3.32, 95% CI: 1.14–9.66) (Figure 1E). Finally, the associations of composite global CVH metrics with dementia, AD, and VaD were not significant in either age stratum.
Discussion
To the best of our knowledge, this is the first population-based study that investigates the associations of composite CVH metrics with dementia and main subtypes of dementia in the elderly Chinese population. In this cross-sectional study of rural residents aged 65 years and older, we found that ideal global CVH metrics (vs poor CVH metrics) were associated with a lower likelihood of dementia and VaD, but not with AD. Furthermore, the reduced likelihoods of dementia and VaD associated with ideal biological CVH were pronounced only among young–old adults, whereas the reduced likelihoods of dementia and VaD in relation to ideal behavioral CVH metrics were evident mainly among the old–old adults.
Of the six examined CVH components, BMI, blood pressure, and total cholesterol have shown the age-dependent associations with dementia in previous studies such that having high levels of these three factors in midlife or young-old adults (eg, age <75 years) is associated with an increased risk of dementia, whereas having these risk factors in old–old (eg, age ≥75 years) is not related to dementia or even associated with a reduced risk of dementia.31–33 This is partly because neuropathological alterations of dementia or AD in the preclinical phase might affect levels of these factors. For example, individuals with preclinical dementia may gradually lose body weight due to loss of appetite and reduced food intake, and thus low body weight might be an early symptom of preclinical and clinical dementia.34,35 As a result, the reversal causality favors the cross-sectional associations of ideal levels of these CVH metrics with an increased likelihood of dementia. In this regard, the potential reverse causality might partly contribute to our observed associations of ideal BMI and blood pressure with a non-significantly increased likelihood of dementia, and of AD in particular, as well as the association of ideal total cholesterol with an increased likelihood of VaD (Table 2). By contrast, dementia, even in the preclinical phase, might reduce person’s physical activity, which would favor the cross-sectional association of ideal physical activity with a reduced likelihood of dementia. Notably, our data showed that having ideal physical activity was significantly associated with a lower likelihood of VaD, but not with AD. This is plausible given that people with VaD often had a history of clinical stroke in our study, which is a major cause of physical impairment and disability. While the potential reversal causation of high blood glucose and smoking with dementia might be less profound than that of BMI, blood pressure, total cholesterol, and physical activity, but selective survival might play a part, which might favor their cross-sectional associations with dementia towards null. Indeed, ideal (low) blood glucose was associated with a reduced OR of dementia, AD, and VaD, whereas the lack of association of smoking status with dementia and subtype dementia might be partly attributed to selective survival.
Similarly, the interpretations of composite CVH metrics in relation to dementia and subtype dementia need to take into account the potential reverse causation of individual CVH metrics with dementia. For example, we found no significant association of biological and behavioral CVH metrics with dementia, partly due to the potential reverse causations of dementia with some CVH metric components, such that ideal blood pressure and BMI were associated with a high likelihood of dementia, while ideal physical activity was associated with a reduced likelihood of dementia in older people, as previously reported.17,36 We found associations of ideal composite biological CVH metrics with a lower likelihood of dementia and VaD among young–old adults, but not in old-old people, partly because higher blood pressure and serum total cholesterol were associated with a greater likelihood of dementia in the young-old (65–74 years) rather than old–old adults, as previous studies suggested.37,38 In addition, we found that an ideal level of behavioral CVH metrics was associated with a decreased likelihood of dementia and VaD in the old–old group but with an elevated likelihood of AD in the young–old group. Indeed, an ideal BMI was associated with a higher likelihood of dementia and AD both in young–old and old–old adults (supplemental Figure 2A and B), which is in line with previous findings from the systematic review and meta-analysis of cross-sectional and prospective studies.17 In addition, in young–old adults, most people with mild-to-moderate AD had relatively well-preserved physical function.39 Taken together, the observed association of ideal composite behavioral CVH metrics with a higher likelihood of AD in young–old adults needs to be interpreted in the context of the potential reverse causation of the cross-sectional association.
Strengths of this study include a community-based design that targets rural residents, a relatively large study sample, and comprehensive assessments of CVH metrics. However, this study also has limitations. First, the causal or temporal relationship between CVH metrics and dementia cannot be determined owing to the cross-sectional nature of the study design. Thus, the study findings need to be carefully interpreted given the fact that the observed cross-sectional associations might be affected by reverse causation or selective survival as we fully discussed above. Furthermore, we used the modified AHA’s recommendations in defining CVH metrics due to a lack of dietary data, which might underestimate the associations of optimal CVH metrics with dementia. Finally, the study participants were derived from only one rural area of western Shandong Province, characterized by relatively low income and low educational attainments, which should be kept in mind when generalizing the study findings to other rural populations.
Conclusion
In summary, this large-scale community-based study of rural Chinese older adults shows that CVH metrics are cross-sectionally associated with dementia, AD, and VaD. Furthermore, the CVH metrics–dementia associations vary with age and by CVH metric components and dementia subtypes. While the cross-sectional nature of the study complicates the interpretations of the observed associations of modifiable CVH metrics with dementia, future longitudinal studies may help determine their potential causal relationships, which could provide solid evidence base for the development of guidelines and recommendations for preventive interventions to delay dementia onset and maintain brain health in the aging process.
Abbreviations
CVH, cardiovascular health; AHA, American Heart Association; BMI, body mass index; AD, Alzheimer’s disease; VaD, vascular dementia; MIND-China, Multimodal Interventions to delay Dementia and disability in rural China; ChiCTR, Chinese Clinical Trial Registry; FBG, fasting blood glucose; APOE, apolipoprotein E; OR, odds ratio; CI, confidence interval.
Data Sharing Statement
Data supporting the findings from this study will be available from the corresponding authors upon approval by the data management committee of MIND-China.
Acknowledgments
We would like to thank all the participants in the MIND-China study as well as the research group for their collaboration in data collection and management. This study was supported in part by grants from the National Key R&D Program of China (grant no.: 2017YFC1310100), the National Natural Science Foundation of China (grants no.: 82171175 and 8191101618), the Alzheimer’s Association Grant (grant no.: AACSFD-22-922844), the Shandong Provincial Natural Science Foundation (grants no.: ZR2021MH005), and the Taishan Scholar Program of Shandong Province, China. C Qiu received grants from the Swedish Research Council (grants no.: 2017-00740, 2017-05819, and 2020-0157) for the Sino-Sweden Network and Research Projects, the Swedish Foundation for International Cooperation in Research and Higher Education (STINT, grant no.: CH2019-8320) for the Joint China–Sweden Mobility program, and Karolinska Institutet, Stockholm, Sweden.
Disclosure
Dr Erika J Laukka reports grants from Swedish Research Council, during the conduct of the study. The authors report no conflicts of interest in this work.
References
1. Sheladia S, Reddy PH. Age-related chronic diseases and Alzheimer’s disease in Texas: a Hispanic focused study. J Alzheimers Dis Rep. 2021;5(1):121–133. doi:10.3233/adr-200277
2. International AsD. World Alzheimer report 2018: the state of the art of dementia research: new frontiers. World Alzheimer Report 2018; 2018:1–48.
3. Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol. 2015;12(5):267–277. doi:10.1038/nrcardio.2014.223
4. Dhana K, Evans DA, Rajan KB, Bennett DA, Morris MC. Healthy lifestyle and the risk of Alzheimer dementia: findings from 2 longitudinal studies. Neurology. 2020;95(4):e374–e383. doi:10.1212/WNL.0000000000009816
5. Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020;5(12):e661–e671. doi:10.1016/s2468-2667(20)30185-7
6. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396(10248):413–446. doi:10.1016/s0140-6736(20)30367-6
7. Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi:10.1161/CIRCULATIONAHA.109.192703
8. Gaye B, Canonico M, Perier MC, et al. Ideal cardiovascular health, mortality, and vascular events in elderly subjects: the three-city study. J Am Coll Cardiol. 2017;69(25):3015–3026. doi:10.1016/j.jacc.2017.05.011
9. Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and Hispanics: the northern Manhattan study. Circulation. 2012;125(24):2975–2984. doi:10.1161/circulationaha.111.081083
10. Kulshreshtha A, Vaccarino V, Judd SE, et al. Life’s Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44(7):1909–1914. doi:10.1161/STROKEAHA.111.000352
11. Perales J, Hinton L, Burns J, Vidoni ED. Cardiovascular health and cognitive function among Mexican older adults: cross-sectional results from the WHO study on global ageing and adult health. Int Psychogeriatr. 2018;30:12. 1827–1836. doi:10.1017/s1041610218000297
12. Crichton GE, Elias MF, Davey A, Alkerwi A. Cardiovascular health and cognitive function: the Maine-Syracuse Longitudinal Study. PLoS One. 2014;9(3):e89317. doi:10.1371/journal.pone.0089317
13. Gardener H, Wright CB, Dong C, et al. Ideal cardiovascular health and cognitive aging in the northern Manhattan study. J Am Heart Assoc. 2016;5(3):e002731. doi:10.1161/JAHA.115.002731
14. Joosten H, van Eersel ME, Gansevoort RT, Bilo HJ, Slaets JP, Izaks GJ. Cardiovascular risk profile and cognitive function in young, middle-aged, and elderly subjects. Stroke. 2013;44(6):1543–1549. doi:10.1161/STROKEAHA.111.000496
15. Eastwood SV, Tillin T, Chaturvedi N, Hughes AD. Ethnic differences in associations between blood pressure and stroke in South Asian and European Men. Hypertension. 2015;66(3):481–488. doi:10.1161/hypertensionaha.115.05672
16. Fayosse A, Nguyen DP, Dugravot A, et al. Risk prediction models for dementia: role of age and cardiometabolic risk factors. BMC Med. 2020;18(1):107. doi:10.1186/s12916-020-01578-x
17. Qu Y, Hu HY, Ou YN, et al. Association of body mass index with risk of cognitive impairment and dementia: a systematic review and meta-analysis of prospective studies. Neurosci Biobehav Rev. 2020;115:189–198. doi:10.1016/j.neubiorev.2020.05.012
18. Corrada MM, Hayden KM, Paganini-Hill A, et al. Age of onset of hypertension and risk of dementia in the oldest-old: the 90+ Study. Alzheimers Dement. 2017;13(2):103–110. doi:10.1016/j.jalz.2016.09.007
19. Kivipelto M, Mangialasche F, Snyder HM, et al. World-wide FINGERS network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16(7):1078–1094. doi:10.1002/alz.12123
20. Wang Y, Han X, Zhang X, et al. Health status and risk profiles for brain aging of rural-dwelling older adults: data from the interdisciplinary baseline assessments in MIND-China. Alzheimers Dement. 2022;8(1):e12254. doi:10.1002/trc2.12254
21. Han X, Jiang Z, Li Y, et al. Sex disparities in cardiovascular health metrics among rural-dwelling older adults in China: a population-based study. BMC Geriatr. 2021;21(1):158. doi:10.1186/s12877-021-02116-x
22. Cong L, Ren Y, Hou T, et al. Use of cardiovascular drugs for primary and secondary prevention of cardiovascular disease among rural-dwelling older Chinese adults. Front Pharmacol. 2020;11:608136. doi:10.3389/fphar.2020.608136
23. Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi:10.1249/MSS.0b013e31821ece12
24. Sabia S, Fayosse A, Dumurgier J, et al. Association of ideal cardiovascular health at age 50 with incidence of dementia: 25 year follow-up of Whitehall II cohort study. BMJ. 2019;366:l4414. doi:10.1136/bmj.l4414
25. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
26. Liang Y, Ngandu T, Laatikainen T, et al. Cardiovascular health metrics from mid- to late-life and risk of dementia: a population-based cohort study in Finland. PLoS Med. 2020;17(12):e1003474. doi:10.1371/journal.pmed.1003474
27. Jiang Z, Han X, Wang Y, et al. Red cell distribution width and dementia among rural-dwelling older adults: the MIND-China study. J Alzheimers dis. 2021;83(3):1187–1198. doi:10.3233/jad-210517
28. Association AP. Diagnostic and Statistical Manual of Mental Disorders.
29. Jack CR
30. Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. report of the NINDS-AIREN international workshop. Neurology. 1993;43(2):250–260. doi:10.1212/wnl.43.2.250
31. Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487–499. doi:10.1016/s1474-4422(05)70141-1
32. Karlsson IK, Lehto K, Gatz M, Reynolds CA, Dahl Aslan AK. Age-dependent effects of body mass index across the adult life span on the risk of dementia: a cohort study with a genetic approach. BMC Med. 2020;18(1):131. doi:10.1186/s12916-020-01600-2
33. Stewart R, White LR, Xue QL, Launer LJ. Twenty-six-year change in total cholesterol levels and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2007;64(1):103–107. doi:10.1001/archneur.64.1.103
34. Alhurani RE, Vassilaki M, Aakre JA, et al. Decline in weight and incident mild cognitive impairment: mayo clinic study of aging. JAMA Neurol. 2016;73(4):439–446. doi:10.1001/jamaneurol.2015.4756
35. Gu Y, Scarmeas N, Cosentino S, et al. Change in body mass index before and after Alzheimer’s disease onset. Curr Alzheimer Res. 2014;11(4):349–356. doi:10.2174/1567205010666131120110930
36. Hughes D, Judge C, Murphy R, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA. 2020;323(19):1934–1944. doi:10.1001/jama.2020.4249
37. Li G, Rhew IC, Shofer JB, et al. Age-varying association between blood pressure and risk of dementia in those aged 65 and older: a community-based prospective cohort study. J Am Geriatr Soc. 2007;55(8):1161–1167. doi:10.1111/j.1532-5415.2007.01233.x
38. Legdeur N, van der Lee SJ, de Wilde M, et al. The association of vascular disorders with incident dementia in different age groups. Alzheimers Res Ther. 2019;11(1):47. doi:10.1186/s13195-019-0496-x
39. Clemmensen FK, Hoffmann K, Siersma V, et al. The role of physical and cognitive function in performance of activities of daily living in patients with mild-to-moderate Alzheimer’s disease - A cross-sectional study. BMC Geriatr. 2020;20(1):513. doi:10.1186/s12877-020-01926-9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

