Back to Journals » Journal of Inflammation Research » Volume 14
Association of a High Neutrophil-to-Lymphocyte Ratio with Hyperdense Artery Sign and Unfavorable Short-Term Outcomes in Patients with Acute Ischemic Stroke
Authors Lin SK , Chen PY, Chen GC, Hsu PJ, Hsiao CL, Yang FY, Liu CY, Tsou A
Received 26 November 2020
Accepted for publication 12 January 2021
Published 5 February 2021 Volume 2021:14 Pages 313—324
DOI https://doi.org/10.2147/JIR.S293825
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Shinn-Kuang Lin,1,2 Pei-Ya Chen,1,2 Guei-Chiuan Chen,1 Po-Jen Hsu,1 Cheng-Lun Hsiao,1 Fu-Yi Yang,1 Chih-Yang Liu,1 Adam Tsou1
1Stroke Center and Department of Neurology, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan; 2School of Medicine, Tzu Chi University, Hualien, Taiwan
Correspondence: Shinn-Kuang Lin
Stroke Center and Department of Neurology, Taipei Tzu Chi Hospital, No. 289, Jian Guo Road, 231, Sindian District, New Taipei City, Taiwan
Tel +886-2-66289779 ext 3129
Fax +886-2-66289009
Email [email protected]
Purpose: Immune–inflammatory processes are involved in all the stages of stroke. This study investigated the association of the neutrophil-to-lymphocyte ratio (NLR) with the hyperdense artery sign (HAS) observed on brain computed tomography (CT) and with clinical features in patients with acute ischemic stroke.
Methods: We retrospectively enrolled 2903 inpatients with acute ischemic stroke from May 2010 to May 2019. Data collected included imaging studies, risk factors, laboratory parameters, and clinical features during hospitalization.
Results: The HAS was identified in 6% of the 2903 patients and 66% of the 236 patients with acute middle cerebral artery occlusion. Patients with the HAS had a higher NLR. HAS prevalence was higher in men and patients with cardioembolism. The NLR exhibited positive linear correlations with age, glucose and creatinine levels, length of hospital stay, initial National Institutes of Health Stroke Scale (NIHSS) scores, and mRS scores at discharge. The NLR was significantly higher in patients with large-artery atherosclerosis and cardioembolism and was the highest in patients with other determined etiology. Multivariate analysis revealed that an initial NIHSS score of ≥ 10 and an NLR of > 3.5 were significant positive factors, whereas diabetes mellitus and age > 72 years were significant negative factors for the HAS, with a predictive performance of 0.893. An initial NIHSS score of ≥ 5, positive HAS, age > 75 years, diabetes mellitus, an NLR of > 3.5, female sex, a white blood cell count of > 8 × 103/mL, and elevated troponin I were significant predictors of unfavorable outcomes, with a predictive performance of 0.886.
Conclusion: An NLR of > 3.5 enabled an efficient prediction of CT HAS. In addition to conventional risk factors and laboratory parameters, both an NLR of > 3.5 and CT HAS enabled improved prediction of unfavorable stroke outcomes.
Keywords: acute ischemic stroke, hyperdense artery sign, neutrophil-to-lymphocyte ratio, NIHSS, unfavorable outcome
Introduction
Stroke was the fourth leading cause of death in Taiwan from 2000 to 2020 and the leading cause of prolonged disability in older adults. Conventional risk factors for vascular diseases, including old age, hypertension, diabetes mellitus, and heart disease, are prominent comorbidities of stroke. Most studies have emphasized the correlation of these comorbidities with stroke and clinical outcomes. The clinical feature of initial stroke severity has been reported to be a strong predictor of functional outcomes.1,2 In addition, laboratory parameters measured during acute stroke, such as the hemoglobin level,3 blood urea nitrogen-to-creatinine ratio,4 and troponin I level,5 provide valuable information in the investigation of clinical outcomes after stroke. Atherosclerosis is a chronic inflammatory process that occurs in the arterial wall.6 Immunity and inflammation are considered the crucial elements of stroke pathobiology. Immune–inflammatory processes are involved in all the stages of acute stroke, including initial artery occlusion, brain parenchymal damage, subsequent tissue repair, and infectious complications.7 Innate and adaptive are the two main types of immune systems. Innate immunity refers to immune responses present at birth and provides the first rapid defense against invasion. Innate immunity is mainly provided by neutrophils, monocytes, macrophages, natural killer cells, and complement systems.8 Adaptive immunity, also known as acquired immunity, is provided by lymphocytes, which deliver antigen-dependent and -specific responses to invasion. The neutrophil-to-lymphocyte ratio (NLR) can reflect the balance between innate and adaptive immunity.9 A higher NLR has been reported to be associated with poor outcomes in patients with acute stroke and patients with various types of cancer.10–13
The hyperdense artery sign (HAS), caused by the fresh thromboembolic material within the artery, is an early direct sign observed on noncontrast computed tomography (CT) that indicates acute large intracranial artery occlusion. The HAS has been observed in 69% of patients with acute middle cerebral artery (MCA) occlusion within 24 hours and is associated with severe initial neurological deficits, large infarction territory, and poor functional outcomes despite thrombolytic therapy.14,15 In the present study, we investigated the association of the NLR with the HAS and clinical outcomes in patients with acute ischemic stroke.
Patients and Methods
Study Population and Data Collection
The stroke registry database was retrospectively reviewed to identify patients who received stroke treatment in a neurological ward from May 2010 to May 2019. The inclusion criteria were 1) a diagnosis of acute ischemic stroke confirmed by clinical presentation and 2) evidence of an ischemic lesion or the absence of a corresponding intracranial lesion other than infarction according to brain CT or magnetic resonance imaging. Information on sex; age; history of hypertension, diabetes mellitus, hyperlipidemia, heart disease, and prior stroke; smoking status; alcohol consumption; cancer diagnosis; presence of uremia; and length of stay (LOS) in hospital was recorded for analysis. Laboratory data obtained on arrival at the emergency department included the complete blood count with white blood cell differentials as well as platelet, glucose, creatinine, and troponin I levels. The NLR was calculated as the ratio of the neutrophil count to the lymphocyte count. An abnormal elevation in troponin I levels was defined as a blood troponin I level of >0.01 μg/L. Fasting cholesterol and triglyceride levels were recorded in the morning after admission to the ward. For patients admitted to the ward for transient ischemic attack (TIA) during the same period, the NLR was also collected for comparison.
Statement of Ethics
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval for this study was provided by the Institutional Review Board of Taipei Tzu Chi Hospital, New Taipei City (approval no. 09-X-025). Informed written consent was waived because the study was a retrospective data analysis. All data collected and analyzed in this retrospective study were derived from clinical records without any intervention or influence on clinical treatment. To fully protect patient privacy and rights, only clinical data of observations were used for publication, and personal information will not be disclosed to any other third party without the patient’s consent.
Stroke Severity and Clinical Features
Stroke severity was assessed when patients presented to the emergency department after symptom onset according to the National Institutes of Health Stroke Scale (NIHSS). We classified the etiology of ischemic stroke according to the Trial of ORG 10,172 in Acute Stroke Treatment (TOAST) categories, namely large-artery atherosclerosis, small-vessel occlusion, cardioembolism, other determined etiology, and undetermined etiology.16 Urinary tract infection, pneumonia, gastrointestinal bleeding, and seizure were registered as in-hospital stroke complications. Functional outcomes were evaluated using the NIHSS, Barthel index, and modified Rankin Scale (mRS) at discharge. An mRS score of >2 was considered to indicate an unfavorable outcome.
Identification of Acute Artery Occlusion and HAS
All patients underwent noncontrast 4-mm slice brain CT when they presented to the emergency department. All CT scans were reviewed by a stroke neurologist. The initial CT scan results were compared with results of follow-up magnetic resonance angiography (MRA) or CT angiography. Acute artery occlusion was defined as the presence of 1) a large area of infarction without a visible appropriate artery on MRA or CTA in association with prominent corresponding clinical neurologic deficits and 2) a large area of infarction with severe cerebral edema on follow-up brain CT in patients who did not undergo angiography. Given that the HAS most commonly occurred in acute MCA occlusion, patients who had acute MCA occlusion with and without HASs were selected for further comparisons. A hyperdense MCA sign was defined as an MCA denser than its contralateral counterpart. We measured and recorded the highest CT Hounsfield units (HU) of arteries on both the affected side (with HAS) and the contralateral side (without HAS). In addition, we calculated the HU ratio between the affected and contralateral sides. Nonvisualization of an appropriate artery with a small area of infarction corresponding to minor clinical symptoms was considered as arterial occlusion from chronic stenosis and was excluded during the selection of patients with acute MCA occlusion.
Statistical Analysis
Continuous variables are presented as the mean ± standard deviation. The chi-square test and Fisher’s exact test were used for categorical comparisons. Group comparisons of continuous variables were performed using the two-sample t-test or analysis of variance as appropriate. Significant predictors in the univariate analysis that were continuous variables were converted into dichotomous variables, with the optimal cutoff level determined according to the Youden index by using the receiver operating characteristic (ROC) curve for HAS and unfavorable outcomes. The variables were then added to a multiple logistic regression model to identify significant factors associated with HAS and unfavorable outcomes. In addition, we compared the predictive performance of the variables by using the C-statistic for HAS and unfavorable outcomes. A p value of <0.05 was considered to indicate a significant result. All statistical analyses were performed using SPSS (version 24; SPSS Inc, Chicago, IL, USA).
Results
During the study period, 2903 patients with acute ischemic stroke and 457 patients with TIA were enrolled. Of all patients with acute ischemic stroke, 2036 (70%) had valid data of troponin I levels because troponin I was not routinely measured in the emergency department in patients with acute stroke during that period. The average age of patients with acute ischemic infarct and those with TIA was 71.0 ± 13.5 and 70.5 ± 13.2 years, respectively. Of all 2903 patients, the HAS was identified in 172 (6%). The HU ranged from 42 to 74 (mean value = 59 ± 5) for the affected MCA with the HAS and from 30 to 46 (mean value = 38 ± 4) for the normal contralateral MCA; the mean MCA HU ratio was 1.6 ± 0.2. Table 1 compares the clinical features between 2903 patients with and without the HAS. Patients with the HAS were older than those without the HAS. Patients with the HAS had a higher NLR, initial NIHSS score, discharge NIHSS score, and mRS score as well as longer LOS in hospital; however, they had a lower platelet count, triglyceride level, and Barthel index score. They also had higher rates of large-artery atherosclerosis, cardioembolism, heart disease, atrial fibrillation, elevated troponin I, in-hospital complications, receipt of thrombolytic therapy, but lower rates of diabetes mellitus, hyperlipidemia, and prior stroke.
 |
Table 1 Comparison of Clinical Features in 2903 Patients with and without Hyperdense Artery Sign |
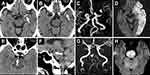
Acute artery occlusion was found in 252 patients; among those, 236 patients had acute MCA or terminal ICA occlusion. The HAS occurred in the first segment of the MCA (M1) in 142 patients, in the distal branch of the MCA (M2) in 12 patients, in the terminal internal carotid artery in 2 patients, in the basilar artery in 14 patients, in the vertebral artery in 1 patient, and in the posterior cerebral artery in 1 patient (Figure 1). Twelve patients had the HAS on the initial brain CT; however, no corresponding artery occlusion was found on follow-up MRA. Of these 12 patients, 2 patients who were classified to have large-artery atherosclerosis received thrombolytic therapy; of the remaining 10 patients who were classified to have cardioembolism, only 5 patients received thrombolytic therapy. Table 2 shows a comparison of clinical features between 236 patients who had acute MCA occlusion with and without the HAS. Of 236 patients, 156 (66%) with acute MCA occlusion exhibited the HAS. The occurrence of the HAS was higher in men than in women. In addition, HAS occurrence was higher in patients with cardioembolism. Patients with the HAS had lower white blood cell and platelet counts as well as lower diabetes mellitus and prior stroke rates.
 |
Table 2 Comparison of Clinical Features Between 236 Patients Who Had Acute Middle Cerebral Artery Occlusion with Positive and Negative Hyperdense Artery Sign |
We compared the NLR among the five groups categorized according to the TOAST classification and patients with TIA (Table 3). Patients with cardioembolism were older and had the highest initial NIHSS scores, whereas patients with small-vessel disease had the lowest initial NIHSS scores. No difference was observed in the NLR between patients with TIA and those with a small-vessel disease or between those with large-artery atherosclerosis and those with cardioembolism. The NLR was significantly higher in patients with large-artery atherosclerosis, cardioembolism, and other determined etiology than in other patients (p < 0.001). The highest NLR was observed in patients with other determined etiology. Table 4 shows the correlation of the NLR with the measured variables and clinical features. The NLR exhibited linear correlations with the measured variables; moreover, it demonstrated positive correlations with the glucose level, creatinine level, age, LOS in hospital, initial and discharge NIHSS scores, and mRS at discharge and negative correlations with the hemoglobin level, cholesterol level, triglyceride level, and Barthel index at discharge. Furthermore, the NLR was higher in patients with elevated troponin I, cancer history, atrial fibrillation, acute MCA occlusion, positive HAS, in-hospital complications, and discharge mRS score of >2 but lower in patients with hyperlipidemia.
 |
Table 3 Mean Values of the Neutrophil-to-Lymphocyte Ratio in Different Groups of TOAST Classification and Transient Ischemic Attack |
 |
Table 4 Correlation Analyses of Neutrophil-to-Lymphocyte Ratio with Measured Variables and Clinical Features in 2903 Patients with Acute Ischemic Stroke |
Table 5 presents the correlations of clinical features with the HAS and unfavorable outcomes. The univariate analyses of continuous variables revealed that old age, high NLR, initial NIHSS score, long LOS in hospital, low platelet count, and low triglyceride level were significantly associated with the HAS. The univariate analyses of dichotomous variables revealed that elevated troponin I, heart disease, atrial fibrillation, thrombolytic therapy, and in-hospital complications were significantly positively associated with the HAS, whereas diabetes mellitus, hyperlipidemia, and prior stroke were negatively correlated with the HAS. The findings of the univariate analysis of continuous variables revealed that old age; high white blood cell count, NLR, and creatinine level; initial NIHSS score; long LOS in hospital; and low hemoglobin, cholesterol, and triglyceride levels were significantly associated with unfavorable outcomes. The findings of the univariate analysis of dichotomous variables revealed that female sex, elevated troponin I, hypertension, diabetes mellitus, heart disease, prior stroke, cancer history, uremia, atrial fibrillation, thrombolytic therapy, in-hospital complications, and a positive HAS was significantly positively associated with unfavorable outcomes. However, hyperlipidemia, smoking status, and alcohol consumption were negatively correlated with favorable outcomes.
 |
Table 5 Univariate Analysis of Factors Affecting Hyperdense Artery Sign and Predictors of Unfavorable Outcomes (mRS Score > 2) in 2903 Patients with Acute Ischemic Stroke |
Through the ROC curve analysis, we identified cutoff points for the NLR and other continuous variables to indicate the HAS and unfavorable outcomes (ie, an mRS score of >2). The cutoff point of the NLR for the HAS was 3.5, which was identified to be the same cutoff point for unfavorable outcomes. The cutoff points of age and the initial NIHSS score were >72 years and ≥10 for HAS, respectively, and >75 years and ≥5 for unfavorable outcomes, respectively. Table 6 presents the results of the multiple regression analysis of the effect of the main significant factors listed in Table 5 on the HAS. An initial NIHSS score of ≥10 (odds ratio [OR]: 144.226; 95% confidence interval [CI]: 19.623–99.673; p < 0.001) and an NLR of >3.5 (OR: 2.146; 95% CI: 1.303–3.534; p = 0.003) were significant positive predictors of the HAS, whereas age > 72 years and diabetes mellitus were significant negative predictors of the HAS. The C-statistics of the regression model for the detection of the HAS comprising the aforementioned four significant factors was 0.893 (95% CI: 0.874–0.912; p < 0.001; Figure 2A). Table 7 presents the results of the regression analysis for the effect of the main significant factors listed in Table 5 on unfavorable outcomes. Initial NIHSS score ≥ 5 (OR: 12.354; 95% CI: 9.715–15.709; p < 0.001), positive HAS (OR: 3.420; 95% CI: 1.727–6.772; p < 0.001), age > 75 years (OR: 2.590; 95% CI: 1.989–3.374; p < 0.001), diabetes mellitus (OR: 1.653; 95% CI: 1.273–2.146; p < 0.001), NLR > 3.5 (OR: 1.512; 95% CI: 1.169–1.954; p = 0.002), female sex (OR: 1.497; 95% CI: 1.133–1.978; p = 0.005), white blood cell count > 8 × 103/mL (OR: 1.446; 95% CI: 1.120–1.866; p = 0.005), and elevated troponin I level (OR: 1.694; 95% CI: 1.139–2.521; p = 0.009) were the significant predictors of unfavorable outcomes. The C-statistics of the regression model for the detection of unfavorable outcomes comprising the aforementioned eight significant factors was 0.886 (95% CI: 0.874–0.898; p < 0.001; Figure 2B).
 |
Table 6 Multivariate Logistic Regression of Factors Affecting Hyperdense Artery Sign in 2903 Patients with Acute Ischemic Stroke |
 |
Table 7 Multivariate Logistic Regression of Predictors for Unfavorable Outcomes (mRS Score > 2) in 2903 Patients with Acute Ischemic Stroke |
Discussion
Atherosclerosis, the primary underlying pathological process in coronary and cerebral arterial diseases, is characterized by chronic inflammation that causes large and medium arterial thromboses.17 When an acute ischemic stroke occurs during artery occlusion, the inflammatory response following the release of danger signals from the damaged brain tissue leads to the activation of the immune system. Innate immunity, including neutrophils, monocytes, macrophages, platelets, and dendritic cells, is rapidly activated with the production of various cytokines. This is followed by the activation of the adaptive immunity involving lymphocytes, which exert an immunosuppressive effect that promotes intercurrent infections (ie, stroke-induced immunodepression).7 These immunological changes may last for weeks and may increase the risk of respiratory or urinary tract infections, particularly in patients with severe stroke, thus affecting clinical outcomes.18 Neutrophils, which are secretory and phagocytic cells, migrate to intraparenchymal perivascular areas within several hours after cerebral ischemia and participate in the early destruction of the blood–brain barrier.19 Lymphocytes accumulate in the brain 3–6 days after stroke and are considered to exhibit a regulatory function by inducing neuroprotection. Persistent lymphopenia after stroke, caused by the redistribution of lymphocytes to lymphatic organs and increased catecholamine and cortisol levels, indicates prolonged brain damage with a high-stress response, which is associated with unfavorable long-term prognosis.20
The HAS is subjectively recognized on noncontrast CT when the artery appears denser than its contralateral counterpart or the surrounding brain density and can be seen within 90 minutes of acute artery occlusion.21 The high attenuation of a thrombus is due to the extrusion of plasma in the thrombus with a subsequent increase in the local hematocrit level, which causes a higher range of 47–61 HU than that of 40–43 HU observed for nonoccluded blood in noncontrast CT.22,23 Red thrombi, usually from acute or cardioembolic clots, are richer in erythrocytes and cause a higher HU, whereas white thrombi, usually from nonacute atherosclerotic clots, are richer in platelets and fibrin and cause a lower HU.24 The presence of the HAS has been reported to predict a poor thrombolytic effect but successful recanalization during thrombectomy.15,25 Several studies have emphasized the precise measurement of thin-slice CT HU values of high attenuation and the bilateral MCA HU ratio to improve the qualitative and quantitative evaluations of sensitivity and specificity as well as the intraobserver and interobserver reliability of the HAS. However, the real-life clinical features of acute stroke (such as the NIHSS score) at the emergency department together with positive CT HAS provided a more accurate diagnostic value and guide for decision-making regarding thrombolytic or thrombectomy therapy.26
In the present study, the HAS was identified in 6% of all patients with acute ischemic stroke and in 66% of patients with acute MCA occlusion. Compared with patients who had an acute ischemic stroke without the HAS, 172 patients with the HAS had higher rates of heart disease, atrial fibrillation, receipt of thrombolytic therapy, in-hospital complications, elevated troponin I levels, long LOS in hospital, poor mRS score on discharge, and high NLR. Similar results were observed in 236 patients who had acute MCA occlusion (with or without the HAS; data not shown). A history of prior stroke typically denotes a chronic atherosclerotic process of intracranial arteries and hence a low probability of an acute large arterial occlusion with the HAS. Among 236 patients with acute MCA occlusion, the HAS was more prevalent in male patients and occurred in patients with the TOAST classification of large-artery atherosclerosis and cardioembolism, with a higher occurrence rate in patients with cardioembolism. Recanalization of the occluded MCA after thrombolytic therapy was observed in two patients with large-artery atherosclerosis and in five patients with cardioembolism who exhibited the HAS on initial CT. Spontaneous recanalization of MCA occlusion occurred in the other five patients with cardioembolism who exhibited the HAS on initial CT but a patent MCA on follow-up MRA. This is compatible with the characteristic that red thrombi from cardioembolism had higher HU on CT and higher potential to break down spontaneously or through thrombolytic therapy.
We found that the NLR was positively correlated with age, glucose levels, creatinine levels, initial NIHSS scores, LOS in hospital, and mRS scores at discharge. The NLR was higher in patients who had elevated troponin I levels, cancer history, atrial fibrillation, positive HAS, acute MCA occlusion, in-hospital complications, and unfavorable outcomes. Cholesterol, triglyceride, and hemoglobin levels showed an inverse correlation with age and the NLR and were also lower in patients with unfavorable outcomes. This result is similar to that reported by Fang et al, who identified that a high total cholesterol level was significantly and independently predictive of lower NIHSS scores and less severe stroke.10 The tendency of older adults to have diets with a low lipid content or experience malnutrition due to chewing disorders may explain this finding. A higher NLR indicated a higher stroke severity and less favorable immune status, resulting in more in-hospital complications, such as pneumonia and urinary tract infections, and prolonged LOS in hospital. Patients with TIA and small-vessel disease had the lowest NLR; this was due to the less severity of the stroke and the small extent of brain tissue damage from small-artery occlusion. Notably, patients with other determined etiology of stroke who tended to be younger and have lower stroke severity compared with those with large-artery atherosclerosis and cardioembolism exhibited the highest NLR. These results slightly differ from those reported by Gökhan et al, who revealed that the NLR was the highest in those with large-artery occlusion.20 No patient was assigned to the group of other determined etiology by Gökhan et al. In the present study, 43 patients were assigned to the other determined etiology group. Most of these patients had prominent immunological, hematological, or systemic disorders associated with acute stroke. Therefore, the NLR was considerably higher in these patients.
The NLR was higher not only in patients with acute MCA occlusion but also in those with the HAS. To the best of our knowledge, these findings have not been reported previously. Acute large intracranial artery occlusion indicates a more severe brain tissue damage and inflammatory response. Occlusion of the MCA must be confirmed through contrast-enhanced CT angiography or MRA. However, the HAS provides an immediate clue for the diagnosis of acute large intracranial occlusion through noncontrast CT. Together with the clinical severity of symptoms, physicians can make a quick decision for further treatment. An initial NIHSS score of ≥10 and an NLR of >3.5 were the two most significant factors for predicting the HAS during the hyperacute stage of stroke. Lim et al reported that the HAS had a high sensitivity of 79% for identifying large-vessel occlusion in acute ischemic stroke patients presenting with an NIHSS score of >10.27
Studies have suggested that the NLR increases in patients with various cancers.12,13 In this study, we found that the NLR was increased in patients with elevated troponin I levels. The risk of stroke increases not only after a new cancer diagnosis but also with time in almost all cancer survivors.28 Cancer and related therapies may cause coagulopathies, such as nonbacterial thrombotic endocarditis, alterations in platelet and endothelial functions, and radiation-induced atherosclerosis. Elevated troponin I levels during acute stroke is a strong independent predictor of both unfavorable outcomes and in-hospital mortality. Mechanisms underlying elevated troponin I levels during acute stroke include ischemic myocardial injury; neurogenic heart syndrome through increased sympathetic activity causing cardiomyopathy; and other systemic conditions such as infection, sepsis, renal failure, and pulmonary embolism.5
Multivariate analyses revealed that the significant predictors of unfavorable outcomes were initial NIHSS score ≥ 5, positive HAS, age > 75 years, diabetes mellitus, NLR > 3.5, female sex, white blood cell count > 8 × 103/mL, and elevated troponin I levels. The predictive performance for unfavorable outcomes of the aforementioned eight factors was up to 0.886. Furthermore, the cutoff point of NLR > 3.5 for the HAS and unfavorable outcomes was the same. Because the NLR was derived from the white blood cell count, which is essential laboratory information during the acute stroke and common routine examinations, the NLR can be selected as a reliable marker for the prediction of both the HAS and unfavorable outcomes.
This study has several limitations. First, this was a retrospective study. We did not have sufficient sequential data during hospitalization for performing a dynamic comparison of the NLR. A dynamic increase in the NLR has been reported to predict 3-month mortality or major disability in patients receiving intravenous thrombolytic treatment.29 Second, we did not investigate the association between the infarct volume and NLR. However, the TOAST classification may partly reflect the infarct size. Third, we did not perform interobserver reliability in recognizing the HAS. Most previous studies have documented the reliability of interobserver agreement in identifying CT HAS. Fourth, because we did not perform a follow-up study after discharge, and only short-term outcomes at discharge were available. A prospective study examining serial NLR and long-term outcomes may provide more prognostic relevance for acute ischemic stroke. Regardless of these limitations, the present results extend the current understanding of the implications of the NLR in patients with acute ischemic stroke.
Conclusion
The HAS occurred in 66% of patients with acute MCA occlusion. An initial NIHSS score of ≥10 and an NLR of >3.5 were the two most significant predictors of the HAS. Initial NIHSS score ≥ 5, positive HAS, age > 75 years, diabetes mellitus, NLR > 3.5, female sex, white blood cell count > 8 × 103/mL, and elevated troponin I were the significant predictors of unfavorable outcomes in patients with acute ischemic stroke. An NLR of >3.5 enabled the improved prediction of CT HAS. Both an NLR of >3.5 and CT HAS enabled the efficient prediction of unfavorable stroke outcomes in addition to conventional risk factors and laboratory parameters.
Acknowledgments
This study was funded by Taipei Tzu Chi Hospital (TCRD-TPE-109-RT-7). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sato S, Toyoda K, Uehara T, et al. Baseline NIH stroke scale score predicting outcome in anterior and posterior circulation strokes. Neurology. 2008;70(Issue 24, Part 2):2371–2377. doi:10.1212/01.wnl.0000304346.14354.0b
2. Rost NS, Bottle A, Lee JM, et al. Stroke severity is a crucial predictor of outcome: an international prospective validation study. J Am Heart Assoc. 2016;5(1):e002433. doi:10.1161/JAHA.115.002433
3. Barlas RS, Honney K, Loke YK, et al. Impact of hemoglobin levels and anemia on mortality in acute stroke: analysis of UK regional registry data, systematic review, and meta-analysis. J Am Heart Assoc. 2016;5(8):e003019. doi:10.1161/JAHA.115.003019
4. Schrock JW, Glasenapp M, Drogell K. Elevated blood urea nitrogen/creatinine ratio is associated with poor outcome in patients with ischemic stroke. Clin Neurol Neurosurg. 2012;114(7):881–884. doi:10.1016/j.clineuro.2012.01.031
5. Su YC, Huang KF, Yang FY, et al. Elevation of troponin I in acute ischemic stroke. PEERJ. 2016;4:e1866. doi:10.7717/peerj.1866
6. Massiot N, Lareyre F, Voury-Pons A, et al. High neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with symptomatic internal carotid artery stenosis. J Stroke Cerebrovas Dis. 2019;28(1):76–83. doi:10.1016/j.jstrokecerebrovasdis.2018.09.001
7. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808. doi:10.1038/nm.2399
8. Opal SM, Esmon CT. Bench-to-bedside review: functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit Care. 2002;7(1):23–38. doi:10.1186/cc1854.
9. Van der Willik KD, Fani L, Rizopoulos D, et al. Balance between innate versus adaptive immune system and the risk of dementia: a population-based cohort study. J Neuroinflamm. 2019;16(1):68. doi:10.1186/s12974-019-1454-z
10. Fang YN, Tong MS, Sung PH, et al. Higher neutrophil counts and neutrophil-to-lymphocyte ratio predict prognostic outcomes in patients after non-atrial fibrillation-caused ischemic stroke. Biomed J. 2017;40(3):154–162. doi:10.1016/j.bj.2017.03.002
11. Lim HH, Jeong IH, An GD, et al. Early prediction of severity in acute ischemic stroke and transient ischemic attack using platelet parameters and neutrophil-to-lymphocyte ratio. J Clin Lab Anal. 2019;33(3):e22714. doi:10.1002/jcla.22714
12. Hirahara T, Arigami T, Yanagita S, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19(1):672. doi:10.1186/s12885-019-5903-y
13. Huszno J, Kolosza Z. Prognostic value of the neutrophil-lymphocyte, platelet-lymphocyte and monocyte-lymphocyte ratio in breast cancer patients. Oncol Lett. 2019;18(6):6275–6283. doi:10.3892/ol.2019.10966
14. Haridy J, Churilov L, Mitchell P, Dowling R, Yan B. Is there association between hyperdense middle cerebral artery sign on CT scan and time from stroke onset within the first 24-hours? BMC Neurol. 2015;15(1):101. doi:10.1186/s12883-015-0358-5
15. Li Q, Davis S, Mitchell P, Dowling R, Yan B. Proximal hyperdense middle cerebral artery sign predicts poor response to thrombolysis. PLoS One. 2014;9(5):e96123. doi:10.1371/journal.pone.0096123
16. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Stroke. 1993;24(1):35–41. doi:10.1161/01.str.24.1.35
17. Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204–212. doi:10.1038/ni.2001
18. Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10(5):471–480. doi:10.1016/S1474-4422(11)70066-7
19. Zhang R, Wu X, Hu W, et al. Neutrophil-to-lymphocyte ratio predicts hemorrhagic transformation in ischemic stroke: a meta-analysis. Brain Behav. 2019;9(9):e01382. doi:10.1002/brb3.1382
20. Gökhan S, Özhasenekler A, Mansur Durgun H, et al. Neutrophil lymphocyte ratios in stroke subtypes and transient ischemic attack. Eur Rev Med Pharmacol Sci. 2013;17(5):653–657.
21. Alshoabi SA, Alsultan KD. Hyperdense artery sign on computed tomography is extremely valuable marker of middle cerebral artery occlusion in early hemiparesis. Am J Diagn Imaging. 2020;6(1):15–17. doi:10.5455/ajdi.20190824024709
22. Koo CK, Teasdale E, Muir KW. What constitutes a true hyperdense middle cerebral artery sign? Cerebrovasc Dis. 2000;10(6):419–423. doi:10.1159/000016101
23. Chieng JSL, Singh DR, Chawla A, Peh WCG. The hyperdense vessel sign in cerebral computed tomography: pearls and pitfalls. Singapore Med J. 2020;61(5):230–237. doi:10.11622/smedj.2020074
24. Liebeskind DS, Sanossian N, Yong WH, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42(5):1237–1243. doi:10.1161/STROKEAHA.110.605576
25. Froehler M, Tateshima S, Duckwiler G, et al. The hyperdense vessel sign on CT predicts successful recanalization with the merci device in acute ischemic stroke. J NeuroInterv Surg. 2013;5(4):289–293. doi:10.1136/neurintsurg-2012-010313
26. Jodaitis L, Ligot N, Chapusette R, Bonnet T, Gaspard N, Naeije G. The hyperdense middle cerebral artery sign in drip-and-ship models of acute stroke management. Cerebrovasc Dis Extra. 2020;10(1):36–43. doi:10.1159/000506971
27. Lim J, Magarik JA, Froehler MT. The CT-defined hyperdense arterial sign as a marker for acute intracerebral large vessel occlusion. J Neuroimaging. 2018;28(2):212–216. doi:10.1111/jon.12484
28. Zaorsky NG, Zhang Y, Tchelebi LT, et al. Stroke among cancer patients. Nat Commun. 2019;10(1):5172. doi:10.1038/s41467-019-13120-6
29. Shi J, Peng H, You S, et al. Increase in neutrophils after recombinant tissue plasminogen activator thrombolysis predicts poor functional outcome of ischemic stroke: a longitudinal study. Eur J Neurol. 2018;25(4):687–745. doi:10.1111/ene.13575
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.