Back to Journals » Infection and Drug Resistance » Volume 12
Association between vitamin D and latent tuberculosis infection in the United States: NHANES, 2011–2012
Authors Wang CY , Hu YL, Wang YH, Chen CH, Lai CC , Huang KL
Received 29 April 2019
Accepted for publication 3 July 2019
Published 22 July 2019 Volume 2019:12 Pages 2251—2257
DOI https://doi.org/10.2147/IDR.S213845
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Cheng-Yi Wang,1,* Yin-Lan Hu,2,* Ya-Hui Wang,3 Cheng-Hsin Chen,1 Chih-Cheng Lai,4 Kun-Lun Huang5
1Department of Internal Medicine, Cardinal Tien Hospital and School of Medicine, College of Medicine, Fu-jen Catholic University, New Taipei City, Taiwan; 2Department of Dentistry, Cardinal Tien Hospital and School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan; 3Medical Research Center, Cardinal Tien Hospital and School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan; 4Department of Intensive Care Medicine, Chi Mei Medical Center, Liouying, Taiwan; 5Division of Pulmonary Medicine, Tri-service General Hospital, Institute of Undersea and Hyperbaric Medicine, National Defense Medical Center, Taipei, Taiwan
*These authors contributed equally to this work
Background: Latent tuberculosis infection (LTBI) is a precursor of active tuberculosis diseases and an important issue in the United States and worldwide. The association between vitamin D deficiency and LTBI is poorly understood.
Methods: From 2011 to 2012, the National Health and Nutrition Examination Survey (NHANES) assessed LTBI (according to tuberculin skin testing and QuantiFERON®,-TB Gold In-Tube) and measured serum levels of vitamin D. We evaluated the association between LTBI and vitamin D using multivariate logistic regression models adjusted for known confounders.
Results: The LTBI group had a lower 25-hydroxyvitamin D [25(OH)D] level than the non-LTBI group (p=0.0012). The adjusted risk of LTBI was significantly higher among participants with serum 25(OH)D levels <12 ng/ml (adjusted OR [aOR], 2.27; 95% CI, 1.40–3.66) and 12–19 ng/ml (aOR, 1.75; 95% CI, 1.25–2.46) compared to those with a level ≥30 ng/ml. The higher risk of LTBI among the participants with serum 25(OH)D levels <12 ng/ml and 12–19 ng/ml remained unchanged in both male and summer season subgroups.
Conclusions: A low serum 25(OH)D level was significantly associated with the risk of LTBI in this US cohort.
Keywords: latent tuberculosis infection, NHANES, risk, vitamin D
Introduction
Tuberculosis (TB) remains a major public health threat worldwide, and its associated mortality is higher than with any other infectious diseases.1 According to the Global Burden of Disease Tuberculosis Collaborators, there were 10.2 million and 10.1 million incident and prevalent TB cases in 2015, respectively, with 1.3 million deaths.2 Several interventions have been developed to eradicate TB, and the early identification of individuals at high-risk of TB is an essential component of TB control.3–5 Latent TB infection (LTBI) is a condition in which Mycobacterium tuberculosis bacteria survive in the host in a dormant state, and individuals with LTBI are both asymptomatic and non-infectious.5 However, individuals with LTBI may develop TB in the near or remote future. Several studies6,7 using genotyping methods have reported that about a signification portion of cases of TB disease are due to the progression of LTBI. Therefore, the early recognition and appropriate treatment of LTBI to reduce the reservoir of M. tuberculosis could be an important public health strategy to reduce or even eradicate TB.
This finding prompted physicians to use oral vitamin D to treat TB in the 1940s, because vitamin D was made in the skin from sunshine. Many relevant and important work done in the 1800s and early 1900s using cod liver oil, sunshine, phototherapy and vitamin D to treat and cure TB infections. After the Nobel Prize in Medicine was awarded in 1903 to Dr Neils Ryberg-Finsen for curing hundreds of previously incurable cases of TB using refracted light rays from an electric arc lamp, UVB phototherapy has become the standard of care in treating TB infections for several decades. The issue of whether or not vitamin D is important in the treatment of TB was answered in the 1940s.8 Multiple reports showed the safety and efficacy of curing TB infections with oral vitamin D using doses of 100,000 to 150,000 units a day for 2 to 3 months, as well as sunshine, phototherapy and cod liver oil.8 Vitamin D is an essential hormone which can help maintain mineral homeostasis in humans.9 In addition to bones, vitamin D deficiency has been inversely associated with several diseases including cardiometabolic diseases,10 hypertension,11 and diabetes.12 Moreover, the immunomodulatory effect of vitamin D has been proven in several in vitro studies,13–15 and some researches have also shown that vitamin D deficiency is significantly associated with an increased risk of active TB.16–21 Although vitamin D deficiency is very common in all age groups,22 only few reports23,24 have examined the prevalence of vitamin D deficiency among individuals with LTBI. However, the association between vitamin D deficiency and LTBI remained unclear. Thus, we conducted this study to investigate the association between vitamin D deficiency and LTBI using a representative sample of the US population – the National Health and Nutrition Examination Survey (NHANES).
Methods
Study design and population
The NHANES is a series of population-based national surveys conducted in the US, and the collected information regarding the health and nutrition are released to the public every 2 years. Using a complex, stratified sampling design, the NHANES can select representative samples of US non-institutionalized civilians including Mexican Americans, African Americans, older adults and those with a lower socioeconomic status. The dataset used in this report was the NHANES 2011–2012. We included participants with available results of tuberculin skin testing (TST) or QuantiFERON®-TB Gold In-Tube (QFT-GIT). In this survey, TST and QFT-GIT were only performed on participants aged 6 years and older, and components of the survey interview used in this study included demographic characteristics, socioeconomic status, and TB questionnaires. NHANES study protocols were approved by the Institutional Review Board of the National Center of Health Statistics, and written informed consent was obtained from all enrolled participants.
Measurement of vitamin D, TST, and QFT-GIF
25-hydroxyvitamin D [25(OH)D] concentrations were detected by LC-MS/MS method.25 For the primary analysis, we categorized the serum 25(OH)D levels into four clinically relevant categories: severely deficient (<12 ng/ml), deficient (12–<20 ng/mL), insufficient (20 to <30 ng/mL), and sufficient (≥30 ng/mL).26 The 25(OH)D levels were converted to ng/ml from nmol/L by multiplying by 0.4.
TST measurements were performed for each participant by technicians trained in the relevant guidelines, and Tubersol (Sanofi, Bridgewater, NJ) PPD was used for all testing. The results of TST were obtained 46–76 hrs after administration to facilitate patient scheduling, and the induration of TST was calculated as the average of up to three recorded TST results. If only one TST result was recorded, this result was used. A positive TST was defined as a reading of induration greater than or equal to 10 mm. QFT-GIT was performed and interpreted in accordance with CDC guidelines for the use of IGRAs.27 HIV testing was also performed in the NHANES among the subset of participants aged 18–59 years.
Covariates
Variables including sex, age, education, race, birthplace, poverty status, smoking status, contact history with active TB patients, size of household site, underlying diseases, renal function, body mass index, alcohol history, season, and associated history of LTBI including BCG scar, past LTBI treatment, previous test for LTBI, and history of TB disease were collected. The primary outcome of interest was the presence of LTBI according to a positive TST or QFT-GIT.
Statistical analysis
Responses coded as “don’t know”, “refused”, or “missing” in the original NHANES data were treated as missing. We accounted for the complex multi-stage stratified sampling technique of the NHANES by using Medical Examination Center sample 2-year weights to adjust for unequal probability of selection and nonresponse; additional weights were calculated for TST or QFT-GIT nonparticipation.28 For descriptive statistics, weighted numbers and percentages for categorical variables were used. The Rao-Scott χ2 test was used to assess differences in LTBI status between categories of each characteristic. In addition, the weighted means and 95% confidence intervals (CIs) of serum 25(OH)D levels were calculated.
We estimated crude odds ratios (ORs) and 95% CIs between serum 25(OH)D levels and LTBI using weighted logistic regression. Model 1 described the association between LTBI and a 10-ng/mL increase in serum 25(OH)D level, while model 2 described the association between LTBI and categorical serum 25(OH)D levels. Adjusted ORs and 95% CIs were calculated using multivariate logistic regression models adjusted for sex, age, race and/or ethnicity, income-to-poverty ratio <1, education, foreign birth, BMI, DM, and hypercholesterolemia.
All analyses were conducted using SAS Survey Procedure software (SAS Institute, version 9.4; Cary, North Carolina). All tests were two-sided, and the level of statistical significance was set at p<0.05.
Results
Participant characteristics
Table 1 summarizes the demographic characteristics of the study population. Overall, 658 (10.8%) of the participants had positive TST or QFT-LTBI. There were significant differences between the LTBI and non-LTBI groups. The LTBI group was older and more predominantly male than the non-LTBI group (both p<0.005). In addition, the LTBI group were more likely to be non-Hispanic black, Mexican American and Asian, and to be born in foreign countries than the non-LTBI group (all p<0.0001). The LTBI group were also more likely to live with active TB patients than the non-LTBI group (p<0.0001). The non-LTBI group had a higher education level and income than the LTBI group (both p<0.005). Smoking status and the prevalence of most underlying conditions including HIV status, malignancy, hypertension, and renal function were similar between the two groups. The LTBI group had a lower 25(OH)D level than the non-LTBI group (p=0.0012).
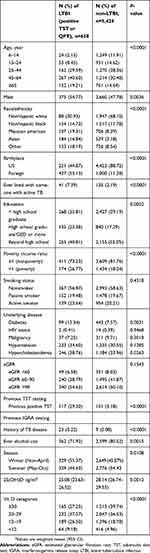 |
Table 1 Demographic of subjects with and without LTBI (n=6,083) |
Association between serum 25(OH)D level and LTBI
Unadjusted analysis of the association between serum 25(OH)D level and LTBI is shown in Table 2. Overall, the risk of LTBI was significantly higher in the participants with serum 25(OH)D levels <12 ng/ml (crude OR, 2.70; 95% CI, 1.59–4.60) and 12–19 ng/ml (crude OR, 2.06; 95% CI, 1.38–3.08) compared to those with a level ≥30 ng/ml. These significant associations remained unchanged in both male and female participants, and summer and winter seasons. In addition, the risk of LTBI was highest among the participants with a serum 25(OH)D level <12 ng/ml, and inverse associations between serum 25(OH)D level and the risk of LTBI among both genders and seasons were noted (Figure 1). The analysis of the association between serum 25(OH)D level and LTBI after adjusting for sex, age, race, poverty, education, birthplace, BMI, DM, and hypercholesterolemia is shown in Table 3. Overall, the adjusted risk of LTBI was significantly higher in the participants with serum 25(OH)D levels <12 ng/ml (adjusted OR (aOR), 2.27; 95% CI, 1.40–3.66) and 12–19 ng/ml (aOR, 1.75; 95% CI, 1.25–2.46) compared to those with a level ≥30 ng/ml. The higher risk of LTBI in the participants with serum 25(OH)D levels <12 ng/ml (adjusted OR (aOR), 2.23; 95% CI, 1.36–3.66) and 12–19 ng/ml remained unchanged in both male and summer season subgroups.
 |
Table 2 Crude association between serum 25-hydroxyvitamin D [25(OH)D] level and latent TB infection |
 |
Table 3 Adjusted the association between serum 25(OH)D level and latent TB infection |
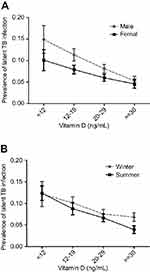 |
Figure 1 The association between the risk of LTBI and serum 25(OH)D level among both genders (A) and seasons (B). |
Discussion
In this study, we showed that serum 25(OH)D levels were inversely associated with the risk of LTBI in a nationally representative sample of US adults. This significant association was demonstrated in several ways. First, the LTBI group had a lower serum level of 25(OH)D and higher frequency of vitamin D deficiency (<30 ng/m) than the non-LTBI group. Second, the adjusted ORs of LTBI risk were about two folds higher in the participants with severe vitamin D deficiency (<12 ng/m) compared to those with a sufficient serum 25(OH)D level (≥30 ng/m). Third, a reciprocal association between serum 25(OH)D level and the risk of LTBI was noted, with a lower serum 25(OH)D level being associated with a higher prevalence of LTBI. Fourth, although a previous study24 showed that the risks of vitamin D deficiency was increased by two-fold in female patients and 1.4-fold in winter, the associations between lower serum 25(OH)D level and higher risk of LTBI remained significant across both genders and was unchanged by seasonality. To the best of our knowledge, this is the first large population-based study to provide recently updated and comprehensive estimates of the association between serum 25(OH)D levels and risk of LTBI. Our findings are consistent with a previous study from Spain, in which Arnedo-Pena et al23 demonstrated that the mean serum 25(OH)D level in TST conversion cases was lower than in controls (17.5±5.6 ng/ml versus 25.9±13.7 ng/ml; p=0.041), and that a sufficient serum 25(OH)D level was a protector against TST conversion. In summary, our findings demonstrated that a low 25(OH)D was a significant risk factor for LTBI in US populations. Since the discovery of antibiotics in the 1940s, the use of vitamin D to treat TB has been forgotten. However, according to our finding and previous study,33 vitamin D may be a useful option to help eradicate TB.
Vitamin D deficiency has long been accepted to be associated with impaired immunity and increased risk of TB.29 Many types of immune cells including monocytes, macrophages, and T-lymphocytes have been proven to play a role in Mycobacterium tuberculosis (MTB) resistance.13,30,31 Correction of vitamin D deficiency allows vitamin D to turn on a gene in white blood cell that makes t cathelicidin in response to sensing TB in the body. The gene can’t be turned on in a sustained way to make adequate amounts of antibiotic if there is insufficient supply of vitamin D in the circulation. It seems apparent that Finsen was able to cure chronic case of TB by causing sustained production of vitamin D, which then led to sustained production of cathelicidin.13 In addition, it has also been reported that vitamin D can induce interleukin-1beta (IL-1β) secretion and further modulate paracrine signaling, which reinforces the role of macrophages in innate immune regulation.30 Another study reported that vitamin D can improve the coordinated response to MTB of monocytes and T-lymphocytes with frequent MTB exposure.29 These findings may suggest that vitamin D plays a role in preventing MTB infection.
To date, the optimal vitamin D level for health and the classification of sufficient versus nonsufficient vitamin D levels remains controversial; however, a serum level of 25(OH)D <12 ng/mL is generally considered to indicate a severe deficiency in vitamin D.32 Although the National Institutes of Health defines a serum 25(OH)D level of ≥20 ng/mL as adequate, some experts suggest that 20–29 ng/mL is still not sufficient, and that ≥30 ng/mL should be considered sufficient.32,33 In this study, we found that the participants with a serum 25(OH)D level of <20 ng/ml had a higher risk of LTBI, and the risk was higher with a serum level of <12 ng/ml. Although this indicates that the optimal level of 25(OH)D to prevent LTBI should be >20 ng/ml, the optimal 25(OH)D levels that it remains unknown whether supplementation would modify risk.
This study has several strengths. First, the NHANES is a representative sample of the US population that rigorously follows very well designed study protocols with extensive quality assurance and quality control. Therefore, the large study population in this analysis was representative of the US population. Moreover, our findings are robust, reliable and generalizable. Second, we used a comprehensive approach to evaluate serum 25(OH)D levels both as continuous and categorical variables. As a result, we not only detected trends in a continuous model but also found supporting evidence of the clinical importance in the categorical classification. Third, many variables, including age, race/ethnicity, and TB exposure status were assessed as potential confounders and adjusted for in the analysis.
The study also has several limitations. First, causal relationships could not be investigated due to the cross-sectional design. Second, the season-at-examination variable used in this analysis was binary, although season and sun exposure are critical to serum 25(OH)D levels. However, that binary variable was the only available temporal information, since the NHANES does not provide dates of examinations. Moreover, even though the seasonality of serum 25(OH)D levels in the NHANES could be a confounder because of the sampling design wherein data are collected from northern states in the summer and southern states in the winter, the association between vitamin D deficiency and LTBI remained constant in different seasons. Third, although we adjusted for many potential confounders, we cannot rule out residual confounding or the effect of unmeasured confounders.
In conclusion, in this large national sample, serum 25(OH)D levels were significantly associated with the risk of LTBI in US adults, and a low 25(OH)D level was a significant risk factor for LTBI. Further studies are warranted to investigate the potential benefits of maintaining optimal vitamin D levels to prevent LTBI.
Acknowledgments
This study was supported by grants from Cardinal Tien Hospital (CTH-104-1-2C01, CTH-104-1-2A08, CTH105A-205, and CTH106A-2B05).
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cox H, Nicol MP. Tuberculosis eradication: renewed commitment and global investment required. Lancet Infect Dis. 2017;18(3):228–2292. doi:10.1016/S1473-3099(17)30692-8
2. GBD Tuberculosis Collaborators. The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;18(3):261–284. doi:10.1016/S1473-3099(17)30703-X
3. Collin SM, Wurie F, Muzyamba MC, et al. Effectiveness of interventions for reducing TB incidence in countries with low TB incidence: a systematic review of reviews. Eur Respir Rev. 2019;28(152):180107. doi:10.1183/16000617.0107-2018
4. Marks SM, Mase SR, Morris SB. Systematic review, meta-analysis, and cost-effectiveness of treatment of latent tuberculosis to reduce progression to multidrug-resistant tuberculosis. Clin Infect Dis. 2017;64(12):1670–1677. doi:10.1093/cid/cix208
5. Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393(10181):1642–1656. doi:10.1016/S0140-6736(19)30308-3
6. Suwanpimolkul G, Jarlsberg LG, Grinsdale JA, et al. Molecular epidemiology of tuberculosis in foreign-born persons living in San Francisco. Am J Respir Crit Care Med. 2013;187(9):998–1006. doi:10.1164/rccm.201212-2239OC
7. Moonan PK, Ghosh S, Oeltmann JE, Kammerer JS, Cowan LS, Navin TR. Using genotyping and geospatial scanning to estimate recent mycobacterium tuberculosis transmission, United States. Emerg Infect Dis. 2012;18(3):458–465. doi:10.3201/eid1803.111107
8. McCullough PJ, Lehrer DS, Vitamin D. cod liver oil, sunshine, and phototherapy: safe, effective and forgotten tools for treating and curing tuberculosis infections - a comprehensive review. J Steroid Biochem Mol Biol. 2018;177:21–29. doi:10.1016/j.jsbmb.2017.07.027
9. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6Suppl):1678s–1688s. doi:10.1093/ajcn/80.6.1678S
10. Al-Khalidi B, Kimball SM, Rotondi MA, Ardern CI. Standardized serum 25-hydroxyvitamin D concentrations are inversely associated with cardiometabolic disease in U.S. adults: a cross-sectional analysis of NHANES, 2001-2010. Nutr J. 2017;16(1):16. doi:10.1186/s12937-017-0237-6
11. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi:10.1161/CIRCULATIONAHA.107.706127
12. Zhang FF, Al Hooti S, Al Zenki S, et al. Vitamin D deficiency is associated with high prevalence of diabetes in Kuwaiti adults: results from a national survey. BMC Public Health. 2016;16:100. doi:10.1186/s12889-016-2758-x
13. Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–2063. doi:10.4049/jimmunol.179.4.2060
14. Martineau AR, Wilkinson KA, Newton SM, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178(11):7190–7198. doi:10.4049/jimmunol.178.11.7190
15. Shapira Y, Agmon-Levin N, Shoenfeld Y. Mycobacterium tuberculosis, autoimmunity, and vitamin D. Clin Rev Allergy Immunol. 2010;38(2–3):169–177. doi:10.1007/s12016-009-8150-1
16. Zeng J, Wu G, Yang W, et al. A serum vitamin D level <25 nmol/l pose high tuberculosis risk: a meta-analysis. PLoS One. 2015;10(5):e0126014.
17. Joo MH, Han MA, Park SM, Shin HH. Vitamin D deficiency among adults with history of pulmonary tuberculosis in Korea based on a nationwide survey. Int J Environ Res Public Health. 2017;14:4. doi:10.3390/ijerph14040399
18. Huang SJ, Wang XH, Liu ZD, et al. Vitamin D deficiency and the risk of tuberculosis: a meta-analysis. Drug Des Devel Ther. 2017;11:91–102. doi:10.2147/DDDT.S79870
19. Arnedo-Pena A, Juan-Cerdan JV, Romeu-Garcia MA, et al. Vitamin D status and incidence of tuberculosis infection conversion in contacts of pulmonary tuberculosis patients: a prospective cohort study. Epidemiol Infect. 2015;143(8):1731–1741. doi:10.1017/S0950268814002386
20. Kim JH, Park JS, Cho YJ, et al. Low serum 25-hydroxyvitamin D level: an independent risk factor for tuberculosis? Clin Nutr. 2014;33(6):1081–1086. doi:10.1016/j.clnu.2013.11.014
21. Ho-Pham LT, Nguyen ND, Nguyen TT, et al. Association between vitamin D insufficiency and tuberculosis in a Vietnamese population. BMC Infect Dis. 2010;10:306. doi:10.1186/1471-2334-10-306
22. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi:10.1210/jc.2011-0385
23. Arnedo-Pena A, Juan-Cerdan JV, Romeu-Garcia A, et al. Latent tuberculosis infection, tuberculin skin test and vitamin D status in contacts of tuberculosis patients: a cross-sectional and case-control study. BMC Infect Dis. 2011;11:349. doi:10.1186/1471-2334-11-208
24. Wingfield T, Schumacher SG, Sandhu G, et al. The seasonality of tuberculosis, sunlight, vitamin D, and household crowding. J Infect Dis. 2014;210(5):774–783. doi:10.1093/infdis/jiu121
25. Schleicher RL, Sternberg MR, Lacher DA, et al. A method-bridging study for serum 25-hydroxyvitamin d to standardize historical radioimmunoassay data to liquid chromatography-tandem mass spectrometry. Nati Health Stat Reports. 2016;25(93):1–13.
26. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi:10.1016/j.annepidem.2007.12.001
27. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59(Rr–5):1–25.
28. Ghassemieh BJ, Attia EF, Koelle DM, Mancuso JD, Narita M, Horne DJ. Latent tuberculosis infection test agreement in the National Health and Nutrition Examination Survey. Am J Respir Crit Care Med. 2016;194(4):493–500. doi:10.1164/rccm.201508-1560OC
29. Tung YC, Ou TT, Tsai WC. Defective Mycobacterium tuberculosis antigen presentation by monocytes from tuberculosis patients. Int J Tuberc Lung Dis. 2013;17(9):1229–1234. doi:10.5588/ijtld.12.0984
30. Verway M, Bouttier M, Wang TT, et al. Vitamin D induces interleukin-1beta expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog. 2013;9(6):e1003407. doi:10.1371/journal.ppat.1003407
31. Diedrich CR, Mattila JT, Flynn JL. Monocyte-derived IL-5 reduces TNF production by Mycobacterium tuberculosis-specific CD4 T cells during SIV/M. Tuberculosis Coinfection. J Immunol. 2013;190(12):6320–6328.
32. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academy Press; 2010.
33. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi:10.1056/NEJMra070553
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
