Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Association Between Uric Acid to HDL Cholesterol Ratio and Diabetic Complications in Men and Postmenopausal Women
Authors Xuan Y, Zhang W, Wang Y, Wang B, Xia F, Zhang K, Li Q, Wang N, Lu Y
Received 7 September 2022
Accepted for publication 15 December 2022
Published 19 January 2023 Volume 2023:16 Pages 167—177
DOI https://doi.org/10.2147/DMSO.S387726
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Yan Xuan,1,2 Wen Zhang,1 Yuying Wang,1 Bin Wang,1 Fangzhen Xia,1 Kun Zhang,1 Qing Li,1 Ningjian Wang,1 Yingli Lu1
1Institute and Department of Endocrinology and Metabolism, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Endocrinology, Luwan Branch, Ruijin Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, 200020, People’s Republic of China
Correspondence: Yingli Lu, Institute and Department of Endocrinology and Metabolism, Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, 200011, People’s Republic of China, Tel +86-13636352507, Fax +86-21-63136856, Email [email protected]
Aim: Previous studies have implicated the uric acid to high-density lipoprotein cholesterol (HDL-C) ratio (UHR) was associated with type 2 diabetes. However, the association between UHR and diabetes-related vascular damages is still unclear.
Methods: The total of 4551 patients with type 2 diabetes from the cross-sectional Environmental Pollutant Exposure and Metabolic Diseases in Shanghai study (METAL study) were enrolled. UHR was calculated as uric acid to HDL-C ratio. Cardiovascular disease (CVD) was defined as previously diagnosed with stroke, coronary heart disease, or peripheral arterial disease. Chronic kidney disease (CKD) was defined as estimated glomerular filtration rate ≤ 60 mL/min/1.73 m2 and/or urinary albumin to creatinine ratio ≥ 30 mg/g. Fundus image was examined by trained individuals and degree of diabetic retinopathy (DR) was evaluated.
Results: UHR was positively correlated with CVD (OR = 1.28, 95% CI: 1.02– 1.61) and CKD (OR = 1.78, 95% CI: 1.39– 2.27) after adjusting for all confounders. No association was found between UHR and DR. In stratified analyses, UHR was predominantly correlated with CVD in diabetic patients with age older than 65 (OR = 1.41, 95% CI: 1.08– 1.85), female (OR = 1.43, 95% CI: 1.06– 1.94) and BMI≥ 24kg/m2 (OR = 1.57, 95% CI: 1.17– 2.11). A 1-SD increment of UHR was also positively associated with CVD (OR 1.26, 95% CI 1.03, 1.15) and CKD (OR 1.28, 95% CI 1.20,1.39). UHR was positively associated with CKD in all subgroups analysis. No significant interaction effect was observed between UHR and all subgroup variables in CVD and CKD risk.
Conclusion: Our study reported a positive association between the UHR and diabetic-related vascular complications in men and postmenopausal women. The relationship between the UHR and DR seems to be uncertain and requires further investigation. And no significant interaction effect was observed between the UHR and all subgroup variables in CVD and CKD risk.
Keywords: type 2 diabetes, uric acid to HDL cholesterol ratio, cardiovascular disease, chronic kidney disease, diabetic retinopathy, inflammation
Introduction
Diabetes mellitus is known as one of the most common and fastest growing diseases, especially in Asia.1 It is reported to affect 693 million adults worldwide by 2045.2 Complications of diabetes including macrovascular (cardiovascular disease (CVD)) and microvascular (chronic kidney disease (CKD), diabetic retinopathy (DR)) damages are the leading cause of morbidity and mortality in diabetes patients.3 On the other hand, the development trend of diabetes in China is very serious.4
The UA level was associated with cardiovascular diseases,5 diabetes6 and hypertension.7 We previously found that hyperuricemia is an independent risk for diabetes.8 SUA level was confirmed to be positively related with severity of diabetes complications as well.9 But the real role of uric acid in these diseases is still very controversial.10,11 Additionally, menopause is proved to be associated with uric acid levels independently and use of sex hormone replacement therapy is associated with lower UA levels among postmenopausal women.12 Postmenopausal status is related with an increased risk of central obesity, dyslipidemia,13 metabolic syndrome14 and cardiovascular events.15 Therefore, it is particularly important to study the risk factors and disease prevention related to metabolic diseases in postmenopausal women.
High-density lipoprotein cholesterol (HDL-c) is a key component of the cardiovascular diseases and metabolic syndrome (MetS). Low HDL-c is as well as characterized as one of the features of metabolic syndrome, dyslipidemia, hypertriglyceridemia, hypertension, and impaired glucose tolerance.16–18 Diabetes patients usually have low HDL-c.19 However, until now, the biological functions of HDL-c in diabetes patients with vascular complications are still not fully clear.20 The Uric Acid/HDL-c ratio (UHR), combined these two metabolic parameters, is a more powerful predictor of metabolic deterioration.21 The UHR was related with many metabolic-inflammatory diseases such as hypertension,22 thyroiditis,23 hepato-steatosis.24 Type 2 diabetes and its related complications are associated with inflammatory markers as Neuregulin-4 (Nrg-4), a new adipokine released from brown adipose tissue,25,26 which was proved to be a good predictor of early detection of one or more diabetic microvascular complications in patients with diabetes. The researcher found UA and HDL-c were also correlated with serum Nrg-427. Recently, UHR has been discovered to be a significant indicator for the metabolic syndrome in diabetes patients,28 glucose control in type 2 diabetes mellitus (T2DM) patients21 and non-alcoholic fatty liver disease (NAFLD) in lean Chinese population.29 Previous studies suggested utilization of UHR in diagnosis of MS as a novel criteria30 in type 2 diabetes patients. But in fact, whether UHR is associated with diabetes complications remains unclear.
Therefore, our objective is to investigate the associations between the emerging UHR and macrovascular and microvascular damages, including cardiovascular diseases, diabetic kidney disease and diabetic retinopathy, in men and postmenopausal women with T2DM.
Materials and Methods
Study Population
The cross-sectional Environmental Pollutant Exposure and Metabolic Diseases in Shanghai (METAL) study (www.chictr.org.cn, ChiCTR 1800017573) was conducted in 2018. This study aimed to research the associations of diabetes complications with risk factors in Chinese type 2 diabetes patients. The detail about sampling process has been described in the previous studies.31,32 Totally, 4937 diabetes patients underwent an examination. Those missing laboratory results (n = 174), premenopausal women (n = 93), questionnaire data (n = 116) and UHR data (n = 3) were excluded. Thus, a total of 4551 participants were included in the final analyses (Figure 1). Informed consent was obtained from all participants included in our study.
 |
Figure 1 Flowchart of the inclusion and exclusion of participants. |
The study protocol was approved by the Ethics Committee of the Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. The protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected by the a priori approval granted by the appropriate institutional review committee.
Clinical, Anthropometric and Laboratory Measurements
The questionnaire including sociodemographic characteristics, family history, lifestyle factors and medical history was taken during an interview by the experienced personnel involved in the Survey on Prevalence in East China for Metabolic Diseases and Risk Factors (SPECT-China).33,34 Weight, height, waist circumference (WC), blood pressure (BP) and other anthropometric assessments were measured by trained nurses. Waist circumference and height were measured to the nearest 0.1 cm, and weight was recorded to the nearest 0.1 kg while subjects were wearing light clothing without shoes. WC was measured from the distance around the umbilicus horizontally with participants in a standing position. Body mass index (BMI) was defined as weight (in kilograms) divided by height (in meters squared). BMI < 24 kg/m2 was defined as normal weight, while BMI ≥24 kg/m2 was defined as overweight/obese according to the Cooperative Meta-Analysis Group of the Working Group on Obesity in China criteria.35 We defined current smoker as smoked at least 100 cigarettes over a lifetime and still currently smoking.36 We defined current drinkers as having consumed alcohol regularly at least once per week for the past six months.37
We obtained blood samples in the morning after fasting for at least 8 h after phlebotomy and centrifuged within 2 h of collection. Serum samples were aliquoted and frozen at a central laboratory. Fasting plasma glucose (FPG), triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL-C), low-density lipoprotein cholesterol (LDL-C) and serum uric acid were measured by AU680 Chemistry Analyzer (Beckman Coulter, Brea, CA, USA). Glycated hemoglobin (HbA1c) was also measured by a high-performance liquid chromatography (HPLC) automatic HbA1c analyzer (MEDCONN, Huizhong Medical Science and Technology Co., Ltd, Shanghai, China; Shanghai Huachen Biological Reagent Co., Ltd, Shanghai, China). The estimated glomerular filtration rate (eGFR) was determined according to the Chinese modified Chronic Kidney Disease Epidemiology Collaboration.38
The sample of urine albumin and creatinine, using a turbidimetric immunoassay and an enzymatic method, were measured with a Beckman Coulter AU 680 (Brea, USA) in a single spot urine sample respectively and urine albumin/creatinine ratio (ACR) was calculated.
Definition of Variables
Hypertension was diagnosed as self-reported previous physician’s diagnosis of hypertension and/or systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg. Dyslipidemia was diagnosed as a self-reported previous physician’s diagnosis of hyperlipidemia or TG≥ 2.26 mmol/L (200 mg/dL), TC level ≥6.22 mmol/L (240 mg/dL), LDL-C≥4.14 mmol/L (160 mg/dL), HDL-c <1.04 mmol/L (40 mg/dL) according to 2019 ESC/EAS guidelines.39
Macrovascular damage included CVD outcomes including previously diagnosed with stroke, coronary heart disease, or peripheral arterial disease which were notes in the registration platform. Microvascular damage included CKD and DR. CKD included eGFR ≤60 mL/min per 1.73 m2 or urinary albumin to creatinine ratio (UACR) ≥30 mg/g. The fundus image was examined by four trained individuals. DR was defined as one or more as following diagnosis: retinal hemorrhage, hard exudation, microaneurysm formation, cotton flocculus, venous beading, retinal microvascular abnormalities, retinal neovascularization, vitreous hemorrhage, and fibrous hyperplasia.40
Statistical Analyses
Data analyses were performed using IBM SPSS version 25 statistical software (IBM Corp., Armonk, NY, USA). P < 0.05 indicated significance (two-sided). Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are presented as percentages (%) as appropriate. Kolmogorov–Smirnov test was employed to test the normality of the data. We used Pearson’s correlation to assess the correlations between the UHR and cardiometabolic risk factors. UHR levels were divided into quartiles, with the first quartile representing the lowest one (as reference group) and the fourth quartile representing the highest. We further used logistic regression to assess the relationship between the UHR and indicators of macrovascular and microvascular complications. We took subgroup analyses to stratify the patients by quartiles of UHR according to sex, age, BMI and HbA1c levels. Model 1 was adjusted for age, sex, BMI, smoke status and drink status. Model 2 was adjusted for model 1 plus TC, LDL, UA, eGFR (only in CVD and DR group), HbA1c, blood pressure, anti-diabetes agents, hypertension, antihypertension drug.
Results
Participant Characteristics by Quartiles of the UHR
The results are in Table 1; totally, 4551 participants with T2DM were enrolled in this study, including 2109 men and 2442 women, a mean ± standard deviation age of 67.40 ± 8.71 years was enrolled. Men had higher levels of BMI, WC, HbA1c, UA, LDL-C, diastolic blood pressure (DBP), uACR and older to be compared with women, but lower levels of systolic blood pressure (SBP), HDL-C, TG, TC, eGFR at the time of admission (All P < 0.05).
 |
Table 1 Characteristics of the Participants by Gender |
In addition, women had higher prevalence of CVD, CKD as well as hyperlipidemia and overweight/obesity. But men had worse life habits such as smoke and drink than women. (All P < 0.05). Although the prevalence of DR and hypertension was higher in women than in men, there was no statistical difference in the study.
Correlation Between the UHR and Cardiometabolic Risk Factors
We examined the relationship between UHR and established cardiovascular risk factors after adjusted age, sex and BMI. As shown in Table 2, UHR was positively correlated with WC (r = 0.360, P < 0.001), SBP (r = 0.108, P < 0.001), DBP (r = 0.191, P < 0.001), FBG (r = 0.053, p = 0.030), TC (r = 0.186, P < 0.001), LDL (r = 0.165, P < 0.001). The higher UHR was associated with increasing cardiovascular risk, indicating that UHR remained a valid of metabolic risk factors among patients with T2DM.
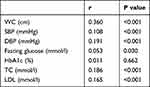 |
Table 2 The Correlation Between UHR and Cardiometabolic Risk Factors After Adjusted by Age, Sex and BMI |
Association Between UHR and Macrovascular /Microvascular Complications
We then conducted multivariate logistic regression to check the relationship between UHR and common complications in all participants. Using Figure 2, the participants were divided into four groups based on their UHR levels: Q1, Q2, Q3, and Q4. When Q1 served as the reference, multivariate logistic regressions were conducted after adjusting for age, sex, BMI, smoke status and drink status. The results showed that the risks in the Q4 quartile of CVD (OR = 1.51, 95% CI: 1.24–1.84, P < 0.001) and CKD (OR = 2.05, 95% CI: 1.65–2.55, P < 0.001) were significantly higher than the results in the Q1 quartile (P < 0.05), but not DR. After further adjusting all confounders, risks in the Q4 quartile of CVD (OR = 1.28, 95% CI: 1.02–1.61, P < 0.05) and CKD (OR = 1.78, 95% CI: 1.39–2.27, P < 0.001) were significantly higher than the results in the Q1 group (P < 0.05) as before. The results showed that an increase in UHR would be associated with macrovascular and microvascular complications.
Further, we compared the association of UHR and diabetes complications with uric acid and HDL-c alone in Supplementary Tables S1–S3. A 1-SD increment of UHR was also positively associated with CVD (OR 1.08, 95% CI 1.01, 1.15) and CKD (OR 1.28, 95% CI 1.20,1.39), but no association with DR (OR 0.92, 95% CI 0.79, 1.08) in Supplementary Table 1. For uric acid alone, the UA level was only positively associated with CKD (OR 1.25, 95% CI 1.16,1.34) in Supplementary Table 2. About HDL-c alone, HDL-c was only associated with CKD (OR 0.85, 95% CI 0.78,0.93) in Supplementary Table 3.
Sensitivity Analysis
We further explored the context in which the UHR was associated with CVD and CKD by sensitivity analyses in Table 3. The association between UHR and CVD was remained strongly in the older participants as compared to those aged younger than 65 (OR: 1.41, CI%1.08,1.85, P = 0.015), suggesting in these aging patients UHR was more correlated with diabetes macrovascular complications. This association was also found in female (OR: 1.57, CI%1.17,2.11, P = 0.003) and BMI ≥24 kg/m2 (OR: 1.43, CI%1.06,1.94, P = 0.022), indicating UHR was null correlated with diabetic macrovascular complications among male and lean participants. Furthermore, the association between UHR and CKD remained always strongly when participants were stratified by all subgroups. No significant interaction effect was observed between the UHR and all subgroup variables in CVD and CKD risk in Table 3.
 |
Table 3 Odds Ratios for Macrovascular and Microvascular Complications According to UHR Quartiles by Various Subgroups |
Discussion
The primary finding in this study was that UHR was highly correlated with CVD and CKD in T2DM patients. The association between UHR and CVD was significant in female participants older than 65 and with BMI≥ 24 kg/m2. The association between UHR and CKD remained significant regardless of age, sex, BMI, and glucose control status. We observe no significant association between UHR and DR. In our study, we explained, at least partly, the role of UHR in chronic vascular complications and showed evidence for guiding the prevention of diabetic complications.
This study reported UHR is associated closely with almost all cardiovascular risk factors as we known just as UA and low HDL-c.41 Then, we presented that UHR level was positively associated with the CVD prevalence. We found that with increasing 1-standard deviation of UHR level, the risk of CVD prevalence increased by 26%. UHR is composed of uric acid and HDL-c, so its effect on diabetes complications may be explained by its compositions. Our previous studies demonstrated the increasing uric acid level was related with a higher prevalence of macrovascular complication.8 Indeed, previous study confirmed the SUA level as a risk factor for CVD.42 Although the relationship between a higher uric acid level and CVD was previously investigated, the epidemiological evidence remains controversial. The follow-up research, over 14.5 years (median), failed to prove a significant association between hyperuricemia and cardiovascular diseases.43 The rate of CVD mortality, had a U-shaped association with uric acid levels only in men, whereas no significant associations were detected in women.42 Our conclusions are similar to those studies results; there is no significant association between increasing UA level and the risk of CVD prevalence. The reasons of the inconsistent results may likely be due to the difference of the number of participants, population characteristics, analysis of confounding factors. Additionally, HDL-c is inversely related with risk of CVD and is a key component of predicting cardiovascular risk.44 In a prospective study, participants with low levels of HDL-c were demonstrated to have double the DM prevalence and a significantly higher risk of CVD compared to those with a normal lipid profile.45 Similar relationships have been reported regarding low HDL and an elevated incidence of stroke especially in elderly patients with T2DM.46 After stratified analyses, the independent positive association of UHR and CVD persisted in the subgroup of age older than 65 years, female and overweight or obesity. This may be due to aging and BMI significantly increase SUA level and decrease HDL-C level, these changes are also associated with atherosclerosis and cardiovascular complications.46,47 In addition, increasing of SUA level and decreasing of HDL-C level in women after menopause are more significant than that in men.48
Our study further researched that the UHR was positively associated with the prevalence of CKD. We found that with increasing 1-standard deviation of UHR level, the risk of CKD prevalence increased by 28%. After stratified analyses, the independent positive relationship of UHR with CKD still persisted across almost all subgroups. In our previous study, we revealed that higher uric acid level was positively related with a higher prevalence of CKD.8 In our study, increasing 1-standard deviation of UA level, the risk of CKD prevalence increased by 25%. The cohort study including 13,964 T2DM patients reported that uric acid level was negatively related with eGFR.49 Furthermore, a meta-analysis showed that an elevated uric acid level was associated with an elevated risk factor of chronic kidney disease.50 Moreover, lower levels of HDL-c were independently and positively associated with the risk of developing diabetic nephropathy in T2DM patients.51 In our study, increasing 1-standard deviation of HDL-c level, the risk of CKD prevalence decreased by 15%. We demonstrated that UHR was positively related with diabetic nephropathy, which may show the importance of adjusting uric acid level and HDL-c levels for preventing diabetic nephropathy.
However, we failed to indicate that the prevalence of DR was correlated with the UHR. Opposed to CVD and CKD, we failed to find the prevalence of DR was associated with the SUA level in the previous study.8 In a cross-sectional study, it failed to be confirmed serum uric acid as an independent risk factor of DR.52 On the other hand, DR in T2DM patients was not found to be significantly associated with serum HDL-C levels, indicating that HDL-C may not have a crucial role to play in these particular diabetic complications.53 Although there is no association between the UHR and DR prevalence found in the present study, our results showed that UHR was associated with the prevalence of CVD and CKD, which indicated relationships of the UHR with the prevalence of CVD and CKD may be stronger than the association of the UHR with the prevalence of DR. Uric acid and HDL-c are very widely used indicators in clinical practice. UHR usage is simple and low cost. It has a strong correlation with diabetic complications and has some predictive power. This allows clinicians to find complications in a timely manner in clinical work to delay or even prevent the development of complications. It will improve the patient’s life and life treatment and save economic costs.
Besides, the UHR was also related with many metabolic-inflammatory diseases. Mehmet et al suggested that elevated Uric acid to HDL cholesterol ratio level be considered a useful tool in diagnosing hepatic steatosis, due to the inexpensive and easy-to-assess nature of Uric acid to HDL cholesterol ratio.24 UHR was an independent risk factor for poor BP control in hypertension subjects and the researchers thought assessment of UHR might be useful in hypertension patients since elevated UHR levels could be associated with poor blood pressure control in this population.22 Ozge showed UHR was significantly and positively correlated with thyroid stimulating hormone (TSH) and negatively correlated with free T4 (FT4).23 UHR is a reliable and useful marker for Hashimoto’s thyroiditis. Therefore, it may be helpful in establishing the diagnosis of Hashimoto’s thyroiditis in addition to other diagnostic tools. To sum up, there is a strong correlation between UHR and metabolic-inflammatory diseases including diabetes, it is worth promoting in clinical applications in the future.
However, there were some limitations in this study. First, the causality of the association between the UHR and diabetic complications cannot be established as a cross-sectional study; we should take further prospective studies to confirm our findings. Second, our study population came from seven different communities in Shanghai, and selection bias may exist. Finally, because all participants were Chinese people, the applicability and utility of the UHR should be confirmed in other ethnic populations.
Conclusion
In conclusion, this study found positive correlations between the UHR and diabetic macrovascular and microvascular complications were found in men and postmenopausal women with diabetes except the relationship of UHR and DR, which suggests that measuring and lowering UHR in a timely manner may be important to prevent and manage diabetic vascular complications. The relationship between the UHR and DR should be further confirmed.
Data Sharing Statement
The electronic medical record data retrieved from the Shanghai Ninth People’s Hospital was anonymized for this study. Summary data that were used to support the findings of this study may be requested from the correspondent author.
Ethics Committee Statement
The protocol of study was approved by the Ethics Committee of the Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine.
The protocol was according to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected by the a priori approval granted by the appropriate institutional review committee. The participants included in our study obtained informed consent.
Informed Consent Statement
Informed consent was received from all subjects involved in the study.
Acknowledgments
The authors would like to thank all the participants in this study. The authors thank all team members and participants in the METAL study. This study was supported by National Natural Science Foundation of China (82120108008, 91857117); Shanghai Municipal Human Resources and Social Security Bureau (2020074); the Major Science and Technology Innovation Program of Shanghai Municipal Education Commission (2018YFC1705103); Shanghai Municipal Huangpu District Commission (HLQ202004). Science and Technology Commission of Shanghai Municipality (18410722300, 19140902400, 20015800400, 20ZR1432500); Clinical Research Plan of SHDC (SHDC2020CR4006); Commission of Health and Family Planning of Pudong District (PWZxq2017-17, PW2015D-5); The funders played no role in the design or conduct of the study, collection, management, analysis, or interpretation of data or in the preparation, review, or approval of the article.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors in this study declare no conflicts of interest.
References
1. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi:10.1038/nrendo.2017.151
2. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
3. Price HI, Agnew MD, Gamble JM. Comparative cardiovascular morbidity and mortality in patients taking different insulin regimens for type 2 diabetes: a systematic review. BMJ Open. 2015;5:e006341. doi:10.1136/bmjopen-2014-006341
4. Wang T, Lu J, Shi L, et al. Association of insulin resistance and beta-cell dysfunction with incident diabetes among adults in China: a nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol. 2020;8:115–124. doi:10.1016/S2213-8587(19)30425-5
5. Fenech G, Rajzbaum G, Mazighi M, et al. Serum uric acid and cardiovascular risk: state of the art and perspectives. Joint Bone Spine. 2014;81:392–397. doi:10.1016/j.jbspin.2014.01.008
6. Katsiki N, Dimitriadis GD, Mikhailidis DP. Serum uric acid and diabetes: from pathophysiology to cardiovascular disease. Curr Pharm Des. 2021;27:1941–1951. doi:10.2174/1381612827666210104124320
7. Lee SW, Kim HC, Nam C, et al. Age-differential association between serum uric acid and incident hypertension. Hypertens Res. 2019;42:428–437. doi:10.1038/s41440-018-0168-4
8. Wan H, Wang Y, Chen Y, et al. Different associations between serum urate and diabetic complications in men and postmenopausal women. Diabetes Res Clin Pract. 2020;160:108005.
9. Chen D, Sun X, Zhao X, et al. Associations of serum uric acid and urinary albumin with the severity of diabetic retinopathy in individuals with type 2 diabetes. BMC Ophthalmol. 2020;20:467.
10. Grassi D, Ferri L, Desideri G, et al. Chronic hyperuricemia, uric acid deposit and cardiovascular risk. Curr Pharm Des. 2013;19:2432–2438. doi:10.2174/1381612811319130011
11. Li X, Meng X, Timofeeva M, et al. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. 2017;357:j2376. doi:10.1136/bmj.j2376
12. Ioannou GN, Boyko EJ. Effects of menopause and hormone replacement therapy on the associations of hyperuricemia with mortality. Atherosclerosis. 2013;226:220–227. doi:10.1016/j.atherosclerosis.2012.10.044
13. Du P, Zhang B, Wang HJ, et al. The prevalence and secular trends of abdominal obesity among Chinese adults, 1993-2011. Ann Epidemiol. 2015;25:797–799. doi:10.1016/j.annepidem.2015.06.082
14. Nakhjavani M, Imani M, Larry M, et al. Metabolic syndrome in premenopausal and postmenopausal women with type 2 diabetes: loss of protective effects of premenopausal status. J Diabetes Metab Disord. 2014;13:102.
15. Lejskova M, Alusik S, Valenta Z, et al. Natural postmenopause is associated with an increase in combined cardiovascular risk factors. Physiol Res. 2012;61:587–596. doi:10.33549/physiolres.932313
16. Castro-Barquero S, Ruiz-Leon AM, Sierra-Perez M, et al. Dietary strategies for metabolic syndrome: a comprehensive review. Nutrients. 2020;12:2983. doi:10.3390/nu12102983
17. Hopkins AL, Lamm MG, Funk JL, et al. Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: a comprehensive review of animal and human studies. Fitoterapia. 2013;85:84–94. doi:10.1016/j.fitote.2013.01.003
18. Hoy SM. Pitavastatin: a review in hypercholesterolemia. Am J Cardiovasc Drugs. 2017;17:157–168. doi:10.1007/s40256-017-0213-8
19. Athyros VG, Doumas M, Imprialos KP, et al. Diabetes and lipid metabolism. Hormones. 2018;17:61–67. doi:10.1007/s42000-018-0014-8
20. Wong NKP, Nicholls SJ, Tan JTM, et al. The role of high-density lipoproteins in diabetes and its vascular complications. Int J Mol Sci. 2018;19:1680. doi:10.3390/ijms19061680
21. Aktas G, Kocak MZ, Bilgin S, et al. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male. 2020;23:1098–1102. doi:10.1080/13685538.2019.1678126
22. Aktas G, Khalid A, Kurtkulagi O, et al. Poorly controlled hypertension is associated with elevated serum uric acid to HDL-cholesterol ratio: a cross-sectional cohort study. Postgrad Med. 2022;134:297–302. doi:10.1080/00325481.2022.2039007
23. Kurtkulagi O, Tel BMA, Kahveci G, et al. Hashimoto’s thyroiditis is associated with elevated serum uric acid to high density lipoprotein-cholesterol ratio. Rom J Intern Med. 2021;59:403–408. doi:10.2478/rjim-2021-0023
24. Kosekli MA, Kurtkulagii O, Kahveci G, et al. The association between serum uric acid to high density lipoprotein-cholesterol ratio and non-alcoholic fatty liver disease: the abund study. Rev Assoc Med Bras. 1992;67:549–554.
25. Kocak MZ, Aktas G, Erkus E, et al. Neuregulin-4 is associated with plasma glucose and increased risk of type 2 diabetes mellitus. Swiss Med Wkly. 2019;149:w20139. doi:10.4414/smw.2019.20139
26. Kocak MZ, Aktas G, Atak BM, et al. Is Neuregulin-4 a predictive marker of microvascular complications in type 2 diabetes mellitus? Eur J Clin Invest. 2020;50:e13206. doi:10.1111/eci.13206
27. Chen LL, Peng MM, Zhang JY, et al. Elevated circulating Neuregulin4 level in patients with diabetes. Diabetes Metab Res Rev. 2017;33:e2870. doi:10.1002/dmrr.2870
28. Yazdi F, Baghaei MH, Baniasad A, et al. Investigating the relationship between serum uric acid to high-density lipoprotein ratio and metabolic syndrome. Endocrinol Diabetes Metab. 2022;5:e00311. doi:10.1002/edm2.311
29. Zhang YN, Wang QQ, Chen YS, et al. Association between serum uric acid to HDL-cholesterol ratio and nonalcoholic fatty liver disease in lean Chinese adults. Int J Endocrinol. 2020;2020:5953461.
30. Kocak MZ, Aktas G, Erkus E, et al. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev Assoc Med Bras. 1992;65:9–15.
31. Wang Y, Zhang W, Xia F, et al. Moderation effect of economic status in the association between early life famine exposure and MAFLD in adulthood. Liver Int. 2021;2021:5.
32. Wan H, Wang Y, Xiang Q, et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol. 2020;19:118.
33. Wang N, Chen Y, Ning Z, et al. Exposure to famine in early life and nonalcoholic fatty liver disease in adulthood. J Clin Endocrinol Metab. 2016;101:2218–2225. doi:10.1210/jc.2016-1076
34. Ning Z, Zhang K, Zhao L, et al. Exacerbation of liver steatosis following exposure to famine and overnutrition. Mol Nutr Food Res. 2017;61. DOI:10.1002/mnfr.201700097
35. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9:373–392. doi:10.1016/S2213-8587(21)00045-0
36. Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi:10.1001/jama.2013.168118
37. Qi H, Hu C, Wang S, et al. Early life famine exposure, adulthood obesity patterns and the risk of nonalcoholic fatty liver disease. Liver Int. 2020;40:2694–2705. doi:10.1111/liv.14572
38. Ji H, Zhang H, Xiong J, et al. eGFRs from Asian-modified CKD-EPI and Chinese-modified CKD-EPI equations were associated better with hypertensive target organ damage in the community-dwelling elderly Chinese: the Northern Shanghai Study. Clin Interv Aging. 2017;12:1297–1308. doi:10.2147/CIA.S141102
39. Authors/Task Force M, Guidelines ESCCfP, Societies ESCNC. ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205.
40. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290–294. doi:10.4239/wjd.v4.i6.290
41. Ndrepepa G. Uric acid and cardiovascular disease. Clin Chim Acta. 2018;484:150–163. doi:10.1016/j.cca.2018.05.046
42. Chang DY, Wang JW, Chen M, et al. Association between serum uric acid level and mortality in China. Chin Med J. 2021;134:2073–2080. doi:10.1097/CM9.0000000000001631
43. Zalawadiya SK, Veeranna V, Mallikethi-Reddy S, et al. Uric acid and cardiovascular disease risk reclassification: findings from NHANES III. Eur J Prev Cardiol. 2015;22:513–518. doi:10.1177/2047487313519346
44. Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384(9943):618–625. doi:10.1016/S0140-6736(14)61217-4
45. Ahmed HM, Miller M, Nasir K, et al. Primary low level of high-density lipoprotein cholesterol and risks of coronary heart disease, cardiovascular disease, and death: results from the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2016;183:875–883. doi:10.1093/aje/kwv305
46. Kaze AD, Santhanam P, Musani SK, et al. Metabolic dyslipidemia and cardiovascular outcomes in type 2 diabetes mellitus: findings from the look AHEAD study. J Am Heart Assoc. 2021;10:e016947. doi:10.1161/JAHA.120.016947
47. Kostara CE, Ferrannini E, Bairaktari ET, et al. Early signs of atherogenic features in the HDL lipidomes of normolipidemic patients newly diagnosed with type 2 diabetes. Int J Mol Sci. 2020;21:8835. doi:10.3390/ijms21228835
48. Cheatham CL, Vazquez-Vidal I, Medlin A, et al. Blueberry consumption affects serum uric acid concentrations in older adults in a sex-specific manner. Antioxidants. 2016;29:43. doi:10.3390/antiox5040043
49. De Cosmo S, Viazzi F, Pacilli A, et al. Serum uric acid and risk of CKD in type 2 diabetes. Clin J Am Soc Nephrol. 2015;10:1921–1929. doi:10.2215/CJN.03140315
50. Xu Y, Zhu J, Gao L, et al. Hyperuricemia as an independent predictor of vascular complications and mortality in type 2 diabetes patients: a meta-analysis. PLoS One. 2013;8:e78206. doi:10.1371/journal.pone.0078206
51. Russo GT, De Cosmo S, Viazzi F, et al. Plasma triglycerides and HDL-C levels predict the development of diabetic kidney disease in subjects with type 2 diabetes: the AMD annals initiative. Diabetes Care. 2016;39:2278–2287. doi:10.2337/dc16-1246
52. Cai X, Chen Y, Yang W, et al. The association of smoking and risk of diabetic retinopathy in patients with type 1 and type 2 diabetes: a meta-analysis. Endocrine. 2018;62:299–306. doi:10.1007/s12020-018-1697-y
53. Morton J, Zoungas S, Li Q, et al. Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: results of the ADVANCE study. Diabetes Care. 2012;35:2201–2206. doi:10.2337/dc12-0306
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

