Back to Journals » International Journal of General Medicine » Volume 14
Association Between Triglyceride-Glucose Index and Risk of Periodontitis: A Cross-Sectional Study
Authors Li L, Li L, Zhou Y, Chen X, Xu Y
Received 17 September 2021
Accepted for publication 1 December 2021
Published 16 December 2021 Volume 2021:14 Pages 9807—9816
DOI https://doi.org/10.2147/IJGM.S339863
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Lili Li,1– 3 Lu Li,1– 3 Yi Zhou,1– 3 Xu Chen,1– 3 Yan Xu1– 3
1Department of Periodontology, The Affiliated Stomatological Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, People’s Republic of China; 2Jiangsu Province Key Laboratory of Oral Diseases, Nanjing Medical University, Nanjing, Jiangsu Province, People’s Republic of China; 3Jiangsu Province Engineering Research Center of Stomatological Translational Medicine, Nanjing, Jiangsu Province, People’s Republic of China
Correspondence: Yan Xu
Department of Periodontology, The Affiliated Stomatological Hospital of Nanjing Medical University, No. 136, Hanzhong Road, Gulou District, Nanjing, Jiangsu Province, 210029, People’s Republic of China
Tel/Fax +86 25-69593041
Email [email protected]
Background: The purpose of this study was to investigate the TyG index in the occurrence of periodontitis among the United States (US) population.
Methods: We analyzed clinical data from 4813 participants in the National Health and Nutrition Examination Survey (NHANES) from 2009 to 2014. the TyG index was calculated as ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]. Dose–response curves, univariate and multivariate logistic analyses were used to analyze the adjusted odds ratio (aOR) and 95% confidence interval (CI) between TyG index and periodontitis. In addition, we performed 1:1 propensity score matching (PSM) for periodontitis and no periodontitis participants to further explore the relationship between TyG and periodontitis.
Results: A total of 4813 participants were included in our study, of which 1353 (28.1%) reported periodontitis and 3460 (71.9%) no periodontitis. The dose–response curves showed a non-linear positive association between TyG and periodontitis, with the risk of periodontitis increased with increasing TyG. In addition, similar results were still observed after subgroup analysis and PSM analysis. After adjusting for confounding variables, multivariate logistic analysis showed that TyG was associated with an increased risk of periodontitis (aOR =1.153; 95% CI 1.006– 1.322, p=0.034).
Conclusion: Elevated TyG index was significantly associated with a high risk of periodontitis, and people with a high TyG index should be aware of the risk of periodontitis progression in order to establish lifestyle changes at an early stage.
Keywords: periodontitis, triglyceride-glucose index, NHANES, propensity score matching
Introduction
Periodontitis is a chronic bacterial infection that occurs in the supporting tissues of the periodontium, with plaque as the initiating factor.1 Periodontitis is characterized by gingival inflammation, periodontal pocket formation, attachment loss and alveolar bone resorption, which can eventually lead to tooth loosening and tooth loss, and seriously affects the oral health of patients.2,3 2009~2010 national epidemiological survey in the United States showed that the proportions of mild, moderate and severe periodontitis were 8.7%, 30.0% and 8.5%, respectively.4 Periodontitis, with its high incidence, high risk, complex etiology, recurrent pathological process and difficulty in healing, is the primary cause of tooth loss in adults and has become an important public health problem in China and worldwide.5,6
Numerous epidemiological studies have shown that periodontitis was associated with several diseases including metabolic syndrome (MetS), which was based on the pathophysiology of obesity, diabetes or abnormal glucose tolerance, hypertension and dyslipidemia.7–10 Insulin resistance (IR) plays a key role in the pathogenesis of MetS and diabetes mellitus.11 IR is characterized by reduced sensitivity of cells in the body to the action of insulin, which in turn leads to the development of diabetes-related complications.12 In addition, IR, which is closely associated with obesity, is more common in patients with type 2 diabetes mellitus and is an important cause of hyperglycemia.13,14
The triglyceride-glucose (TyG) index derived from fasting triglycerides (TG) and fasting blood glucose (FBG) is considered a simple and inexpensive method to assess IR and has been shown to perform better than the homeostasis model assessment of IR (HOMA-IR),15–18 and our previous study found that the TyG index was independently associated with kidney stones.19 Some studies have shown that IR is closely associated with periodontitis.20,21 However, no studies have examined the correlation between TyG index and the occurrence of periodontitis. Therefore, we conducted the present study to clarify the relationship between TyG index and periodontitis using samples from the National Health and Nutrition Examination Survey (NHANES) database.
Materials and Methods
Study Design and Patients
The data used in this study were obtained from the NHANES database. NHANES is conducted annually by the National Center for Health Statistics (NCHS), a division of the Centers for Disease Control and Prevention (CDC), to assess the health nutritional status and health behaviors of the unstructured population in the United States.22 A complex, multi-stage probability sampling design is used in the NHANES survey to obtain representative data.23 All NHANES protocols are implemented in accordance with the United States Department of Health and Human Services (HHS) human research subject protection policy and are reviewed and standardized annually by the NCHS Research Ethics Review Committee. All subjects participating in the survey have signed informed consent. All data used in this study were published free of charge by NHANES and required no additional authorization or ethical review.
In this study, we selected data from three survey cycles (2009–2010, 2011–2012 and 2013–2014) of NHANES for a cross-sectional study. Total 30,468 participants were included in NHANES from 2009–2014, of which 11,753 had completed periodontal examinations. In addition, we excluded 1091 participants with missing teeth, 5714 with unknown triglyceride, 16 with unknown glucose, 97 with unknown LDL cholesterol and 22 with unknown body mass index (BMI). Ultimately, 4813 participants were included in the study. Figure 1 shows additional details of the overall study design, sampling and exclusion criteria
 |
Figure 1 Schematic flowchart of exclusion criteria in our study. |
Study Variables and Outcome
Fasting glucose and lipid levels were the main observables in this study, including serum TG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). In addition, other covariates were included, such as age (≤50 and >50 years), sex (male and female), education level (less than high school, high school or equivalent, college or higher and other), race (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic and other race), marital status (married and unmarried/other), BMI (<25.0 kg/m2 and ≥25.0 kg/m2), hypertension (yes and no/unknown), diabetes mellitus (yes, prediabetes and no/unknown), smoking status (never, former and current), vigorous activity (yes and no) and moderate activity (yes and no).
Periodontal Examination
In NHANES, adults aged 30 years and older were eligible for a full-mouth periodontal examination (FMPE) that included assessment of probing pocket depths (PPD) and clinical attachment loss (CAL). Prevalence was reported using the CDC/AAP recommended case definition for periodontitis surveillance. The oral health examination was performed by dental examiners and a full-mouth periodontal evaluation is performed on six areas of each tooth (distal-buccal, mid- facial, mesio-facial, disto-lingual, mid-lingual, and mesio-lingual). Periodontitis was defined as follows: severe periodontitis: two or more interproximal sites with CAL ≥ 6 mm (not on the same tooth) and one or more interproximal sites with PPD ≥ 5 mm; moderate periodontitis: two or more interproximal sites with clinical CAL ≥ 4 mm (not on the same tooth) or two or more interproximal sites with PPD ≥ 5 mm (not on the same tooth); mild periodontitis: the presence of at least 2 or more interproximal sites with CAL ≥ 3mm and PPD ≥ 4mm (not on the same tooth), or one site with PPD ≥ 5mm.24
Statistical Analysis
The TyG index was calculated as ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2] as previously described.18 Continuous variables were expressed as mean and standard deviation, and t-tests for slope were used in the generalized linear model. Categorical variables were expressed as numbers and percentages and analyzed by chi-square test. Based on the median of the TyG index (8.5), we divided participants into high and low TyG groups. Univariate and multivariate logistic regression were used to assess the relationship between TyG index and periodontitis, and the associated adjusted odds ratio (aOR) and 95% confidence interval (CI) were calculated. In addition, we adjusted for all confounding variables and used restricted cubic spline functions to describe the dose-response relationship between TyG index and periodontitis. Subsequently, we further assessed the relationship between TyG index and periodontitis in the subgroup. To eliminate the possible influence of other variables, we performed a 1:1 propensity score matching (PSM) for periodontitis and no periodontitis participants, and explored the relationship between TyG index and periodontitis using dose-response curves. PSM refers to the screening of the experimental and control groups by certain statistical methods so that the screened study subjects in terms of clinical characteristics (adjusting for potential confounders) comparable.25 Statistical analyses were performed using SPSS software (version 24.0) and R software (version 3.6.2). P value < 0.05 was considered statistically significant.
Results
A total of 4813 eligible participants were retrieved from 2009 to 2014 for inclusion in the final study. Based on the status of periodontitis, Table 1 shows the general characteristics of all participants. Of the 4813 participants, 1353 participants (28.1%) had periodontitis, of which 463 (9.6%) had mild periodontitis and 890 (18.5) had moderate/severe periodontitis. Chi-square test revealed significant differences between the periodontitis and no periodontitis groups for sex, age, race, education level, marital status, BMI, hypertension, diabetes mellitus smoking status, vigorous activity and moderate activity. Participants with periodontitis were predominantly male (62.3% vs 43.3%), low education (59.5% vs 39.1%), unmarried (47.5% vs 38.5%), current smoking (27.5% vs 14.3%), no vigorous activity (86.8% vs 78.3%), no moderate activity (65.4% vs 55.4%), and combined with hypertension (41.5% vs 36.3%) or diabetes mellitus (14.4% vs 10.9%) compared to participants without periodontitis. In addition, participants with periodontitis had higher levels of missing teeth, PD ≥ 4 mm teeth, PD ≥ 5 mm teeth, AL ≥ 3 mm teeth, AL ≥ 4 mm teeth, glucose, triglycerides, and TyG than those without periodontitis and increased with increasing periodontitis; HDL-C was lower than those without periodontitis and decreased with increasing periodontitis (Table 1).
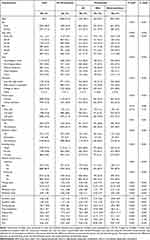 |
Table 1 Baseline Characteristics of 4813 Participants According to Periodontal Status |
We used univariate and multivariate logistic analyses to assess the risk of TyG levels and periodontitis (Table 2). Univariate logistic regression showed that TyG was associated with an increased risk of periodontitis (aOR =1.343; 95% CI 1.212–1.489, p<0.001). In addition, after adjusting for sex, age, race, education level, marital status, BMI, hypertension, diabetes mellitus, smoking status, vigorous activity and moderate activity, multivariate logistic analysis showed that TyG remained an independent risk factor for periodontitis (aOR =1.153; 95% CI 1.006–1.322, p=0.034).
 |
Table 2 Adjusted Odds Ratios for Associations Between the TyG and the Risk of Periodontitis in NHANES 2009–2014 |
To assess the relationship between TyG index and periodontitis, dose-response curve was used. In all participants, after adjusting for sex, age, race, education level, marital status, BMI, hypertension, diabetes mellitus, smoking status, vigorous activity and moderate activity, dose-response analysis curve with restricted cubic spline functions showed that periodontitis risk increased with TyG index and that TyG index was nonlinearly and positively associated with periodontitis risk (Figure 2). In addition, the optimal TyG level was 7.84 when the OR was 1 (Figure 2). Furthermore, similar results were observed in the subgroup analysis (Figure 3). To eliminate the possible influence of other variables, we performed a 1:1 PSM for participants with and without periodontitis (Figure S1). The general characteristics of all participants after PSM are shown in Table S1. After PSM, the dose-response curve showed a non-linear positive correlation between TyG index and periodontitis risk (Figure 4).
 |
Figure 2 Dose–response curve between triglyceride-glucose (TyG) index and periodontitis before propensity score matching (PSM). |
 |
Figure 3 Dose–response curve between triglyceride-glucose (TyG) index and periodontitis were analyzed in subgroups before propensity score matching (PSM). |
 |
Figure 4 Dose–response curve between triglyceride-glucose (TyG) index and periodontitis after propensity score matching (PSM). |
Discussion
In the present large sample cross-sectional study, we evaluated the relationship between TyG index and periodontitis using the nationally representative NHANES database. In this study, we found that high TyG levels were associated with increased odds of periodontitis in the US population ≥30 years of age. The dose-response curves showed a non-linear positive association between TyG index and the risk of periodontitis. Furthermore, this result remained consistent after different subcomponent stratification analyses and PSM analyses were performed. To the best of our knowledge, this is the first study to explore the relationship between TyG index and periodontitis in a normal population.
Several previous studies have shown varying degrees of association between periodontitis and various components of MetS (like obesity, increased TG, decreased HDL-C, hypertension, hyperglycemia).20,26,27 Numerous epidemiological studies have shown that the core pathogenesis of MetS is IR.28 Diabetes mellitus is an important component of MetS. It has been shown that diabetic patients have three times higher risk of developing periodontal disease than non-diabetic patients.29 Periodontitis is now considered to be the sixth major complication of diabetes.30 The longer the duration of diabetes and the poorer the glycemic control, the higher the incidence of periodontal disease. In our study, we found that 195 (34.1%) out of 572 diabetic participants had periodontitis and 1119 (27.2%) out of 4108 prediabetic participants had periodontitis. In addition, the mean value of TyG index for participants with diabetes mellitus and periodontitis was 9.07, which was significantly higher than the mean value of TyG index for all participants (8.56).
The TyG index based on TG and fasting glucose was first proposed in 2008 by Simental-Mendia et al17 in a clinical study including 748 healthy individuals, and several subsequent studies have found that the TyG index can be considered as the simplest and most effective marker for clinical assessment of IR.31,32 The association between TyG index and MetS has also been demonstrated. Angoorani et al33 found that TyG index can be a valid indicator of MetS in children and adolescents by including 14,400 students aged 7–18 years. Raimi et al34 assessed the physical status of 473 Nigerian adults and found that TyG index was effective in identifying MetS and the product of TyG index and anthropometric indices improved the identification and prediction of MetS. In addition, Aslan et al35 demonstrated that the TyG index is a better marker than HOMA-IR to determine the risk of MetS. A recent study involving 1542 Chinese population found that the TyG index had the strongest predictive value for the development of MetS over a 5-year period.36
The mechanistic details of the relationship between TyG index and periodontitis remain to be further explored and may be related to the following explanations. First, patients with periodontitis have poor IR.20,21 Second, reverse causality could also explain the association between high levels of TyG and higher odds of periodontitis. A higher TyG index indicates not only IR, but also poor health status.37,38 Third, inflammation and oxidative stress may play an important role in the association of TyG index with periodontitis. Elevated lipids have been found to modulate the release of inflammatory mediators and growth factors, causing damage to the vascular endothelium. These would exacerbate the periodontal tissue inflammatory response and increase the destruction of periodontal tissue.39,40
Although the current study found a positive association between TyG index and the risk of periodontitis, some limitations of this study need to be acknowledged. First, the current study was a cross-sectional study and the causal relationship between TyG index and periodontitis could not be confirmed. Second, the present study was based on the US population and may not be applicable to other regions. Lastly, the present investigation was conducted as a retrospective study, and further prospective studies are needed.
Conclusion
Our study found a non-linear positive association between TyG index and periodontitis after adjusting for confounders in the US population, and a high TyG index was associated with an increased odd of periodontitis. This finding suggests that the TyG index would be a novel biomarker for predicting individualized clinical treatment.
Data Sharing Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethical Approval Statement
This study used previously collected deidentified data, which was deemed exempt from review by the Ethics Committee of the Affiliated Stomatological Hospital of Nanjing Medical University.
Acknowledgments
The authors thank the National Health and Nutrition Examination Survey (NHANES) staff and investigators. A special thanks to all who participated in the NHANES survey for making this study possible through their participation.
Funding
This work is supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions, Grant/Award Number: PAPD, 2018-87; Key projects of social development of Jiangsu Department of Science and Technology, Grant/Award Number: BE2020707.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–155. doi:10.1016/S0140-6736(04)16627-0
2. Hienz SA, Paliwal S, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res. 2015;2015:615486. doi:10.1155/2015/615486
3. Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. doi:10.1038/nri3785
4. Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Cdc periodontal disease surveillance workgroup: James Beck GDRP. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–920. doi:10.1177/0022034512457373
5. Bhattarai KR, Junjappa R, Handigund M, Kim HR, Chae HJ. The imprint of salivary secretion in autoimmune disorders and related pathological conditions. Autoimmun Rev. 2018;17(4):376–390. doi:10.1016/j.autrev.2017.11.031
6. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. doi:10.1038/nrdp.2017.38
7. Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(Suppl 4):3–10. doi:10.1111/j.1469-0691.2007.01798.x
8. Daudt LD, Musskopf ML, Mendez M, et al. Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. Braz Oral Res. 2018;32:e35. doi:10.1590/1807-3107bor-2018.vol32.0035
9. Gobin R, Tian D, Liu Q, Wang J. Periodontal diseases and the risk of metabolic syndrome: an updated systematic review and meta-analysis. Front Endocrinol. 2020;11:336. doi:10.3389/fendo.2020.00336
10. Fiorillo L, Cervino G, Laino L, et al. Porphyromonas gingivalis, periodontal and systemic implications: a systematic review. Dent J. 2019;7(4):114.
11. Pandit K, Mukhopadhyay P, Chatterjee P, Majhi B, Chowdhury S, Ghosh S. Assessment of insulin resistance indices in individuals with lean and obese metabolic syndrome compared to normal individuals: a population based study. J Assoc Physicians India. 2020;68(10):29–33.
12. Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19–39.
13. Zhang H, Huang Y, Chen S, et al. Hydrogen sulfide regulates insulin secretion and insulin resistance in diabetes mellitus, a new promising target for diabetes mellitus treatment? A review. J Adv Res. 2021;27:19–30. doi:10.1016/j.jare.2020.02.013
14. Gu X, Al Dubayee M, Alshahrani A, et al. Distinctive metabolomics patterns associated with insulin resistance and type 2 diabetes mellitus. Front Mol Biosci. 2020;7:609806. doi:10.3389/fmolb.2020.609806
15. Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi:10.1210/jc.2010-0288
16. Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57–63. doi:10.2337/diacare.23.1.57
17. Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. doi:10.1089/met.2008.0034
18. Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16(1):108. doi:10.1186/s12933-017-0589-4
19. Jiang H, Li L, Liu J, et al. Triglyceride-glucose index as a novel biomarker in the occurrence of kidney stones: a cross-sectional population-based study. Int J Gen Med. 2021;14:6233–6244. doi:10.2147/IJGM.S334821
20. Benguigui C, Bongard V, Ruidavets JB, et al. Metabolic syndrome, insulin resistance, and periodontitis: a cross-sectional study in a middle-aged French population. J Clin Periodontol. 2010;37(7):601–608. doi:10.1111/j.1600-051X.2010.01571.x
21. Gurav AN. Periodontitis and insulin resistance: casual or causal relationship? Diabetes Metab J. 2012;36(6):404–411. doi:10.4093/dmj.2012.36.6.404
22. Mao W, Wu J, Zhang Z, Xu Z, Xu B, Chen M. Neutrophil-lymphocyte ratio acts as a novel diagnostic biomarker for kidney stone prevalence and number of stones passed. Transl Androl Urol. 2021;10(1):77–86. doi:10.21037/tau-20-890
23. Mao W, Hu Q, Chen S, et al. Polyfluoroalkyl chemicals and the risk of kidney stones in US adults: a population-based study. Ecotoxicol Environ Saf. 2021;208:111497. doi:10.1016/j.ecoenv.2020.111497
24. Eke PI, Dye BA, Wei L, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86(5):611–622. doi:10.1902/jop.2015.140520
25. Austin PC. The performance of different propensity score methods for estimating marginal odds ratios. Stat Med. 2007;26(16):3078–3094. doi:10.1002/sim.2781
26. Watanabe K, Cho YD. Periodontal disease and metabolic syndrome: a qualitative critical review of their association. Arch Oral Biol. 2014;59(8):855–870. doi:10.1016/j.archoralbio.2014.05.003
27. Hatipoglu H, Yaylak F, Gungor Y. A brief review on the periodontal health in metabolic syndrome patients. Diabetes Metab Syndr. 2015;9(2):124–126. doi:10.1016/j.dsx.2015.02.007
28. Bagry HS, Raghavendran S, Carli F. Metabolic syndrome and insulin resistance: perioperative considerations. Anesthesiology. 2008;108(3):506–523. doi:10.1097/ALN.0b013e3181649314
29. Daniel R, Gokulanathan S, Shanmugasundaram N, Lakshmigandhan M, Kavin T. Diabetes and periodontal disease. J Pharm Bioallied Sci. 2012;4(Suppl 2):S280–S282. doi:10.4103/0975-7406.100251
30. Devanoorkar A, Kathariya R, Guttiganur N, Gopalakrishnan D, Bagchi P. Resistin: a potential biomarker for periodontitis influenced diabetes mellitus and diabetes induced periodontitis. Dis Markers. 2014;2014:930206. doi:10.1155/2014/930206
31. Vasques AC, Novaes FS, de Oliveira Mda S, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–e100. doi:10.1016/j.diabres.2011.05.030
32. Guerrero-Romero F, Villalobos-Molina R, Jimenez-Flores JR, et al. Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch Med Res. 2016;47(5):382–387. doi:10.1016/j.arcmed.2016.08.012
33. Angoorani P, Heshmat R, Ejtahed HS, et al. Validity of triglyceride-glucose index as an indicator for metabolic syndrome in children and adolescents: the CASPIAN-V study. Eat Weight Disord. 2018;23(6):877–883. doi:10.1007/s40519-018-0488-z
34. Raimi TH, Dele-Ojo BF, Dada SA, et al. Triglyceride-glucose index and related parameters predicted metabolic syndrome in Nigerians. Metab Syndr Relat Disord. 2021;19(2):76–82. doi:10.1089/met.2020.0092
35. Aslan Cin NN, Yardimci H, Koc N, Ucakturk SA, Mehtap Akcil OK. Triglycerides/high-density lipoprotein cholesterol is a predictor similar to the triglyceride-glucose index for the diagnosis of metabolic syndrome using International Diabetes Federation criteria of insulin resistance in obese adolescents: a cross-sectional study. J Pediatr Endocrinol Metab. 2020;33(6):777–784. doi:10.1515/jpem-2019-0310
36. Lin HY, Zhang XJ, Liu YM, Geng LY, Guan LY, Li XH. Comparison of the triglyceride glucose index and blood leukocyte indices as predictors of metabolic syndrome in healthy Chinese population. Sci Rep. 2021;11(1):10036. doi:10.1038/s41598-021-89494-9
37. Zhang M, Wang B, Liu Y, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: the Rural Chinese Cohort Study. Cardiovasc Diabetol. 2017;16(1):30. doi:10.1186/s12933-017-0514-x
38. Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14(5):575–585. doi:10.1016/j.cmet.2011.07.015
39. Nam KW, Kwon HM, Jeong HY, Park JH, Kwon H, Jeong SM. High triglyceride-glucose index is associated with subclinical cerebral small vessel disease in a healthy population: a cross-sectional study. Cardiovasc Diabetol. 2020;19(1):53. doi:10.1186/s12933-020-01031-6
40. Leong XF, Ng CY, Badiah B, Das S. Association between hypertension and periodontitis: possible mechanisms. ScientificWorldJournal. 2014;2014:768237. doi:10.1155/2014/768237
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
