Back to Journals » Cancer Management and Research » Volume 12
Association Between the HER2 Protein Expression Level and the Efficacy of Neoadjuvant Chemotherapy in HER2-Positive Breast Cancer
Authors Yan H, Xiao H, Zhu J, Zhang J, Liu Z
Received 25 August 2020
Accepted for publication 26 November 2020
Published 10 December 2020 Volume 2020:12 Pages 12715—12722
DOI https://doi.org/10.2147/CMAR.S278694
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Hui Yan, Hui Xiao, Jiujun Zhu, Jingyang Zhang, Zhenzhen Liu
Department of Breast Surgery, Breast Cancer Center, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
Correspondence: Zhenzhen Liu
Department of Breast Surgery, Breast Cancer Center, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
Tel +86 13603862755
Email [email protected]
Objective: This study aimed to assess the relationship between human epidermal growth factor receptor-2 (HER2) protein expression level and clinicopathological features of HER2-positive breast cancer, and to analyze whether the expression level of HER2 protein could predict the response to anti-HER2 therapy.
Methods: The present study included 296 patients with HER2-positive breast cancer receiving neoadjuvant chemotherapy (NAC) containing trastuzumab between January 2014 and November 2019. The univariate comparisons of the differences in clinicopathological parameters between different HER2 protein expression groups, and the association between HER2 protein expression level and efficacy of NAC, were made using a X2 test or Mann–Whitney U-test. Multivariate analyses of the differences in clinicopathological parameters between different HER2 protein expression groups, and the association between HER2 protein expression level and efficacy of NAC, were performed using logistic regression analysis.
Results: A total of 110 patients achieved a pathological complete response (pCR) after NAC. The pCR rate was 37.2%. The study showed that patients who were HR-negative, AR-positive, and CK5/6-negative had significantly higher expression level of HER2 protein [odds ratio (OR) = 0.183, P < 0.001; OR = 6.414, P = 0.004; OR = 0.261, P = 0.004, respectively]. Patients with HER2 3+ detected by immunohistochemistry (IHC) had significantly higher pCR rates compared with patients with HER2 2+. The HER2 protein expression level might effectively predict the efficacy of NAC in patients with HER2-positive breast cancer (OR = 3.520, P = 0.003).
Conclusion: The HER2 protein expression level was related to multiple clinical features in patients with HER2-positive breast cancer. For example, hormone receptor, androgen receptor, cytokeratin5/6, and HER2 protein expression level may be used to predict the response to NAC in patients with HER2-positive breast cancer and may serve as a predictive factor for NAC efficacy.
Keywords: breast neoplasm, human epidermal growth factor receptor-2, neoadjuvant chemotherapy, pathologic complete response
Introduction
Breast cancer is the most common malignant tumor among woman in China. Its incidence has been on the rise, and the onset age tends to be young.1 Breast cancer with human epidermal growth factor receptor-2 (HER2) overexpression is an aggressive subtype of breast cancer, promoting cancer cell growth. HER2-positive breast cancer accounts for about 15%–20% of patients with breast cancer.2 Neoadjuvant chemotherapy (NAC) largely increases the chances of operative and the chances of breast-conserving surgery. NAC combined with preoperative anti-HER2 therapy is the standard treatment for HER2-positive breast cancer, which can improve the pathologic complete response (pCR) rate. The overall survival (OS) and disease-free survival (DFS) of patients who had achieved pCR after receiving NAC significantly improved compared with the OS and DFS of patients without pCR.3–6 Therefore, developing predictive factors for NAC efficacy was important to select candidates with an improved predicted prognosis. Studies have found that high HER2 protein expression in patients receiving anti-HER2 treatment may indicate a better prognosis. For example, the analysis of the CLEOPATRA Phase III trial showed that HER2 3+ by immunohistochemistry (IHC) was associated with an improvement in progression-free survival (PFS) and OS (P = 0.05) in both the trastuzumab plus docetaxel groups dividing by pertuzumab or placebo.7 A previous study explored the response to NAC depending on the HER2 protein expression level. It showed that the expression level of HER2 protein was related to the clinical response rate following NAC (P = 0.01), but not related to the pCR rate (P = 0.05); however, the sample size is small (n=40).8 The studies on the association between HER2 protein expression and NAC efficacy are relatively few. In the present study, the data of 296 patients with HER2-positive breast cancer, who were treated at the Breast Cancer Center of Henan Cancer Hospital from January 2014 to November 2019, were retrospectively collected. The HER2 protein expression level and the correlation between clinicopathological features were analyzed, and the predictive value of the pCR rate after NAC was further discussed.
Patients and Methods
Patients and Samples
This retrospective study included 296 female patients with breast cancer, who were treated at the Breast Cancer Center of Henan Cancer Hospital from January 2014 to November 2019. The inclusion criteria were as follows: (1) ultrasound-guided core needle biopsy performed in patients with invasive breast cancer prior to chemotherapy; (2) date on estrogen receptor (ER), progesterone receptor (PR), androgen receptor (AR), HER2, Ki-67, cytokeratin5/6 (CK5/6), and epidermal growth factor receptor (EGFR) available for HER2-positive breast cancer; and (3) the cT stage determined by the tumor size during the clinical physical examination according to the seventh edition of American Joint Committee on Cancer (AJCC) clinical staging of patients with breast cancer; the status confirmed by core needle biopsy If axillary lymph nodes were palpated positively or imaging studies were suspicious for metastasis; (4) no history of radiotherapy, chemotherapy, endocrine therapy, or other antitumor treatments; (5) no contraindications of chemotherapy and NAC; and (6) breast-conserving surgery or modified radical mastectomy after receiving four to eight cycles of standard regimen containing trastuzumab, and dissection of the axillary lymph nodes. The exclusion criteria were as follows: (1) male sex; (2) HER2-negative or inflammatory breast cancer; (3) distant metastases detected via the imaging examination; (4) surgery not conducted, resulting in failure to assess response to chemotherapy; (5) incomplete medical records; (6) patients with severe cardiopulmonary dysfunction; and (7) patients with another malignant tumors (Figure 1).
 |
Figure 1 Flow chart of patients screening. |
Neoadjuvant Chemotherapy
Of the 296 patients, 175 received 6 cycles of the TCH regimen (docetaxel + carboplatin + herceptin), 28 received 6 cycles of the PH regimen (paclitaxel + herceptin), and 93 received 4 cycles of the EC regimen (epirubicin + cyclophosphamide) following 4 cycles of the TH regimen (docetaxel + herceptin).
Immunostaining
The tissue samples were fixed with 10% neutral formaldehyde, embedded in paraffin, and cut into 4‐μm‐thick continuous slices for IHC (to determine ER, PR, AR, HER2, Ki-67, CK5/6, and EGFR statuses). The criteria were as follows: (1) The criterion for determining ER and PR positivity was that 1% or more of tumor cells exhibited positive nuclear staining by IHC, positivity for either One or both was defined as hormone receptor (HR) positive, and both were negative at the same time as HR-negative. (2) The criterion for determining AR positivity was that 10% of tumor cells exhibited positive nuclear staining by IHC. (3) According to the recommendations of the American Society of Clinical Oncology-College of American Pathologists (ASCO-CAP) in 2013, HER2 positivity was defined as 3+ by IHC or amplification by fluorescence in situ hybridization (FISH). (4) The average ratio of Ki-67-positive cells to all tumor cells was used as a quantitative indicator after selecting 10 high-power visual fields for each section. The Ki67 expression threshold was 30% (high expression, ≥30%; low expression, <30%). (5) Complete pathological response was defined as the absence of invasive breast cancer cells in the breast and lymph nodes after NAC (ypT0ypN0).9
Statistical Analysis
All statistical analyses were performed using SPSS 22.0 (SPSS, Inc.). Patients were divided into HER2 2+ and HER2 3+ groups by IHC. Univariate comparisons of the difference in clinicopathological parameters (age, menopausal status, HR status, AR status, Ki67 index, CK5/6 status, and EGFR status) between the two groups, and the association between HER2 protein expression level and efficacy of NAC was analyzed using the X2 test, and the difference in clinicopathological parameters (cT stage and cN stage) between the two groups was determined using the Mann–Whitney U-test. Multivariate analyses of the difference in clinicopathological parameters between the two groups, and the association between HER2 protein expression level and efficacy of NAC, were performed using logistic regression analysis. Factors with P<0.2 in the univariate comparisons were included in the multivariate analysis model. A P value of<0.05 indicated a statistically significant difference.
Result
Patient Characteristics
A total of 296 patients with HER2-positive breast cancer were recruited, all of whom were woman. The median age was 47.5 years (range, 23‒72 years). Further, 189 (63.9%) patients were premenopausal.
Also, 218 (73.6%) patients were cT1/2 and 184 (62.2%) patients were HR-positive. Of the 296 patients, 57 (19.3%) were assorted into the HER2 2+ group and 239 (80.7%) into the HER2 3+ group. A pCR was noted in 110 (37.2%) patients but not in 186 (62.8%) patients.
Associations Between HER2 Protein Expression Level and Clinicopathological Parameters
HR-positive patients were more likely to be HER2 2+ compared with HR-negative patients (24.7% vs 10.0%; P = 0.002). The HER2 2+ rate was lower in the AR-positive group (17.4%) than in the AR-negative group (57.1%; P = 0.001). The CK5/6-positive group exhibited a higher HER2 3+ rate compared with the CK5/6-negetive group (60.0% vs 83.5%; P = 0.001). The Clinicopathological features, such as age, menopausal status, cT stage, cN stage, Ki67 index, and EGFR status, were not significantly different in patients with different HER2 protein expression levels (P > 0.05; Table 1). The results of multivariate analysis showed that HR status (P < 0.001), AR status (P = 0.004), and CK5/6 status (P = 0.004) were all related to the HER2 protein expression level. The results of multivariate analysis showed that patients who were HR-negative, AR-positive, and CK5/6-negative had a significantly higher expression level of HER2 protein (OR = 0.183, P < 0.001; OR = 6.414, P = 0.004; OR = 0.261, P = 0.004, respectively; Table 2).
 |
Table 1 Univariate Analyses for the Associations Between HER2 Protein Expression Level and Clinicopathological Characteristics |
 |
Table 2 Multivariate Analyses for the Associations Between HER2 Protein Expression Level and Clinicopathological Characteristics |
Pathological Response After NAC
The pCR rate was 37.2%. pCR was achieved in 11 of 57 patients in the HER2 2+ group, with a pCR rate of 19.3%. Also, pCR was achieved in 99 of 139 patients in the HER2 3+group, with a pCR rate of 41.4%. Significant differences in the pCR rate depending on the HER2 protein expression level are shown in Table 3. The univariate regression analysis in both groups revealed that the pCR rate in patients with high HER2 protein expression and those with a low cT stage was significantly higher (P = 0.002; P < 0.001, respectively). No significant association was found between pCR and other clinicopathological features including age, menstrual status, cN stage, HR status, AR status, Ki67 index, CK5/6 status, and EGFR status (Table 3). Factors with P<0.2 in univariate comparisons were included in the multivariate logistic regression analysis. The multivariate analysis revealed that the HER2 protein expression level was an independent predictive factor for pCR (OR = 3.520, P = 0.003). Additionally, The cT stage was also an independent predictive factor for pCR (P = 0.003; Table 4).
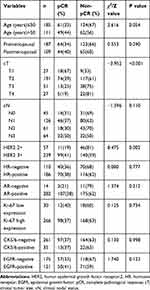 |
Table 3 Univariate Analyses for the Predictive Factors of Pathological Complete Response |
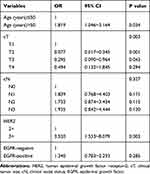 |
Table 4 Multivariate Analysis of Factors Affecting the Efficacy of Neoadjuvant Chemotherapy for HER2-Positive Breast Cancer |
Discussion
The overexpression and/or amplification of HER2 is not only correlated with disease progression and poor prognosis but also the only biomarker to decide anti-HER2 therapy.10 The emergence of trastuzumab has significantly improved the prognosis of patients with HER2-positive breast cancer,11 However, no apparent clinical benefit has been achieved in a considerable proportion of patients. The heterogeneity of HER2 protein expression and its predictive value for prognosis and neoadjuvant efficacy have received attention.7,12 The results of this study revealed that HR-negative, AR-positive, and CK5/6-negative patients had a higher expression level of HER2 protein. Patients with HER2 3+ detected by IHC had a higher pCR rate compared with patients with HER2 2+. Higher HER2 protein expression was predictive of pCR to NAC.
In this study, the expression level of HER2 protein was significantly higher in the HR-negative group than in the HR-positive group. Previous reports suggested some correlation between ER and HER2 signaling pathways. ER inhibited HER2 expression under the action of estrogen, and HER2 expression was enhanced when ER expression decreased.13 The study by Liu et al showed that HR expression negatively correlated with HER2 expression (r = −0.338, p = 0.000).14 The result of this study was consistent with previous findings. Some previous studies on the relationship between AR and HER2 expression showed no correlation between the expression of AR and HER2,15,16 some other studies revealed that the HER2 protein expression level was lower in the AR-positive group than in the AR-negative group.17 However, The present study showed that the HER protein expression level was higher in the AR-positive group than in the AR-negative group. These differences in results might be due in large part to the different molecular subtype composition ratios of the study population and the different distribution of clinicopathological characteristics. In addition, the assessment methods for AR, such as IHC, TMA, RIA, RPPA, were not uniform. And IHC also had different criteria for the definition of AR-positivity, including positive cells ≥10%, ≥5% and ≥1%.16 In this study, AR-positivity was defined as ≥10%.
In this study, pCR rates of patients with HER2 3+ detected by IHC was significantly higher. The data showed that the HER2 protein expression level in patients with HER2-positive breast cancer might effectively predict response to trastuzumab therapy. Three mechanisms were proposed for trastuzumab antitumor activity: (1) antibody-dependent cytotoxicity (ADCC); (2) cell cycle arrest caused by cascade inhibition of MAPK and PI3K signals; and (3) increased production of anti-angiogenic factors.18,19 In the whole-body context, natural killer cell‒mediated ADCC is stimulated by the binding of trastuzumab on the surface of HER2-overexpressing cells, which binds to the extracellular domain IV of HER2.20 Laboratory studies have also indicated that the degree of the ADCC response may be proportional to the HER2 protein expression level.21,22 Several basic studies theoretically supported the present study. However, no evidence is available to show that the other two mechanisms are related to the HER2 protein expression level. According to the ASCO-CAP guidelines in 2013, tumors with the HER2/CEP17 ratio ≥2.0 were considered HER2-positive when using FISH. Patients in the BCIRG-006 trial, whose breast cancers had an HER2/CEP17 ratio ≥2.0 but HER2 copy number >4 signals per cell, could not benefit from trastuzumab therapy (HR, 1.10; 95% CI, 0.31‒3.89; P = 0.886).23 Patients of this group might be a reason why patients with HER2 2+ had a lower pCR rate compared with patients with HER2 3+.
In a study from Burstein at al., IHC was used to detect the expression level of HER2 protein and analyze its value in predicting the efficacy of NAC.8 The results showed that the clinical response rate of HER2 2+ breast cancer was significantly lower than that of HER2 3+ breast cancer (38% vs 84%), but no difference in pCR rate was observed (P = 1.0), which might be because of the small sample size (n = 40). Another study showed that pCR occurred significantly more frequently in HER2 3+ tumors (P = 0.009).24 In a study from Scaltriti et al.12 HER2 protein expression strongly correlated with pCR in both HR-negative (ratio of 2.5; 95% CI, 1.44–4.20; P = 0.001) and HR-positive (ratio of 3.1; 95% CI, 1.61–5.81 P = 0.001) groups of patients treated with the combination of Lapatinib and Trastuzumab. This study used HERmark analysis to quantify the expression level of HER2 protein, which was more accurate and sensitive compared with IHC. The results of the present study were consistent with previous findings. In previous studies, HR-negative patients had more chances to achieve pCR compared with HR-positive patients.25,26 The present study showed that the HR status was not related to pCR because of the small sample size.
This study showed that the HER2 protein expression level in patients with HER2-positive breast cancer might effectively predict the response to NAC. Interestingly, the predictive value of HER2 expression for the efficacy of NAC has been described at several levels. A study indicated that the expression level of HER2 mRNA significantly correlated with pCR in HER2-positive and ER-positive breast cancer (P = 0.004), but had no correlation in ER-negative and HER2-positive breast cancer.27 A study including 14,597 patients demonstrated that the association between HER2/CEP17 ratio and pCR to NAC was linear (P < 0.001).28 Patients with HER2 heterogeneity seemed to be less responsive to anti-HER2 targeted therapy, as demonstrated by a lower achievement of pCR following NAC containing trastuzumab.29,30 Therefore, it is important to increase the accuracy of HER2 testing and explore the intrinsic subtypes of HER2-positive breast cancer to suggest rational therapeutic strategies and predict the efficacy of anti-HER2 treatment.
This study, using IHC to determine the expression level of HER2 protein and analyze its value in predicting the efficacy of NAC, had the largest sample size to date, thus providing an important basis for the exploration of more accurate HER2 testing, intrinsic subtype of HER2-positive breast cancer, and individualized treatment. However, the study had several limitations: it was retrospective; the analysis could only be performed in a subpopulation; it was subject to the misclassification bias or errors in record keeping; and it did not demonstrate whether OS and DFS were improved after achieving this increase in pCR rates. The results of this study showed the clinical utility of the HER2 protein expression level as a predictive factor for the prognosis of patients receiving NAC, which can be further validated by increasing the sample size and involving multicenter collaboration.
In summary, the present study showed that the HER2 protein expression level was related to multiple clinical features, such as HR, AR, and CK5/6, in patients with HER2-positive breast cancer. Also, the HER2 protein expression level might serve as an independent predictor of pCR to NAC.
Abbreviations
ADCC, antibody-dependent cellular cytotoxicity; AJCC, American Joint Committee on Cancer; AR, androgen receptor; ASCO-CAP, American Society of Clinical Oncology-College of American Pathologists; CK5/6, cytokeratin5/6; DFS, disease-free survival; EGFR, epidermal growth factor receptor; ER, estrogen receptor; FISH, fluorescence in situ hybridization; HER2, epidermal growth factor receptor-2; HR, hormone receptor; IHC, immunohistochemistry; NAC, neoadjuvant chemotherapy; OS, overall survival; pCR, pathological complete response; PFS, progression-free survival; PR, progesterone receptor.
Ethics Approval and Consent to Participate
This study was complied with the Declaration of Helsinki and approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University. The Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University did not require patients to agree to review their medical records (medical records were written by doctors and belonged to the medical records room. On the premise of not disclosing the privacy of patients, doctors can use it for clinical research). All data analyzed is anonymous, patient data are kept strictly confidential.
Acknowledgments
We thank the Department of Pathology, Affiliated Cancer Hospital of Zhengzhou University for providing us with the data of tissue.
Funding
This work was supported by a grant from Henan province science development plan project (202102310415).
Disclosure
The authors have no competing interests to declare.
References
1. Fan L, Strasser-Weippl K, Li -J-J, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–e289. doi:10.1016/S1470-2045(13)70567-9
2. Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–2429. doi:10.1016/S0140-6736(16)32417-5
3. Mauri D, Pavlidis N, Ioannidis J. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. doi:10.1093/jnci/dji021
4. Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet (London, England). 2010;375:377–384.
5. Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from national surgical adjuvant breast and bowel project B-18. J Clin Oncol. 1997;15(7):2483–2493. doi:10.1200/JCO.1997.15.7.2483
6. Buzdar A, Ibrahim N, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–3685. doi:10.1200/JCO.2005.07.032
7. Baselga J, Cortes J, Im SA, et al. Biomarker analyses in Cleopatra: a Phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32(33):3753–3761. doi:10.1200/JCO.2013.54.5384
8. Burstein HJ, Harris LN, Gelman R, et al. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol. 2003;21(1):46–53. doi:10.1200/JCO.2003.03.124
9. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi:10.1016/S0140-6736(13)62422-8
10. Godoy-Ortiz A, Sanchez-Munoz A, Chica Parrado MR, et al. Deciphering HER2 breast cancer disease: biological and clinical implications. Front Oncol. 2019;9:1124. doi:10.3389/fonc.2019.01124
11. Valachis A, Mauri D, Polyzos NP, et al. Trastuzumab combined to neoadjuvant chemotherapy in patients with HER2-positive breast cancer: a systematic review and meta-analysis. Breast. 2011;20(6):485–490. doi:10.1016/j.breast.2011.06.009
12. Scaltriti M, Nuciforo P, Bradbury I, et al. High HER2 expression correlates with response to the combination of lapatinib and trastuzumab. Clin Cancer Res. 2015;21:569–576.
13. Kunisue H, Kurebayashi J, Otsuki T, et al. Anti-HER2 antibody enhances the growth inhibitory effect of anti-oestrogen on breast cancer cells expressing both oestrogen receptors and HER2. Br J Cancer. 2000;82(1):46–51. doi:10.1054/bjoc.1999.0875
14. Liu X, Zheng Y, Qiao C, et al. Expression of SATB1 and HER2 in breast cancer and the correlations with clinicopathologic characteristics. Diagn Pathol. 2015;10(1):50. doi:10.1186/s13000-015-0282-4
15. Fu WF, Li JJ, Kang SH, et al. The expression, clinicopathologic characteristics, and prognostic value of androgen receptor in breast cancer: a bioinformatics analysis using public databases. DNA Cell Biol. 2020;39(5):864–874. doi:10.1089/dna.2019.5192
16. Vera-Badillo F, Templeton A, de Gouveia P, et al. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(1):djt319. doi:10.1093/jnci/djt319
17. Collins LC, Cole KS, Marotti JD, et al. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2011;24(7):924–931. doi:10.1038/modpathol.2011.54
18. Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215.
19. Zhang X, Chen J, Weng Z, et al. A new anti-HER2 antibody that enhances the anti-tumor efficacy of trastuzumab and pertuzumab with a distinct mechanism of action. Mol Immunol. 2020;119:48–58. doi:10.1016/j.molimm.2020.01.009
20. Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27(34):5838–5847. doi:10.1200/JCO.2009.22.1507
21. Cooley S, Burns LJ, Repka T, et al. Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neu. Exp Hematol. 1999;27(10):1533–1541. doi:10.1016/S0301-472X(99)00089-2
22. Mimura K, Kono K, Hanawa M, et al. Trastuzumab-mediated antibody-dependent cellular cytotoxicity against esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11(13):4898–4904. doi:10.1158/1078-0432.CCR-04-2476
23. Press M, Sauter G, Buyse M, et al. HER2 gene amplification testing by Fluorescent in situ Hybridization (FISH): comparison of the ASCO-College of American Pathologists Guidelines with FISH scores used for enrollment in breast cancer international research group clinical trials. J Clin Oncol. 2016;34(29):3518–3528. doi:10.1200/JCO.2016.66.6693
24. Arnould L, Arveux P, Couturier J, et al. Pathologic complete response to trastuzumab-based neoadjuvant therapy is related to the level of HER-2 amplification. Clin Cancer Res. 2007;13(21):6404–6409. doi:10.1158/1078-0432.CCR-06-3022
25. Robidoux A, Tang G, Rastogi P, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised Phase 3 trial. Lancet Oncol. 2013;14(12):1183–1192. doi:10.1016/S1470-2045(13)70411-X
26. Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28(12):2024–2031. doi:10.1200/JCO.2009.23.8451
27. Denkert C, Huober J, Loibl S, et al. HER2 and ESR1 mRNA expression levels and response to neoadjuvant trastuzumab plus chemotherapy in patients with primary breast cancer. Breast Cancer Res. 2013;15(1):R11. doi:10.1186/bcr3384
28. Greenwell K, Hussain L, Lee D, et al. Complete pathologic response rate to neoadjuvant chemotherapy increases with increasing HER2/CEP17 ratio in HER2 overexpressing breast cancer: analysis of the National Cancer Database (NCDB). Breast Cancer Res Treat. 2020;181(2):249–254. doi:10.1007/s10549-020-05599-1
29. Lee HJ, Seo AN, Kim EJ, et al. HER2 heterogeneity affects trastuzumab responses and survival in patients with HER2-positive metastatic breast cancer. Am J Clin Pathol. 2014;142(6):755–766. doi:10.1309/AJCPIRL4GUVGK3YX
30. Hou Y, Nitta H, Wei L, et al. HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res Treat. 2017;166(2):447–457. doi:10.1007/s10549-017-4453-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
