Back to Journals » Breast Cancer: Targets and Therapy » Volume 9
Association between telomere length and CYP19 TTTA repetition polymorphism in healthy and breast cancer-diagnosed women
Authors Murillo-Ortiz B , Martínez-Garza S , Suárez García D , Castillo Valenzuela RDC , García Regalado JF, Cano Velázquez G
Received 22 October 2016
Accepted for publication 14 December 2016
Published 13 January 2017 Volume 2017:9 Pages 21—27
DOI https://doi.org/10.2147/BCTT.S125431
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pranela Rameshwar
Blanca Murillo-Ortiz,1 Sandra Martínez-Garza,1 David Suárez García,1 Rosa del Carmen Castillo Valenzuela,2 Juan Francisco García Regalado,2 Gerardo Cano Velázquez1
1Institute Mexican of Social Security, Department Oncology, Unit of Research in Clinical Epidemiology, 2Department of Medical Sciences, University of Guanajuato, León Guanajuato, Mexico
Introduction: Several studies have reported an increase in breast cancer (BC) risk when patients are carriers of the CYP19 TTA polymorphism with ≥10 repeats; moreover, it has been reported that telomere length is associated with a higher susceptibility of developing cancer.
Objective: The objective of this study was to understand the relationship between CYP19 TTTA repetition polymorphism and telomere length and its effects on serum estradiol, estrone and follicle-stimulating hormone (FSH).
Materials and methods: A total of 180 postmenopausal healthy and 70 BC-diagnosed women were included. Telomere length was determined through real-time quantitative polymerase chain reaction, and aromatase polymorphism was analyzed through DNA; both samples were obtained from circulating leukocytes. Serum estrone, estradiol and FSH were determined by enzyme-linked immunosorbent assay.
Results: Patients with a BC diagnosis showed >10 repetitions more frequently, compared with that of healthy women (50% vs 23%, Χ2 =11.44, p=0.0007). A significant difference in telomere length between healthy and BC women was observed (5,042.7 vs 2,256.7 pb, Z=4.88, p<0.001). CYP19 TTTA repeat polymorphism was associated with serum levels of estradiol and estrone in both groups, being higher in those with >10 repeats. Moreover, telomere length showed an inverse relationship with the number of repeats of the aromatase polymorphism in healthy women (R2=0.04, r= -0.24); in contrast, BC patients did not display this relationship. In addition, telomere length presented an inverse relationship with serum levels of estradiol and estrone in BC patients (p=0.02).
Conclusion: Telomere length is shorter in BC patients than in healthy patients. The CYP19 TTTA repeat polymorphism is associated with serum levels of estradiol and estrone in both healthy women and BC patients, being higher in those with polymorphism carriers >10 repeats. Telomere length has an inverse correlation with the number of repeats of the aromatase polymorphism in healthy women but not in BC women. Estradiol and estrone levels in BC women have an inverse relationship with telomere length.
Keywords: telomere length, CYP19 polymorphism, breast cancer
Introduction
High serum levels of estrogens have been related to the risk of developing breast cancer (BC).1 Recently, a meta-analysis that included 14 studies with 20,098 subjects in total demonstrated a strong relationship between CYP19 aromatase polymorphism and BC, although it concluded that more studies are required to evaluate the role of CYP19 in the various carcinogenic mechanisms of BC. Furthermore, it was demonstrated that the higher concentrations of estradiol in situ in BC are due to catalyzing production by intratumoral aromatase.2 Several studies have reported an increase in BC risk when the patients present ≥10 allelic repeats and that TTTA3 is far more frequent in BC patients compared to healthy controls.4–6 Huang et al7 found a higher survival in premenopausal women with BC, positive estrogen receptors and aromatase repetition polymorphism, reporting a survival rate of 89% vs 68% (p = 0.003) at 8 years compared to those who do not present the repetition variant polymorphism.
Telomeres are specialized structures on the region of each end of the human chromosomes, and their role in genome integrity is essential. Previous studies have reported that shortening of telomere length is associated with a higher risk of developing cancer.8,9 Shen et al10 demonstrated that telomere shortening and a low antioxidant diet increase the risk of developing BC. As a result, progressive shortening of the telomere generates instability of the genomic material and by losing its protective portion eventually resulting in carcinogenesis.11
In fact, 51 studies were analyzed looking for the relationship of telomere length with the risk of developing cancer. A total of 23,379 cases and 68,792 controls were included. The findings suggest that telomeres play different roles in each cancer type, and, moreover, their length is a risk factor in developing digestive tract cancer and head and neck cancer.12
Objective
The objective of this study was to understand the relationship between CYP19 TTTA repetition polymorphism and telomere length and its effects on serum estradiol, estrone and follicle-stimulating hormone (FSH).
Materials and methods
We included a total of 180 healthy women who were volunteers recruited from the family medicine department and 70 BC-diagnosed women from the Oncology Department of the High Specialty Medical Unit No. 1 Bajio, Instituto Mexicano del Seguro Social (IMSS), León, Guanajuato, México. The study was performed in accordance with the Declaration of Helsinki of the World Medical Association and was approved by the local bioethics committee of the High Specialty Medical Unit No. 1 Bajio, IMSS, with the registration number R 2009-1001-35. All patients signed an informed consent form for this investigation.
Telomere length was determined through quantitative real-time polymerase chain reaction (PCR), and aromatase polymorphism was analyzed through genomic DNA; both samples were obtained from circulating leukocytes. The serum levels of estrone, estradiol and FSH were determined by enzyme-linked immunosorbent assay (ELISA). Each group was analyzed according to individual characteristics.
Hormone levels
The serum levels of estrone, estradiol and FSH were determined by ELISA on a blood draw taken in fasting state of 8 hours.
Telomere length and genotyping of the CYP19 aromatase polymorphism
For the measurement of telomere length and genotyping of the CYP19 aromatase polymorphism, we used DNA samples that were extracted from white blood cells. The telomere length was determined by quantitative real-time PCR telomere assay. Genotyping of the CYP19 aromatase polymorphism was determined through PCR.
Telomere measurement
DNA samples were extracted from circulating white blood cells obtained from a simple blood draw. The ratio of telomere repeat copy number to a single gene copy number (T/S) was determined by a previously described modified version of the quantitative real-time PCR telomere assay.3 We performed PCR amplification with oligonucleotide primers designed to hybridize to the TTAGGG and CCCTAA repeats. The final concentrations of reagents in the PCR were 0.2 SYBR Green 1 (Molecular Probes), 15 mM Tris–HCl pH 8.0, 50 mM KCl, 2 mM MgCl2, 0.2 mM each dNTP, 5 mM dithiothreitol, 1% dimethyl sulfoxide and 1.25 U AmpliTaq Gold DNA Polymerase. The final telomere primer concentrations were tel 1,270 nM and tel 2,900 nM. The final 36B4 (single copy gene) primer concentrations were 36B4u, 300 nM and 36B4d, 500 nM. The primer sequences (written 5′→3′) were tel 1, GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT; tel 2, TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA; 36B4u, CAGCAAGTGGGAAGGTGTAATCC and 36B4d, CCCATTCTATCATCAACGGGTACAA. All PCRs were performed on a LightCycler® 1.5 (Hoffman-La Roche Ltd.). The thermal cycling profile for both amplicons began with a 95°C incubation for 3 min to activate the AmpliTaq Gold DNA Polymerase. The telomere PCR conditions were 40 cycles at 95°C for 15 s and at 54°C for 2 min; for 36B4 PCR, the conditions were 40 cycles at 95°C for 15 s and at 58°C for 1 min. The LightCycler® 1.5 was then used to generate the standard curve for each run and to determine the dilution factors of standards corresponding to the amounts of T and S in each sample.
DNA isolation and genotyping
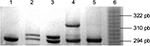
DNA was isolated from white cells using standard phenol–chloroform method. PCR was performed in 25 µL reaction containing 80 ng of genomic DNA, 1× PCR buffer, 200 nm dNTP, 2 mM de MgCl2,100 pmol of each primer (5′-GCAGGTACTTAGTTAGCTAC-3′ and 5′-TTACAGTGAGCCAAGGTGCT-3′) and 2 units of Taq DNA polymerase (Thermo Fisher Scientific). The amplification conditions were as follows: 5 minutes of the initial denaturation at 94oC followed by 30 cycles at 94oC for 1 min, at 53oC for 1 min and at 72oC for 4 min and a final extension at 72oC for 10 min. After the PCR process, an aliquot was electrophoresed through a 10% polyacrylamide denaturing gel to separate fragments followed by silver staining. The size of each allele was determined using CSF1PO as allelic ladder (Figure 1).
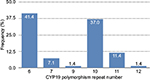  | Figure 1 CYP19 aromatase polymorphism frequency by number of repeats in BC-diagnosed women. Abbreviation: BC, breast cancer. |
Statistical analysis
Fisher’s exact test was used to determine the association between patients with BC and >10 repeats, which were more common compared to healthy women. Linear regression analysis was performed to determine the relationship between telomere length and estrone, estradiol and FSH levels. Correlations were tested by means of Spearman’s rank correlation coefficients, comparing the telomere length between healthy women and BC patients. Mann–Whitney U test was used to compare the telomeric length between healthy women and BC-diagnosed women. p < 0.05 was considered significant.
Results
The mean age of the BC patients was 58.74 ± 12 years, and the body mass index (BMI) was 30 kg/m2 (21.69–37.10); 40% of them were classified as obese and 51% as overweight. BC clinical stages 2 and 3 were the most frequent at diagnosis (37.14% and 41.42%, respectively). The most frequent histological diagnoses were ductal carcinoma (60%), lobular carcinoma (24.28%) and others (11.0%). Healthy women had a mean age of 59 ± 11 years and a BMI of 29.8 ± 7.1 kg/m2. The clinical characteristics and hormone levels of the patients are shown in Table 1.
  | Table 1 Clinical parameters of BC-diagnosed women and healthy women. Abbreviations: BC, breast cancer; BMI, body mass index; FSH, follicle-stimulating hormone. |
Distribution and frequency of the CYP19 aromatase polymorphism
A total of ≥10 repeats were more common in BC patients compared with the healthy women (50% vs 23%, χ2 = 11.44, p = 0.0007).
In the healthy women’s group, the most common polymorphism was 6 repeats followed by 7 repeats; 23% of healthy women had >10 repeats and 71% had <10 repeats of the CYP19 polymorphism. In BC patients also, the most common polymorphism was 6 repeats (41.4%), followed by 10 repeats (37%; Figure 1); in fact, 50.1% had >10 repeats of TTTA and 49.9% had <10 repeats of TTTA (χ2 = 11.44, p = 0.0007; Figure 2). The different number of repetitions found in each group of the acrylamide gel is shown in Figure 3.
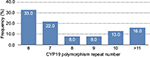  | Figure 2 CYP19 aromatase polymorphism frequency by number of repeats in healthy women. |
Telomeric length
A significant difference was obtained when the telomere length was compared between healthy and BC patients (5,042.7 vs 2,256.7 pb, Z = 4.88, p < 0.001), which is shown in Figure 4. We proved the existence of a significantly and anticipated telomere shortening in BC patients. Spearman’s coefficient analysis applied on the healthy women’s group demonstrated an inverse correlation between the number of repetitions and telomere length (R2 = 0.04, r = -0.24); on the other hand, this correlation was not observed in the analysis of BC patients’ group.
Hormone levels
Estradiol, estrone and FSH serum levels were determined, and the relationship between them, telomere length and the number of repeats were obtained through linear regression models.
Hormone levels in healthy women
In healthy women with >10 repeats of the polymorphism, the levels of estrone (Z = 1.74, p = 0.04) and estradiol (Z = 1.78, p = 0.03) were far higher than those in patients with less repetitions.
A correlation analysis between telomere length and estrone levels showed a positive relationship (R2 = 0.13, β = 55.93, t = 2.5, p = 0.01); there was no evidence of relationship between telomere length and estradiol levels (R2 = 0.32, β = -22.31, t = -1.16, p > 0.05) as shown in Figure 5. There was no significant relationship with FSH levels (β = 7.21, t = 0.37, p > 0.05).
  | Figure 5 The relationship between telomere length and serum levels of (A) estradiol (pg/mL) and (B) estrone (pg/mL) in healthy women. |
Hormone levels in BC-diagnosed women
Higher levels of estrone (p = 0.04) and estradiol (p = 0.02) were also observed in the BC patients’ group with >10 repeats compared to those who had <10 repeats.
Telomere length showed an inverse and significant relationship with serum estrone levels (p = 0.02).
Discussion
In the present study, we demonstrated that in healthy and BC-diagnosed women, a significant relationship between serum estrone and estradiol levels and >10 repeats of CYP19 aromatase polymorphism exists (Z = 1.74, p = 0.04, Z = 1.78, p = 0.03).
We also found evidence that suggests that estrone levels and telomere length had a positive relationship, which is consistent with previous reports that proposed that telomere length is directly determined by the influence of estrogen levels, due to its action on telomerase activity and on the telomerase reverse transcriptase gene expression in cancer culture cells.8
Several studies report shorter telomeres in BC patients than in healthy controls.13,14 We could also ascertain a difference of 2,700 base pairs in length between healthy and BC patients, with the difference being higher in the former ones (p = 0.001). The current study demonstrates that the telomere length in healthy patients, without external influence such as genetic disruption that is found in cancer or its treatment, is directly influenced by the levels of circulating estrogens; furthermore, the levels of the latter are related to the number of repeats of aromatase polymorphism, which implies that in healthy women, telomeric length is influenced by TTTA polymorphism.
There is evidence that demonstrates that hormones can regulate telomerase activity in specific tissues, especially in the endometrium, which expresses telomerase activity even though its somatic origin is tightly regulated during menstrual cycle phases, suggesting a control mechanism of the telomerase by sexual steroids.15 This influence has been reported since 1999 by Kyo et al who demonstrated that telomere length in healthy premenopausal women was longer than males paired by age, suggesting that the difference between them was due to estrogen influence.
It has been demonstrated in mouse models that the cell’s telomeric length of the adrenal gland is shortened when estrogen knockout mice are studied, and moreover, this effect is reversed by replacement therapy with external estrogens for 3 weeks, which reactivate telomerase activity.16 Again, it has been reported that postmenopausal women under treatment of hormonal replacement therapy had longer telomeres than postmenopausal women without this treatment. The influence of estrogens on the progression of cancer, especially BC, is also well known. In postmenopausal women, the origin of these estrogens is the peripheral conversion of adrenal androgens to estrone, catalyzed by aromatase enzyme, and furthermore, the CYP19 polymorphism with TTTA tandem repeats of >10 is related to an increase in the risk of developing BC. Previously, Haiman et al17 showed that CYP19 haplotypes correlated with circulating estrogen levels (r = 0.83–0.466), although a relationship between the latter and cancer development could not be demonstrated.
We found that estrone levels on patients with more than 10 repeats on aromatase’s polymorphism were significantly higher than those with less than 10 repetitions (p = 0.04), association that also was obtained with estradiol levels (p = 0.03), we suggest an association between these hormones levels and number of repetitions on telomere’s length, given that a higher repetition results on higher levels of circulating estrone and subsequently a longer telomere’s length on healthy women (r = 0.2, p = 0.01).
As previously reported by several studies regarding the relationship between CYP19 polymorphism and BC10,18–23; we ascertain this relationship in BC patients with >10 repeats in the aromatase gene (p = 0.05). Moreover, the results found in the current study demonstrate that BC patients have significantly shorter telomeres than healthy women with a mean difference of 2,700 base pairs. The latter situation has been practically taken as a consensus by most researchers.
Taking this fact as a prognostic factor for the development of cancer, it seems that telomeric shortening is an important part in cancer progression from the point that it gets to a critical moment as the repair and senescence mechanisms fail, ending in chromosome instability and carcinogenesis, to which follows telomerase reactivation that aids in perpetuation of cellular immortality, which in fact generates shorter telomeres than in healthy cells.24 Nevertheless, recently some reports demonstrate that in early stages, the telomeres found on BC and renal cell carcinoma patients are larger than those in healthy controls,8,9,13,14 which apparently contradicts the results found by De Vivo, Shen, McGrath, but Pooley K et al, but is similar to the results of Pooley et al,9 in which there is a difference in telomere length while considering retrospective designs.25 It has been proposed, as a justification for these differences, that this is due to telomere shortening influenced by cancer treatment, given that both chemotherapy and radiotherapy cause DNA damage. Until now, there is no certainty on the effect and degree of these therapies on lymphocytes and their sub populations, considering that this study did not show differences in the measurement on patients who received chemotherapy, hormonal therapy or radiotherapy.
This is the first report of a study designed with an aim of establishing a relationship between telomere length in healthy postmenopausal women and BC-diagnosed patients and CYP19 aromatase gene polymorphism. Telomeric length and aromatase polymorphism have been considered separately as risk predictors for developing BC, but the association between them has not been investigated. More studies analyzing both factors are needed.
Conclusion
First and foremost, there is a significant difference in telomere length between healthy women and BC-diagnosed women. The CYP19 TTTA polymorphism is associated with serum levels of estradiol and estrone in healthy and BC-diagnosed women, being higher in those with >10 tandem repeats. Notably, telomere length presents an inverse correlation with the number of repetitions in healthy women but not in BC patients. Moreover, telomere length has an inverse relationship with estradiol and estrone levels in BC patients. The current study contributes to support the important evidence on the role that fatty acid aromatization has on the elevation of serum levels of both estrone and estradiol and, consequently, a direct effect on telomere length, which is an important risk factor for the development of BC.
Disclosure
The authors report no conflicts of interest in this work.
References
Kitahara CM, Flint AJ, Berrington de Gonzalez A, et al. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLOS Med. 2014. | ||
Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol. 2003;86(3–5):219–224. | ||
Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):47e. | ||
Pineda B, García-Pérez MÁ, Cano A, Lluch A, Eroles P. Associations between aromatase CYP19 rs 10046 polymorphism and breast cancer risk: from a case-control to a meta-analysis of 20,098 subjects. PLoS One. 2013;8(1):e53902. | ||
Kristensen VN, Harada N, Yoshimura N, et al. Genetic variants of CYP19 (aromatase) and breast cancer risk. Oncogene. 2000;19(10):1329–1333. | ||
Zhang I, Gu I, Qian B, et al. Association of genetic polymorphisms of ER-alpha and the estradiol-synthesizing enzyme genes CYP17 and CYP19 with breast cancer risk in Chinese women. Breast Cancer Res Treat. 2009;114(2):327–338. | ||
Huang CS, Kuo SH, Lien HC, et al. The CYP19 TTTA repeat polymorphism is related to the prognosis of premenopausal stage I-II and operable stage III breast cancers. Oncologist. 2008;13(7):751–760. | ||
De Vivo I, Prescott J, Wong JY, Kraft P, Hankinson SE, Hunter DJ. A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1152–1156. | ||
Pooley KA, Sandhu MS, Tyrer J, et al. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res. 2010;70(8):3170–3176. | ||
Shen J, Gammon MD, Terry MB, et al. Telomere length, oxidative damage, antioxidants and breast cancer risk. Int J Cancer. 2009;124(7):1637–1643. | ||
Zhu X, Han W, Xue W, et al. The association between telomere length and cancer risk in population studies. Sci Rep. 2016;6:22243. | ||
Wu X, Amos CI, Zhu Y, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003;95(16):1211–1218. | ||
McGrath M, Wong J, Michaud D, Hunter D, Vivo D. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16(4):815–819. | ||
Shen J, Terry M, Gurvich I, Liao Y, Senie R, Santella R. Short telomere length and breast cancer risk: a study in sister sets. Cancer Res. 2007;67(11):5538–5544. | ||
Kyo S, Takakura M, Kanaya T, et al. Estrogen activates telomerase. Cancer Res. 1999;59(23):5917–5921. | ||
Bayne S, Jones M, Li H, Pinto A, Simpson E, Liu J. Estrogen deficiency leads to telomerase inhibition, telomere shortening and reduced cell proliferation in the adrenal gland of mice. Cell Res. 2008;18(11):1141–1150. | ||
Haiman C, Dossus L, Setiawan V, et al. Genetic variation at the CYP19A1 locus predicts circulating estrogen levels but not breast cancer risk in postmenopausal women. Cancer Res. 2007;67(5):1893–1897. | ||
Ahsan H, Chen Y, Whittemore AS, et al. A family-based genetic association study of variants in estrogen-metabolism genes COMT and CYP1B1 and breast cancer risk. Breast Cancer Res Treat. 2004;85(2):121–131. | ||
Hu M, Xie W, Xiong B, et al. Study on the relationship between polymorphisms of genes (CYP17, CYP19 and SULT1A1) and susceptibility to breast cancer in Chinese women. Zhonghua Liu Xing Bing Xue Za Zhi. 2006;27(4):351–355. | ||
Miyoshi Y, Iwao K, Ikeda N, Egawa C, Noguchi S. Breast cancer risk associated with polymorphism inCYP19 in Japanese women. Int J Cancer. 2000;89(4):325–328. | ||
Okobia MN, Bunker CH, Zmuda JM, et al. Simple tandem repeat (TTTA) n polymorphism in CYP19 (aromatase) gene and breast cancer risk in Nigerian women. J Carcinog. 2006;5(1):12. | ||
Ribeiro FS, da Fonte de Amorim LM, de Almeida Simão T, Mendonça GA, de Moura Gallo CV, Pinto LFR. CYP19 (TTTA)n polymorphism and breast cancer risk in Brazilian women. Toxicol Lett. 2006;164(1):90–95. | ||
Saharia A, Guittat L, Crocker S, et al. Flap endonuclease 1 contributes to Telomere stability. Curr Biol. 2008;18(7):496–500. | ||
McGlynn LM, Stevenson K, Lamb K. Cellular senescence in pretransplant renal biopsies predicts postoperative organ function. Aging Cell. 2009;8(1):45–51. | ||
Svenson U, Nordfjall K, Stegmayr B, et al. Breast cancer survival is associated with Telomere length in peripheral blood cells. Cancer Res. 2008;68(10):3618–3623. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.