Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Association Between Sleep Duration and Albuminuria in Patients with Type 2 Diabetes: A Cross-Sectional Study in Ningbo, China
Authors Li X , Chattopadhyay K , Qian X, Yu J, Xu M , Li L , Sun J , Li J
Received 11 March 2022
Accepted for publication 24 May 2022
Published 30 May 2022 Volume 2022:15 Pages 1667—1675
DOI https://doi.org/10.2147/DMSO.S366064
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Xueyu Li,1,2,* Kaushik Chattopadhyay,3,* Xingjun Qian,4 Jingjia Yu,1 Miao Xu,1 Li Li,1 Jing Sun,5,6 Jialin Li1
1Department of Endocrinology and Metabolism, Ningbo First Hospital, Ningbo, People’s Republic of China; 2Medical School, Ningbo University, Ningbo, People’s Republic of China; 3Lifespan and Population Health Academic Unit, School of Medicine, University of Nottingham, Nottingham, UK; 4Health Management Centre, Ningbo First Hospital, Ningbo, People’s Republic of China; 5School of Medicine and Dentistry, Griffith University, Gold Coast, Queensland, Australia; 6Menzies Health Institute Queensland, Griffith University, Gold Coast, Queensland, Australia
*These authors contributed equally to this work
Correspondence: Jialin Li; Jing Sun, Email [email protected]; [email protected]
Purpose: Type 2 diabetes mellitus (T2DM) can lead to microvascular complications including diabetic kidney disease. Albuminuria is an important marker to diagnose kidney injury in T2DM patients and healthy sleep duration is important for maintaining good health in patients with T2DM. However, the association between sleep duration and albuminuria in T2DM patients is unclear. Thus, this study aimed to investigate the association between sleep duration and albuminuria in patients with T2DM in Ningbo, China.
Methods: A cross-sectional study was conducted at National Metabolic Management Centre (MMC) - Ningbo First Hospital from March 2018 to February 2021. Adult patients with T2DM were included in the study. The sleep duration (daytime and nocturnal) was self-reported. Albuminuria was defined as the presence of urinary albumin-creatinine ratio ≥ 30 mg/g. Logistic regression analyses were performed to identify the association.
Results: There were 2688 T2DM patients in the study. In the unadjusted model (1), the odds of albuminuria increased with the daytime sleep duration (31– 60 minutes: OR 1.36, 95% CI 1.09– 1.71; ≥ 61 minutes: 1.73, 1.33– 2.24). Similarly, after adjusting for age and sex (model 2), the odds of albuminuria increased with the daytime sleep duration (31– 60 minutes: 1.34, 1.07– 1.68; ≥ 61 minutes: 1.69, 1.30– 2.20). After adjusting for age, sex, physical activity, smoking, alcohol drinking, overweight/obesity, hypertension, hyperuricaemia, duration of T2DM, glycated haemoglobin, angiotensin-converting enzyme inhibitors/angiotensin receptor blocker usage and nocturnal sleep duration (model 3), the odds of albuminuria increased with the daytime sleep duration (31– 60 minutes: 1.33, 1.04– 1.71; ≥ 61 minutes: 1.71, 1.29– 2.26). However, no relationship was found between nocturnal sleep duration and albuminuria.
Conclusion: Longer daytime sleep is found to be associated with albuminuria in patients with T2DM in Ningbo, China but no association is found between nocturnal sleep duration and albuminuria. The findings are exploratory, and there is a need for longitudinal studies on this topic.
Keywords: type 2 diabetes mellitus, sleep, albuminuria, China
Introduction
Type 2 diabetes mellitus (T2DM) is a serious chronic condition. In China, around 140 million people have diabetes, the largest T2DM population in the world.1 T2DM can lead to microvascular complications like diabetic kidney disease.2 In fact, T2DM is the primary cause of chronic kidney disease and can finally lead to end-stage kidney disease.3 Diabetic kidney disease is common in China, especially among middle-aged and older adults.4 A meta-analysis reported the prevalence of diabetic kidney disease in China is 22%.5 Albuminuria, defined as the urinary albumin-creatinine ratio (UACR) ≥30 mg/g, is not only one of the diagnostic indicators of diabetic kidney disease but also plays a key role in the progression of diabetic kidney disease.6 T2DM patients with an increased UACR level have a higher risk of end stage kidney diseases (ie, estimated glomerular filtration rate (eGFR)< 15mL/min/1.73m2 or renal replacement therapy).7
The duration of sleep is associated with a range of health conditions including T2DM.8–10 Adults are recommended to sleep seven to eight hours per night.11 Sleep deprivation is common in China, and a meta-analysis of 47 studies reported that 36% of older adults had sleep disturbances,12 and 23% of T2DM patients sleep for less than six hours per day compared to only 12% of healthy people in China.13
Previous studies have found an association between sleep duration and albuminuria in the general population.14,15 Similar studies conducted in patients with T2DM have predominantly considered nocturnal sleep duration or total sleep duration but not daytime napping16–18 considering daytime napping is a common practice in China,19,20 and the findings have been inconsistent.16–18,21 To the best of the authors’ knowledge, no such study on daytime napping has been conducted in patients with T2DM in China. Thus, the study aimed to investigate the association between daytime as well as nocturnal sleep duration and albuminuria in patients with T2DM in Ningbo, China. The findings could support the need for appropriate actions to address this issue.
Methods
Study Design, Site, Population, Data Source and Period
A cross-sectional study was conducted and data were collected through the National Metabolic Management Centre (MMC) - Ningbo First Hospital from 1st March 2018 to 28th February 2021. Please see STROBE checklist (Supplementary Material). MMC is a multi-hospital-based programme running across mainland China to provide standardised management for metabolic diseases and led by Ruijin Hospital, Shanghai.22 A total of 3170 patients with metabolic diseases were registered and managed at this MMC in the study. The study inclusion criteria were patients aged 18 to 75 years, visiting this MMC for the first time and diagnosed with T2DM based on the World Health Organization (WHO) criteria (1999).23 In the present study, patients <18 years of age (n=19) and >75 years of age (n=15) and with type 1 diabetes (n=65), gestational diabetes (n=5), other types of diabetes (n=76) and missing data on the exact disease condition (n=87), sleep duration (n=48) and UACR (n=167) were excluded. The rest of the 2688 patients were eligible and included in the present study.
Data Collection and Study Variables
A standardised questionnaire developed and piloted by MMC was used for this purpose, and the physiological, anthropometric and biochemical parameters were measured/analysed by the trained nurse/laboratory staff using the MMC standardised protocol.22 In this study, the following variables were used: (a) self-reported sociodemographic factors, namely, age (18 to 39 years, 40 to 59 years or ≥60 years) and sex (male or female); (b) self-reported lifestyle factors, namely, physical activity (low, medium or high; assessed using the Chinese version of the International Physical Activity Questionnaire-short (IPAQ),24 smoking and alcohol drinking (current status); (c) health conditions, namely, overweight/obesity (yes or no; defined as body mass index (BMI) ≥24kg/m2;25 body weight and height were measured with light clothes and without shoes in standing position using a calibrated automatic digital weight and height scale (HNH-318, Omron, Japan); weight was measured to the nearest 0.1 kg, height was measured to the nearest 0.5 cm and BMI was calculated as weight in kg divided by height in m2), hypertension (yes or no; defined as systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg on the day of visit;26 measured using an automated blood pressure monitor (HBP-1100U, Omron, Japan) in a seated position), hyperuricaemia (yes or no; defined as serum uric acid >7 mg/dL;27 measured using an enzymatic method (AU5800, Beckman Coulter, USA)), duration of T2DM (<5 years, ≥5 to <10 years or ≥10 years)28 and glycated hemoglobin (HbA1c; <7% (< 53 mmol/mol) or ≥7% (≥ 53 mmol/mol);29 measured using the high-performance liquid chromatographic (HPLC) method (D-10 Hemoglobin Analyzer, Bio-Rad, USA)); (d) usage of medicines associated with kidney functions and urinary albumin, namely, angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blocker (ARB) (yes or no); (e) self-reported sleep duration, namely, daytime sleep (none, ≤30 minutes, 31 to 60 minutes or ≥61 minutes)21,30 and nocturnal sleep (<7 hours, ≥7 to <8 hours, ≥8 to <9 hours or ≥9 hours);31 and (f) albuminuria (yes or no; defined as the presence of UACR ≥30 mg/g;32 UACR was calculated by dividing the urinary albumin value by the urinary creatinine value; urinary albumin and creatinine were measured in the spot urine sample using the immunonephelometry and enzymatic methods, respectively (AU5800, Beckman Coulter, USA)).
Ethics
Ethics approval was obtained from the Research Ethics Committees of Ruijin Hospital (2017-42) and Ningbo First Hospital (2019-R057). Written informed consent was obtained from all the patients.
Statistics Analyses
Numbers and percentages are reported for categorical data, means and standard deviations (SD) for normally distributed continuous data and median and interquartile range (IQR) for skewed continuous data. To identify any association between sleep duration and increased albuminuria, the following models were created: in model 1, simple logistic regression analyses were performed. The dependent variable was albuminuria (yes or no; defined as the presence of UACR ≥30 mg/g), and the independent variable was sleep duration (daytime sleep or nocturnal sleep); in model 2, multiple logistic regression analyses were performed and adjusted for age and sex; and in model 3, multiple logistic regression analyses were performed and adjusted for age, sex, physical activity, smoking, alcohol drinking, overweight/obesity, hypertension, hyperuricaemia, duration of T2DM, HbA1c, ACEI/ARB usage and sleep duration (in the daytime sleep analysis, the nocturnal sleep was adjusted for and vice versa). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Data missing (unknown) on the adjusted variables were included in the adjusted models. A p-value ≤0.05 was considered statistically significant. IBM SPSS statistics version 28.0 for Windows was used for data analyses.
Results
There were 2688 T2DM patients in the study. Table 1 reports the characteristics of the study participants. The mean age was 51 years (SD ±12), and 65% were men. The median daytime sleep duration was 10 minutes (IQR 60 minutes). The mean nocturnal sleep duration was 8 hours (SD ±1). 25.8% of them had albuminuria.
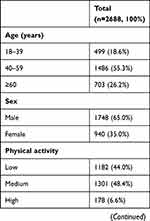 | 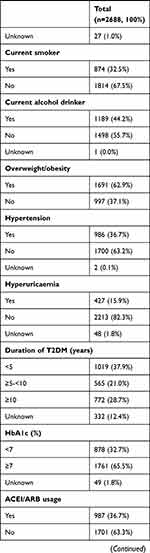 | 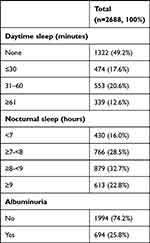 |
Table 1 Characteristics of T2DM Patients in the Study |
Table 2 reports the association between sleep duration and albuminuria in T2DM patients. In model 1, the odds of albuminuria increased with the increasing daytime sleep duration (31 to 60 minutes: OR 1.36, 95% CI 1.09 to 1.71; ≥61 minutes: OR 1.73, 95% CI 1.33 to 2.24). Similarly, in model 2, the odds of albuminuria increased with the daytime sleep duration (31 to 60 minutes: OR 1.34, 95% CI 1.07 to 1.68; ≥61 minutes: OR 1.69, 95% CI 1.30 to 2.20). In model 3, the odds of albuminuria increased with the daytime sleep duration (31 to 60 minutes: OR 1.33, 95% CI 1.04 to 1.71; ≥61 minutes: OR1.71, 95% CI 1.29 to 2.26). However, no relationship was found between nocturnal sleep duration and albuminuria.
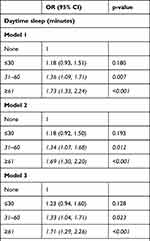 |
Table 2 Association Between Sleep Duration and Albuminuria in Patients with T2DM |
Discussion
In the present study, longer daytime sleep was associated with albuminuria among T2DM patients in Ningbo, China. Considering the adverse effects of longer daytime sleep, a maximum of 30 minutes of daytime sleep can be allowed and anything more than this is not recommended.33 The study finding is consistent with a study conducted among T2DM patients in Japan (beta coefficient 0.01, 95% CI 0.01 to 0.02).21 Another study conducted in the general Chinese population found that those who slept during the daytime were at higher risk of albuminuria compared to those who did not sleep during the daytime (0–1 h/day: OR 1.55, 95% CI 1.44 to 1.67; 1–1.5 h/day: OR 1.30, 95% CI 1.14 to 1.49; >1.5 h/day: OR 1.57, 95% CI 1.35 to 1.81).14 Among newly diagnosed T2DM patients in Turkey, daytime sleep was found to be associated with 24-h urinary albumin excretion (beta coefficient 0.04, p-value 0.043).17 Studies conducted in Japan and Singapore have reported a U-shaped association between total sleep duration and albuminuria in T2DM patients.16,18 This dissimilarity could be due to the fact that they focused on the total sleep duration, and the present study focused separately on daytime and nocturnal sleep durations.
The underlying mechanism behind the association between daytime sleep duration and albuminuria in patients with T2DM remains unclear. It could be due to the following reasons: first, longer sleep duration is likely to indicate less physical activity and energy consumption, which are known to be associated with hyperglycaemia34 and overweight and obesity.35 These health conditions can lead to endothelial dysfunction36 and finally kidney injury.37 Second, most renal physiological activities follow a circadian rhythm, including renal blood flow, GFR, sodium and water excretion.38 Longer sleep may disrupt the sleep-wake cycle, affect the circadian rhythm and lead to kidney dysfunction.39 Circadian disorganisation can cause albuminuria, glomerulosclerosis and renal fibrosis.40 Third, blood pressure varies throughout the day and is maintained at different times by different control systems. A prolonged daytime sleep could lead to sympathetic reactivation, resulting in a rapid increase in blood pressure41 and finally leading to kidney injury.42 Fourth, longer daytime sleep may indicate insufficient nocturnal sleep or poor quality of sleep.43 The poor quality of sleep increases the inflammatory markers, which contributes to albuminuria.44 In the present study, nocturnal sleep duration was adjusted for, however, the quality of sleep was not recorded and adjusted for.
In our previous study, shorter nocturnal sleep duration was found to be associated with higher body weight, BMI and visceral fat area.45 Considering the close relationship between visceral fat accumulation and urinary albumin,46 the present study explored the relationship between nocturnal sleep duration and albuminuria in T2DM patients. However, no association was found between nocturnal sleep duration and albuminuria. This is also consistent with the study conducted among T2DM patients in Japan.21 Nocturnal sleep was negatively associated with 24-h urinary albumin excretion in the study conducted on newly diagnosed T2DM patients in Turkey.17 A study conducted among T2DM patients in China found an association between <6 hours of nocturnal sleep and diabetic kidney disease (defined as the presence of eGFR <60 mL/min/1.73 m2/UACR >30 mg/g).47 In these previous studies, different definitions of nocturnal sleep were used and daytime sleep was not adjusted for, and these could be the reasons for different study findings.
The present study has several strengths and weaknesses. To the best of the authors’ knowledge, this was the first study to examine the association between daytime sleep duration and albuminuria among T2DM patients in China. The study included a relatively large number of T2DM patients, and the routinely collected data quality was high. Missing data on adjusted variables were low in the study, and multiple logistic regression analyses included the sample with missing data. The sleep duration was self-reported by T2DM patients which may not provide an objective measure of sleep duration. A previous study reported a good correlation between self-reported sleep duration and polysomnography results.48 Nevertheless, objective measures of sleep should be used in future studies. The UACR level in the body, used to define albuminuria, is affected by multiple factors.49 Using the average of multiple assessments over a few months could have provided a more accurate assessment of albuminuria. However, the data in the study were coming from a single one-time cross-sectional assessment. Since it was a cross-sectional study, the causal relationship between sleep duration and albuminuria could not be determined, and there is a need for longitudinal studies. The present study did not consider the influence of sleep quality and obstructive sleep apnoea/hypopnoea syndrome, and these need to be explored in future studies.
Conclusions
In the present cross-sectional study, longer daytime sleep is found to be associated with albuminuria in patients with T2DM in Ningbo, China but no association is found between nocturnal sleep duration and albuminuria. The findings are exploratory, and there is a need for longitudinal studies on this topic.
Data Sharing Statement
The dataset will be available upon request unless there are legal or ethical reasons for not doing so.
Ethics Approval
The Research Ethics Committee of Ruijin Hospital, China (2017-42) and the Research Ethics Committee of Ningbo First Hospital, China (2019-R057) approved the study.
Funding
The work was supported by the Medical Health Science and Technology Project of Zhejiang Province (Grant No. 2019KY562) and the Major Program of Social Development of Ningbo Science and Technology Bureau (Grant No. 2019C50094).
Disclosure
Xueyu Li and Kaushik Chattopadhyay are co-first authors for this study. The authors report no conflicts of interest in this work.
References
1. Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109–119. doi:10.1016/j.diabres.2021.109119
2. Jin P, Peng J, Zou H, et al. A five-year prospective study of diabetic retinopathy progression in Chinese type 2 diabetes patients with “well-controlled” blood glucose. PLoS One. 2015;10(4):e0123449. doi:10.1371/journal.pone.0123449
3. Zhang L, Long J, Jiang W, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375(9):905–906. doi:10.1056/NEJMc1602469
4. Hou JH, Zhu HX, Zhou ML, et al. Changes in the spectrum of kidney diseases: an analysis of 40,759 biopsy-proven cases from 2003 to 2014 in China. Kidney Dis. 2018;4(1):10–19. doi:10.1159/000484717
5. Zhang XX, Kong J, Yun K. Prevalence of diabetic nephropathy among patients with type 2 diabetes mellitus in China: a Meta-Analysis of Observational Studies. J Diabetes Res. 2020;2020:2315607. doi:10.1155/2020/2315607
6. Jia Y, Zheng Z, Xue M, et al. Extracellular vesicles from albumin-induced tubular epithelial cells promote the M1 macrophage phenotype by targeting klotho. Mol Ther. 2019;27(8):1452–1466. doi:10.1016/j.ymthe.2019.05.019
7. Oshima M, Toyama T, Hara A, et al. Combined changes in albuminuria and kidney function and subsequent risk for kidney failure in type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9(1):e002311. doi:10.1136/bmjdrc-2021-002311
8. Lu H, Yang Q, Tian F, et al. A meta-analysis of a cohort study on the association between sleep duration and type 2 diabetes mellitus. J Diabetes Res. 2021;2021:8861038. doi:10.1155/2021/8861038
9. Wang Y, Zeng Y, Zhang X, et al. Daytime napping duration is positively associated with risk of hyperuricemia in a Chinese population. J Clin Endocrinol Metab. 2021;106(5):e2096–e2105. doi:10.1210/clinem/dgab043
10. Park S, Lee S, Kim Y, et al. Short or long sleep duration and CKD: a Mendelian Randomization Study. J Am Soc Nephrol. 2020;31(12):2937–2947. doi:10.1681/ASN.2020050666
11. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. doi:10.1016/j.sleh.2014.12.010
12. Lu L, Wang SB, Rao W, et al. The prevalence of sleep disturbances and sleep quality in older Chinese adults: a comprehensive meta-analysis. Behav Sleep Med. 2019;17(6):683–697. doi:10.1080/15402002.2018.1469492
13. Wang F, Chow IHI, Li L, et al. Sleep duration and patterns in Chinese patients with diabetes: a meta-analysis of comparative studies and epidemiological surveys. Perspect Psychiatr Care. 2019;55(2):344–353. doi:10.1111/ppc.12353
14. Ye Y, Zhang L, Yan W, et al. Self-reported sleep duration and daytime napping are associated with renal hyperfiltration and microalbuminuria in an apparently healthy Chinese population. PLoS One. 2019;14(8):e0214776. doi:10.1371/journal.pone.0214776
15. Yu JH, Han K, Kim NH, et al. U-shaped association between sleep duration and urinary albumin excretion in Korean adults: 2011–2014 Korea National Health and Nutrition Examination Survey. PLoS One. 2018;13(2):e0192980. doi:10.1371/journal.pone.0192980
16. Ohkuma T, Fujii H, Iwase M, et al. Association between sleep duration and urinary albumin excretion in patients with type 2 diabetes: the Fukuoka diabetes registry. PLoS One. 2013;8(11):e78968. doi:10.1371/journal.pone.0078968
17. Afsar B. The relationship between self-reported nocturnal sleep duration, daytime sleepiness and 24-h urinary albumin and protein excretion in patients with newly diagnosed type 2 diabetes. Prim Care Diabetes. 2013;7(1):39–44. doi:10.1016/j.pcd.2013.01.002
18. Tan NYQ, Chan J, Cheng CY, Wong TY, Sabanayagam C. Sleep duration and diabetic kidney disease. Front Endocrinol. 2019;9:808. doi:10.3389/fendo.2018.00808
19. Fu J, Zhang X, Moore JB, et al. Midday nap duration and hypertension among middle-aged and older Chinese adults: a Nationwide Retrospective Cohort Study. Int J Environ Res Public Health. 2021;18(7):3680. doi:10.3390/ijerph18073680
20. Zhao H, Gui W, Huang H, et al. Association of long-term sleep habits and hypertension: a cross-sectional study in Chinese adults. J Hum Hypertens. 2020;34(5):378–387. doi:10.1038/s41371-019-0225-8
21. Franke FJ, Arzt M, Kroner T, et al. Daytime napping and diabetes-associated kidney disease. Sleep Med. 2019;54:205–212. doi:10.1016/j.sleep.2018.10.034
22. Zhang Y, Wang W, Ning G. Metabolic Management Center: an innovation project for the management of metabolic diseases and complications in China. J Diabetes. 2019;11(1):11–13. doi:10.1111/1753-0407.12847
23. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi:10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
24. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi:10.1249/01.MSS.0000078924.61453.FB
25. China Expert Panel of Medical Nutrition Therapy for Overweight/Obesity. Expert consensus on medical nutrition therapy for overweight/obesity in China. Chin J Diabetes Mellitus. 2016;9:525–540.
26. Writing Group of 2018 Chinese Guidelines for the Management of Hypertension. 2018 Chinese guidelines for the management of hypertension. Chin J Cardiovasc Med. 2019;24(1):24–56.
27. Chinese Society of Endocrinology. Guideline for the diagnosis and management of hyperuricemia and gout in China (2019). Clin J Endocrinol Metab. 2020;36:1–13.
28. Wang JQ, Li J, Wen CD, et al. Predictors of poor glycemic control among type 2 diabetes mellitus patients treated with antidiabetic medications: a cross-sectional study in China. Medicine. 2021;100(43):e27677. doi:10.1097/MD.0000000000027677
29. Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin J Endocrinol Metab. 2021;37(4):311–398.
30. Kowall B, Lehnich AT, Strucksberg KH, et al. Associations among sleep disturbances, nocturnal sleep duration, daytime napping, and incident prediabetes and type 2 diabetes: the Heinz Nixdorf Recall Study. Sleep Med. 2016;21:35–41. doi:10.1016/j.sleep.2015.12.017
31. Ge YJ, Xin SM, Luan DC, et al. Association of physical activity, sedentary time, and sleep duration on the health-related quality of life of college students in Northeast China. Health Qual Life Outcomes. 2019;17(1):124. doi:10.1186/s12955-019-1194-x
32. KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–S154. doi:10.1053/j.ajkd.2006.12.005
33. Mayo Clinic. Napping: do’s and don’ts for the healthy adults. Available from: https://www.mayoclinic.org/healthy-lifestyle/adult-health/in-depth/napping/art-20048319.
34. Baoying H, Hongjie C, Changsheng Q, et al. Association of napping and night-time sleep with impaired glucose regulation, insulin resistance and glycated haemoglobin in Chinese middle-aged adults with no diabetes: a cross-sectional study. BMJ Open. 2014;4(7):e004419. doi:10.1136/bmjopen-2013-004419
35. Loredo JS, Weng J, Ramos AR, et al. Sleep patterns and obesity: Hispanic Community Health Study/Study of Latinos Sueño Ancillar Study. Chest. 2019;156(2):348–356. doi:10.1016/j.chest.2018.12.004
36. Bakker W, Eringa EC, Sipkema P, et al. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2009;335(1):165–189. doi:10.1007/s00441-008-0685-6
37. Malyszko J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin Chim Acta. 2010;411(19–20):1412–1420. doi:10.1016/j.cca.2010.06.019
38. Solocinski K, Gumz ML. The circadian clock in the regulation of renal rhythms. J Biol Rhythms. 2015;30(6):470–486. doi:10.1177/0748730415610879
39. Hill AM, Crislip GR, Stowie A, et al. Environmental circadian disruption suppresses rhythms in kidney function and accelerates excretion of renal injury markers in urine of male hypertensive rats. Am J Physiol Renal Physiol. 2021;320(2):F224–F233. doi:10.1152/ajprenal.00421.2020
40. Martino TA, Oudit GY, Herzenberg AM, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1675–R1683. doi:10.1152/ajpregu.00829.2007
41. Stergiou GS, Mastorantonakis SE, Roussias LG. Intraindividual reproducibility of blood pressure surge upon rising after nighttime sleep and siesta. Hypertens Res. 2008;31(10):1859–1864. doi:10.1291/hypres.31.1859
42. Ruiz-Hurtado G, Ruilope LM. Microvascular injury and the kidney in hypertension. Hipertens Riesgo Vasc. 2018;35(1):24–29. doi:10.1016/j.hipert.2017.03.002
43. Gandhi KD, Mansukhani MP, Silber MH, et al. Excessive daytime sleepiness: a clinical review. Mayo Clin Proc. 2021;96(5):1288–1301. doi:10.1016/j.mayocp.2020.08.033
44. Meng L, Ding Y, Li J, et al. Impact of inflammatory markers on the relationship between sleep quality and diabetic kidney disease. Sleep Breath. 2022;26(1):157–165. doi:10.1007/s11325-021-02380-6
45. Xu M, Chattopadhyay K, Qian X, et al. Association between nocturnal sleep duration and obesity indicators among people with type 2 diabetes: a cross-sectional study in Ningbo, China. Diabetes Metab Syndr Obes. 2022;15:1357–1364. doi:10.2147/DMSO.S350347
46. Wen J, Yuan H. Independent association between the visceral adiposity index and microalbuminuria in patients with newly diagnosed type 2 diabetes. Diabetes Metab Res Rev. 2020;36(1):e3198. doi:10.1002/dmrr.3198
47. Meng LL, Liu Y, Geng RN, et al. Association of diabetic vascular complications with poor sleep complaints. Diabetol Metab Syndr. 2016;8(1):80. doi:10.1186/s13098-016-0195-8
48. Kushida CA, Chang A, Gadkary C, et al. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi:10.1016/S1389-9457(00)00098-8
49. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA consensus conference. Am J Kidney Dis. 2014;64(4):510–533. doi:10.1053/j.ajkd.2014.08.001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
