Back to Journals » Neuropsychiatric Disease and Treatment » Volume 14
Association between platelet distribution width and poor outcome of acute ischemic stroke after intravenous thrombolysis
Authors Gao F , Chen C, Lyu J , Zheng J, Ma XC , Yuan XY, Huo K, Han JF
Received 12 April 2018
Accepted for publication 30 May 2018
Published 3 September 2018 Volume 2018:14 Pages 2233—2239
DOI https://doi.org/10.2147/NDT.S170823
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Wai Kwong Tang
Fan Gao,1 Chen Chen,2 Jun Lyu,1 Jie Zheng,1 Xian-Cang Ma,1,3 Xing-Yun Yuan,2 Kang Huo,2 Jian-Feng Han2
1Clinical Research Center, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China; 2Department of Neurology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China; 3Department of Psychiatry, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China
Purpose: The platelet distribution width (PDW) reflects the status of platelet activity and may be useful for early predictions of the clinical outcome of stroke patients. The purpose of the study was to determine the associations between PDW and clinical outcomes after intravenous thrombolysis in stroke patients.
Patients and methods: Acute ischemic stroke patients who received intravenous treatment with recombinant tissue-type plasminogen activator were selected for inclusion in the retrospective cohort of this study. The relations between PDW at admission and clinical outcomes were analyzed, including a poor outcome as assessed using the modified Rankin Scale at 3 months, early neurological improvement, and any hemorrhage. The effect of PDW at admission on a poor outcome at 3 months was analyzed using a multivariable logistic regression model with adjustment for potential confounders. The optimal PDW cutoff for predicting poor outcome at 3 months was determined by analyzing the receiver operating characteristics curve.
Results: PDW was significantly higher for a good outcome than a poor outcome (p=0.005), with median (interquartile range) values of 16.2 (13.2–17.2) and 13.6 (12.5–15.9), respectively. PDW was also higher in patients with early neurological improvement than in patients without improvement (p=0.020) and did not differ between hemorrhage and nonhemorrhage patients. The association between PDW <16.05% and poor outcome remained in a multivariable logistic regression analysis, with an OR of 6.68 and a 95% CI of 1.69–26.49 (p=0.007).
Conclusion: Results suggest a novel hypothesis that a lower PDW may be related with a poor outcome at 3 months after intravenous thrombolysis in acute ischemic stroke patients.
Keywords: acute ischemic stroke, platelet distribution width, intravenous thrombolysis, prognosis
Introduction
Intravenous thrombolysis with recombinant tissue-type plasminogen activator (r-tPA) has been approved as a reperfusion therapy for acute ischemic stroke (AIS) patients by the US Food and Drug Administration for the past 20 years.1 While mechanical thrombectomy has been considered a suitable therapy for AIS patients with large-vessel occlusion in whom treatment can be initiated within 6 h of symptom onset, rapid r-tPA administration is still considered the mainstay early therapy for AIS patients.2 AIS patients benefit from the effectiveness of timely restoration of blood flow achieved by r-tPA treatment, but potential limitations accompanying r-tPA have also received attention, such as a narrow time window, higher risks of intracerebral hemorrhage and other complications, lower recanalization rates, and numerous patients having contradictions for r-tPA.3 Predicting the prognostic outcome of intravenous r-tPA treatment at admission is crucial so that clinicians understand when intravenous r-tPA treatment is appropriate and how to improve the prognosis after AIS.
Platelets participate the process of coagulation, inflammation, thrombosis, and atherosclerosis, which are activated in the early thromboembolic phase of ischemic stroke.4,5 Activated platelets will release thromboxane A2 and high expression of glycoprotein Ib and glycoprotein IIb/IIIa receptors, which contribute to thrombosis and stroke.5,6 Several investigators found that both mean platelet volume (MPV) and platelet distribution width (PDW) were indicators of platelet activation.7–9 The PDW represents the heterogeneity of platelet size,4,5 with an elevated PDW indicating a patient still having activation of platelets.4,5,7,10 Furthermore, PDW is a more direct marker to represent platelet reactivity than MPV.8 Studies have already confirmed the association between MPV and stroke outcome.11–13 Nevertheless, previous studies of thromboembolic diseases have generally neglected PDW as a platelet index in comparison with MPV.8 No previous study has explored the association between PDW and the prognosis of stroke after intravenous thrombolysis. Therefore, the objectives of the current study were to determine 1) the relationship between PDW at admission and a poor outcome at 3 months after intravenous thrombolysis in AIS patients and 2) if PDW is correlated with other clinical outcomes, such as early neurological improvement (ENI), symptomatic intracerebral hemorrhage (sICH), and extracranial hemorrhage.
Patient and methods
Patients
All of the AIS patients included in this retrospective study visited the Department of Neurology at the First Affiliated Hospital of Xi’an Jiaotong University between May 2013 and October 2016. The following inclusion criteria were applied: 1) diagnosed as ischemic stroke according to the World Health Organization criteria and confirmed by brain computed tomography or magnetic resonance imaging (MRI) in the hospital and 2) received intravenous r-tPA treatment. The exclusion criteria were 1) <18 years old, 2) >6 h from symptom onset to treatment, 3) having received thrombolysis therapy combined with mechanical thrombectomy, and 4) missing data for PDW at admission or functional outcome at 3 months. Intravenous thrombolysis in our hospital was conducted in accordance with international and institutional guidelines.14 Informed consent was not required due to the retrospective design of the study, which was approved by the ethics committee for medical research at the First Affiliated Hospital of Xi’an Jiaotong University. Data were analyzed anonymously in this study.
Baseline characteristics
Baseline information was collected for all patients, including demographic data, vascular risk factors, time from symptom onset to treatment, blood pressure, body temperature, National Institutes of Health Stroke Scale (NIHSS) score at admission, Glasgow Coma Scale score at admission, findings of laboratory tests, the Trial of ORG 10172 in Acute Stroke Treatment classification, and vessel occlusion site.
All blood samples were collected from patients upon their arrival at hospital. Data from the first-time sample were obtained if multiple sampling was performed within 24 h. All venous blood samples were tested in the Clinical Laboratory at the First Affiliated Hospital of Xi’an Jiaotong University. Laboratory values were determined using an automatic biochemistry analyzer (BJ-G188, Hitachi Ltd., Tokyo, Japan).
Definitions
Hypertension was defined as a prior diagnosis, ongoing antihypertensive medications, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg.4,15 Diabetes mellitus (DM) was defined as a previous diagnosis of and receiving treatment for DM, or fasting plasma glucose ≥7.0 mmol/L (126 mg/dL).4,8 Hyperlipidemia was defined as total cholesterol ≥6.22 mmol/L or triglycerides ≥2.26 mmol/L or low-density lipoprotein cholesterol ≥4.14 mmol/L or high-density lipoprotein cholesterol <1.04 mmol/L.16 Atrial fibrillation was defined according to the self-reported history or diagnosed on a standard 12-lead electrocardiogram.17 Myocardial infarction was defined based on the self-reported history.17 Coronary artery disease was defined by previous onset of acute myocardial infarction or angina pectoris.18 Brain MRI was used to determine the vessel occlusion site using an echo planar instrument operating at 3.0 or 1.5 T. The stroke etiology was classified according to Trial of ORG 10172 in Acute Stroke Treatment criteria.19
Outcomes
The primary outcome was the functional outcome as assessed by the modified Rankin Scale (mRS) score at 3 months after stroke (mRS score =0–2, good outcome; mRS =3–6, poor outcome). The secondary outcomes included ENI, sICH, other bleeding, death in hospital, and duration of hospitalization. ENI was defined as an improvement in the NIHSS score of ≥4 at 24 h compared with baseline.20 sICH was defined as any intracranial hemorrhage indicated in brain imaging within 24 h accompanied with an NIHSS score ≥4 points higher than that at admission or the lowest score during the first 7 days or hemorrhage causing death.21–23 Other bleeding referred to any extracranial hemorrhage within 24 h. The recovery status after discharge was queried during a routine follow-up, and the mRS score at 3 months was evaluated by trained neurologists via the telephone.
Statistical analyses
Patients were stratified into 2 groups according to good and poor outcomes at 3 months. Differences in basic characteristics and clinical outcomes between these 2 groups were compared using Student’s t-test or the Mann–Whitney U test for continuous variables, and the Pearson χ2 test or Fisher’s exact test for categorical variables. The Mann–Whitney U test was used to detect differences in PDW according to the clinical outcome.
A receiver operating characteristics (ROC) curve was used to determine the optimal cutoff point for the continuous PDW and to calculate the sensitivity and specificity for the cutoff value in predicting a poor outcome at 3 months after stroke. The optimal cutoff value was determined by maximizing the Youden index. PDW was then transformed into a dichotomous variable based of its optimal cutoff value. Univariate and multivariable logistic regression models were applied to identify the association between poor outcome and PDW.
OR and 95% CI values were calculated after adjusting for the following potential confounders in a multivariable logistic regression model: age, NIHSS score at admission, and fasting glucose at admission.13,24 A probability value of p<0.05 was treated as statistically significant. All of the statistical analyses were implemented using R software (version 3.3.1, The R Foundation, Vienna, Austria).
Results
Eight of the 82 patients who met the inclusion criteria of the study were excluded due to having received mechanical thrombectomy combined with intravenous r-tPA or due to their PDW values at admission being unknown. Therefore, 74 patients were finally enrolled in the study.
The baseline characteristics and clinical properties of the study population are presented in Table 1. The age of the study population was 62.1±12.6 years (mean ± SD), and 47 patients (63.5%) were male. The outcome was poor in 34 (45.9%) of the 74 patients. The median PDW in the total population was 14.7%, with an interquartile range (IQR) of 12.7%–16.5%. Patients in the poor-outcome group were significantly older and more likely to have atrial fibrillation or DM than those in the good-outcome group (p<0.05). The NIHSS score and fasting glucose at admission were also higher in patients with poor outcomes (p<0.05). The Glasgow Coma Scale score at admission and PDW were lower in the poor-outcome patients than in the good-outcome patients (p<0.05). None of the other clinical parameters differed significantly between poor- and good-outcome patients.
The clinical outcomes in the 2 groups are listed in Table 2. ENI was achieved within 24 h by 42.5% and 14.7% of those in the good- and poor-outcome groups, respectively (p=0.009). However, sICH, other bleeding, inhospital mortality, and length of hospital stay did not differ between the 2 groups.
  | Table 2 Clinical outcomes of patients after intravenous thrombolysis |
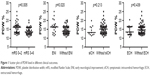
The variation in PDW with clinical outcome is presented in Figure 1. Besides the evident difference in PDW between good- and poor-outcome patients (median [IQR] =16.15 [13.20–17.20] versus 13.55 [12.40–15.93], p=0.005), PDW also showed an increasing trend in patients with ENI compared with those without ENI (16.3 [13.55–17.80] versus 13.9 [12.60–16.20], p=0.020). However, PDW did not differ between hemorrhage and nonhemorrhage patients (including intracranial and extracranial bleeding).
ROC curve analysis was applied to evaluate the utility of PDW in predicting a poor outcome. The Youden index was maximal for a PDW cutoff of 16.05%, with PDW <16.05% producing a sensitivity of 57.5% and a specificity of 79.4%. The area under the ROC curve was 0.689, with 95% CI =0.568–0.809, while the positive predictive value was 61.4% and the negative predictive value was 90% (Figure 2).
The association between PDW <16.05% and a poor outcome at 3 months was assessed using univariate and multivariable logistic regression models. In the univariate logistic regression model, the OR and 95% CI were 5.22 and 1.84–14.78, respectively (p=0.002). The results obtained in the multivariable logistic regression analysis are listed in Table 3. After adjusting for major confounders, there remained an independent relationship between PDW <16.05% and a worse outcome at 3 months (OR =6.68, 95% CI =1.69–26.49, p=0.007). In addition, the baseline NIHSS score (OR =1.18, 95% CI =1.03–1.34, p=0.015) and fasting glucose (OR =1.38, 95% CI =1.03–1.85, p=0.029) were also significantly related to a poor outcome at 3 months.
Discussion
This retrospective cohort study evaluated the associations between PDW at admission and different kinds of clinical outcomes among AIS patients who received r-tPA therapy. The results indicated that a lower PDW at admission was associated with poor outcome at 3 months in AIS patients. The association remained after controlling potential confounders.
The correlation of MPV with the risk or prognosis of stroke has been studied much more extensively than the effect of PDW. The relation between MPV and stroke outcome remains controversial. Two studies performed prior to 1995 found no relation, but 2 subsequent studies by Butterworth and Bath11 and Greisenegger et al12 found that an elevated MPV was related to a worse outcome in AIS patients. Moreover, Peng et al13 reported that an elevated MPV was related to a poor outcome in AIS patients after mechanical thrombectomy.
There have been only 2 studies on the relation between PDW and carotid artery lesions: one suggested that PDW was a predictor of the severity of carotid artery plaques,25 while the other found that a higher PDW would increase the risk of developing symptomatic carotid artery plaques.5 Little is known about the connection between PDW at admission and stroke outcome after intravenous thrombolysis treatment, although it has been confirmed that both MPV and PDW increase during platelet activation.7,26 MPV and PDW provide different angiology information. Several studies have suggested that PDW is a more sensitive marker of the variability in platelet size and provides more comprehensive information about platelet reactivity than does MPV.7,8,27,28 PDW may be influenced by increases in the size and number of platelets and by the presence of pseudopodia.9 The present study identified an obvious difference in PDW between poor- and good-outcome patients, but not in MPV. To the best of our knowledge, the present study represents the first investigation of the relationship between PDW and stroke outcome after intravenous thrombolysis.
The mechanism underlying how PDW influences AIS patients under intravenous thrombolysis is unclear. One conceivable explanation is that PDW level may indicate different types of thrombi. Elevated PDW may reflect that a new thrombus is being formed with higher production of larger active platelets,7,8 while lower PDW may suggest that an old thrombus was previously formed. The activated platelets aggregate around the fibrin matrix in the formation of the structural framework of a blood clot. The reticular structure of a new thrombus is looser than that in an old thrombus. Therefore, intravenous thrombolysis with r-tPA may help dissolving fibrin clots in a new thrombus easily compared with an old thrombus. It can be inferred that patients who have a new thrombus with higher PDW may respond better to intravenous thrombolysis therapy and exhibit a better recovery of neurological function.
Previous studies suggest that a high PDW indicates a higher risk of adverse vascular events, whereas the current study found a positive correlation between PDW and stroke outcome. Possible explanations for this discrepancy are the inclusion of different patients and different times of blood sampling. The population in this study was admitted to hospital within 6 h of stroke onset, and blood samples were drawn promptly upon their arrival at hospital. In contrast, Koklu et al5 obtained blood samples twice with a 15-day interval before stroke onset in patients with carotid artery stenosis. Physiological process may lead to variations of PDW over time. The patients in the studies of Reddy et al6 and Ulucan et al29 had acute coronary syndrome, and so their treatment strategy may differ considerably from the thrombolytic therapy used in the present study. Furthermore, blood samples in the study of Ulucan et al29 were taken within 24 h after the onset of acute coronary syndrome, while the time when blood samples were collected by Reddy et al6 was unclear.
The present study was subject to some limitations. First, the study had a retrospective cohort design, and data collection could not be preplanned or controlled in a strict manner. Second, because of the relatively small number of patients in the study, some significant confounding factors might not have been included in the multivariable logistic regression model. Third, the absence of any relationships between PDW and other clinical outcomes (ENI, bleeding, or mortality) in the multivariable analysis in this study might have been due to too few patients experiencing these outcomes, thereby reducing the statistical power. Finally, information about the dynamic alteration of PDW during hospitalization was not available. Future studies should focus on the changes in PDW with time after thrombolytic therapy and how they are related to clinical outcome in AIS patients.
Conclusion
The results obtained in this study suggest the new hypothesis that PDW plays a positive role in influencing the functional outcome of AIS after intravenous thrombolysis. An admitted AIS patient should be closely monitored in order to determine whether to apply intravenous thrombolysis treatment. A prospective study involving a larger number of patients is needed to confirm the results obtained in this study.
Acknowledgment
We would like to thank all the members of the Clinical Research Center, the First Affiliated Hospital of Xi’an Jiaotong University for their participation in this study. The abstract for this article has been accepted by an annual conference in People’s Republic of China: the 21st National Conference of Neurology. The location is in Shanghai, People’s Republic of China and the conference is held between 6–9 September, 2018.
Author contributions
FG and J-FH came up with the research idea and designed the study; FG, CC, X-YY, and KH acquired the data; FG, JL, JZ, and X-CM carried out the statistical analysis; FG, CC, X-YY, KH, and J-FH interpreted the data; and FG and J-FH drafted the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Tsivgoulis G, Safouris A, Krogias C, Arthur AS, Alexandrov AV. Endovascular reperfusion therapies for acute ischemic stroke: dissecting the evidence. Expert Rev Neurother. 2016;16(5):527–534. | ||
Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020–3035. | ||
Broeg-Morvay A, Mordasini P, Bernasconi C, et al. Direct mechanical intervention versus combined intravenous and mechanical intervention in large artery anterior circulation stroke: a matched-pairs analysis. Stroke. 2016;47(4):1037–1044. | ||
Yilmaz F, Koklu E, Kizilirmak Yilmaz F, Sariönder Gencer E, Alparslan AŞ, Yildirimtürk Ö. Evaluation of mean platelet volume and platelet distribution width in patients with asymptomatic intermediate carotid artery plaque. Kardiol Pol. 2017;75(1):35–41. | ||
Koklu E, Yuksel IO, Arslan S, et al. Predictors of symptom development in intermediate carotid artery stenosis: mean platelet volume and platelet distribution width. Angiology. 2016;67(7):622–629. | ||
Reddy SK, Shetty R, Marupuru S, Yedavalli N, Shetty K. Significance of platelet volume indices in STEMI patients: a case-control study. J Clin Diagn Res. 2017;11(4):LC05–LC07. | ||
Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14(1):28–32. | ||
Cetin M, Bakirci EM, Baysal E, et al. Increased platelet distribution width is associated with ST-segment elevation myocardial infarction and thrombolysis failure. Angiology. 2014;65(8):737–743. | ||
Ozyurtlu F, Yavuz V, Cetin N, Acet H, Ayhan E, Isik T. The association between coronary slow flow and platelet distribution width among patients with stable angina pectoris. Postepy Kardiol Interwencyjnej. 2014;10(3):161–165. | ||
Jindal S, Gupta S, Gupta R, et al. Platelet indices in diabetes mellitus: indicators of diabetic microvascular complications. Hematology. 2011;16(2):86–89. | ||
Butterworth RJ, Bath PM. The relationship between mean platelet volume, stroke subtype and clinical outcome. Platelets. 1998;9(6):359–364. | ||
Greisenegger S, Endler G, Hsieh K, Tentschert S, Mannhalter C, Lalouschek W. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004;35(7):1688–1691. | ||
Peng F, Zheng W, Li F, et al. Elevated mean platelet volume is associated with poor outcome after mechanical thrombectomy. J Neurointerv Surg. 2018;10(1):25–28. | ||
Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. | ||
Nkoke C, Lekoubou A, Balti E, Kengne AP. Stroke mortality and its determinants in a resource-limited setting: a prospective cohort study in Yaounde, Cameroon. J Neurol Sci. 2015;358(1–2):113–117. | ||
Zhong C, Xu T, Xu T, et al. Plasma homocysteine and prognosis of acute ischemic stroke: a gender-specific analysis from CATIS randomized clinical trial. Mol Neurobiol. 2016;54(3):2022–2030. | ||
Buijs JE, Uyttenboogaart M, Brouns R, et al. The effect of age and sex on clinical outcome after intravenous recombinant tissue plasminogen activator treatment in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25(2):312–316. | ||
Huang WY, Weng WC, Peng TI, et al. Association of hyponatremia in acute stroke stage with three-year mortality in patients with first-ever ischemic stroke. Cerebrovasc Dis. 2012;34(1):55–62. | ||
Aoki J, Kimura K, Koga M, et al. NIHSS-time score easily predicts outcomes in rt-PA patients: the SAMURAI rt-PA registry. J Neurol Sci. 2013;327(1–2):6–11. | ||
Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138–1147. | ||
Coutinho JM, Liebeskind DS, Slater LA, et al. Combined intravenous thrombolysis and thrombectomy vs thrombectomy alone for acute ischemic stroke: a pooled analysis of the SWIFT and STAR studies. JAMA Neurol. 2017;74(3):268–274. | ||
Weber R, Nordmeyer H, Hadisurya J, et al. Comparison of outcome and interventional complication rate in patients with acute stroke treated with mechanical thrombectomy with and without bridging thrombolysis. J Neurointerv Surg. 2017;9(3):229–233. | ||
Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. | ||
Hallevi H, Barreto AD, Liebeskind DS, et al. Identifying patients at high risk for poor outcome after intra-arterial therapy for acute ischemic stroke. Stroke. 2009;40(5):1780–1785. | ||
del Zoppo GJ. The role of platelets in ischemic stroke. Neurology. 1998;51(3 Suppl 3):S9–S14. | ||
Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost. 2003;9(3):177–190. | ||
Adam G, Kocak E, Ozkan A, et al. Evaluation of platelet distribution width and mean platelet volume in patients with carotid artery stenosis. Angiology. 2015;66(4):375–378. | ||
De Luca G, Venegoni L, Iorio S, et al. Platelet distribution width and the extent of coronary artery disease: results from a large prospective study. Platelets. 2010;21(7):508–514. | ||
Ulucan S, Keser A, Kaya Z, et al. Association between PDW and long term major adverse cardiac events in patients with acute coronary syndrome. Heart Lung Circ. 2016;25(1):29–34. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.