Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Association Between Lack of Insight and Prefrontal Serotonin Transporter Availability in Antipsychotic-Free Patients with Schizophrenia: A High-Resolution PET Study with [11C]DASB
Authors Kim JH , Son YD , Kim HK, Kim JH
Received 26 August 2021
Accepted for publication 8 October 2021
Published 21 October 2021 Volume 2021:17 Pages 3195—3203
DOI https://doi.org/10.2147/NDT.S336126
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Jeong-Hee Kim,1,2 Young-Don Son,1– 3 Hang-Keun Kim,1– 3 Jong-Hoon Kim1,3,4
1Neuroscience Research Institute, Gachon University, Incheon, Republic of Korea; 2Department of Biomedical Engineering, College of Health Science, Gachon University, Incheon, Republic of Korea; 3Gachon Advanced Institute for Health Sciences & Technology, Gachon University, Incheon, Republic of Korea; 4Department of Psychiatry, Gachon University College of Medicine, Gil Medical Center, Incheon, Republic of Korea
Correspondence: Jong-Hoon Kim
Department of Psychiatry, Gachon University College of Medicine, Gil Medical Center, 1198 Guwol-dong, Namdong-gu, Incheon, 21565, Republic of Korea
Tel +82 32 460 2696
Fax +82 32 472 8813
Email [email protected]; [email protected]
Young-Don Son
Department of Biomedical Engineering, College of Health Science, Gachon University, 191 Hambakmoe-ro, Yeonsu-gu, Incheon, 21936, Republic of Korea
Tel +82 32 820 4416
Email [email protected]
Background: Previous studies suggested a link between serotonergic neurotransmission and impaired insight in schizophrenia. In this study, we examined the relationship between serotonin transporter (SERT) availability in regions of the prefrontal cortex (dorsolateral, ventrolateral, ventromedial, and orbitofrontal cortices) and insight deficits in antipsychotic-free patients with schizophrenia using high-resolution positron emission tomography (PET) with [11C]DASB.
Methods: Nineteen patients underwent [11C]DASB PET and 7-Tesla magnetic resonance imaging scans. To assess SERT availability, the binding potential with respect to non-displaceable compartment (BPND) was derived using the simplified reference tissue model. Patients’ level of insight was assessed using the Insight and Treatment Attitude Questionnaire (ITAQ). The relationship between ITAQ scores and [11C]DASB BPND values was examined using the region-of-interest (ROI)- and voxel-based analyses with relevant variables as covariates. The prefrontal cortex and its four subregions were selected as a priori ROIs since the prefrontal cortex has been implicated as the critical neuroanatomical substrate of impaired insight in schizophrenia.
Results: The ROI-based analysis revealed that the ITAQ illness insight dimension had significant negative correlations with the [11C]DASB BPND in the left dorsolateral, left orbitofrontal, and bilateral ventrolateral prefrontal cortices. The ITAQ treatment insight dimension had significant negative correlations with the [11C]DASB BPND in the bilateral dorsolateral, left orbitofrontal, and bilateral ventrolateral prefrontal cortices. The ITAQ total score showed significant negative correlations with the [11C]DASB BPND in the bilateral prefrontal cortex and three subregions (dorsolateral, ventrolateral, and orbitofrontal cortices). A supplementary voxel-based analysis corroborated a significant negative association between the ITAQ score and the [11C]DASB BPND in the prefrontal cortices.
Conclusion: Our study provides in vivo evidence of significant negative correlations between insight deficits and prefrontal SERT availability in patients with schizophrenia, suggesting significant involvement of prefrontal serotonergic signaling in impaired insight, one of the core symptoms of schizophrenia.
Keywords: insight, schizophrenia, serotonin transporter, prefrontal cortex, [11C]DASB, positron emission tomography
Introduction
Lack of insight is common among patients with schizophrenia and is significantly linked with poor antipsychotic drug adherence and frequent relapse, leading to a poor prognosis.1 Poor insight has long been recognized as a significant barrier to treatment adherence and recovery in schizophrenia.1 Although numerous investigations have been made concerning the clinical correlates of poor insight in patients with schizophrenia, the neurobiological underpinning of lack of insight is still unclear and not well understood.2
Several magnetic resonance imaging (MRI) studies on the neuroanatomical and functional correlates of poor insight in schizophrenia have been performed, which suggest that the prefrontal cortex is involved in the lack of insight in patients with schizophrenia.3–9 A meta-analysis also reported that insight deficits in patients with schizophrenia are significantly correlated with impairment in specific cognitive functioning, such as set-shifting and error monitoring,10 which is primarily subserved by the prefrontal cortex. Another meta-analysis by Nair et al11 reported significant associations between insight into illness and executive function. These findings suggest a strong link between poor insight and prefrontal cortical dysfunction in schizophrenia.
However, the neurochemical underpinning of impaired insight in schizophrenia remains poorly understood. In a previous study, platelet serotonin concentrations were significantly correlated with lack of insight in patients with schizophrenia,12 suggesting a possible involvement of serotonergic neurotransmission in insight deficits in schizophrenia. A genetic association study reported that the functional polymorphism of the serotonin 1A receptor was significantly related to the theory of mind (ToM) performance,13 which was found to be an important factor contributing to impaired insight in schizophrenia.14 In addition, genetic variations of tryptophan hydroxylase-2 (TPH2) and serotonin 2A receptors were significantly associated with social cognitive functions, particularly emotional management components,15,16 which play a substantial role in insight deficits in schizophrenia.17 Moreover, the core pathophysiology in schizophrenia is associated with altered synaptic plasticity in the prefrontal cortex,18 where serotonin plays a significant role as a neuromodulatory transmitter.19 Hence, we suppose that serotonin-related mechanisms in the prefrontal cortex could be involved in impaired insight in patients with schizophrenia. However, no in vivo molecular positron emission tomography (PET) imaging investigations have been reported to elucidate the relationship between serotonergic neurotransmission and impaired insight in schizophrenia.
Therefore, in this study, we examined the relationship between serotonin transporter (SERT) availability in regions of the prefrontal cortex (dorsolateral, ventrolateral, ventromedial, and orbitofrontal cortices) and insight deficits in antipsychotic-free patients with schizophrenia. We employed high-resolution PET with 11C-3-amino-4-(2-dimethylaminomethylphenylthio)benzonitrile ([11C]DASB), a highly selective radioligand with high affinity and good specificity for in vivo SERT imaging in the brain including the cortical regions.20–24 In this study, the prefrontal cortex and its four subregions were chosen a priori based on the aforementioned literature, which implicates the prefrontal cortex as a critical neuroanatomical substrate of impaired insight in schizophrenia.3,6,9,25
Materials and Methods
Subjects
The study protocol was approved by the Institutional Review Board of the Gachon University Gil Medical Center, and all procedures used in the study were conducted in accordance with international ethical standards and the Declaration of Helsinki. Patients were recruited from the schizophrenia clinic at Gachon University Gil Medical Center. Written informed consent was obtained from all participants after providing a full explanation of the study procedures.
The criteria for patient recruitment were as follows: (i) a diagnosis of schizophrenia based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV),26 which was established using the Structured Clinical Interview for DSM-IV (SCID);27 (ii) age between 18 and 55 years; (iii) absence of any psychotropic medication for at least 14 days before the PET scan (with the exception of lorazepam, which was allowed at a maximal dose of 3 mg per day); and (iv) no history of receiving antidepressant medications. Patients were excluded if they (i) met the diagnostic criteria for a psychiatric diagnosis other than schizophrenia; (ii) had a concurrent diagnosis of substance abuse or dependence; (iii) had concurrent medical or neurological disorders; (iv) were acutely psychotic or suicidal/homicidal; (v) were judged to be uncooperative with regard to the study; (vi) did not fully understand the study procedures; or (vii) could not give written informed consents.
Nineteen patients were enrolled in the study (Table 1). The mean Positive and Negative Syndrome Scale (PANSS)28 total score was 84.7 ± 23.6. The mean duration of illness was 2.3 ± 1.8 years. Before the PET scans, all patients underwent urine tests to exclude substance abuse, and female patients had additional urine pregnancy tests to exclude pregnancy. None of the patients had any structural abnormalities on brain MRI, which was confirmed by a board-certified radiologist.
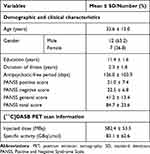 |
Table 1 Demographic/Clinical Characteristics and PET Scan Parameters |
Clinical Assessments
Symptoms of schizophrenia were assessed using the PANSS.28 Patients’ level of clinical insight was assessed using the Insight and Treatment Attitude Questionnaire (ITAQ).29 The ITAQ is a rater-administered 11-item scale that measures clinical insight in two dimensions: awareness of illness (illness insight) and awareness of need for treatment (treatment insight).29 Each item on the ITAQ elicits responses on a Likert type scale scored 0 for no insight, 1 for partial insight and 2 for good insight.29 A higher score is indicative of good insight. A standardized Korean version of the ITAQ was used in this study.30
[11C]DASB PET and MRI Data Acquisition
All patients were scanned with the High Resolution Research Tomograph (HRRT)-PET (Siemens, Knoxville, TN, USA) using [11C]DASB. For attenuation and scatter correction, a transmission scan using a 137Cs point source was performed during the six minutes preceding the start of the PET emission recording.31 Following a mean bolus injection of 582 ± 54 MBq [11C]DASB with a mean molar activity of 83 ± 63 GBq/μmol, a dynamic emission recording lasting 90 minutes was obtained in three-dimensional (3-D) acquisition mode. Following the PET scans, structural MRI images were acquired with 7-Tesla MRI (MAGNETOM 7T, Siemens, Erlangen, Germany) using a 3-D T1-weighted magnetization-prepared rapid gradient echo (T1MPRAGE) sequence. This entailed the following parameters: repetition time = 1900 ms; echo time = 3.73 ms; inversion time = 1100 ms; flip angle = 10°; number of slices = 256; and voxel size = 0.8 × 0.8 × 0.8 mm3. To minimize the patient’s head movement during the MRI and PET scans, all patients’ heads were fixed to the sponges as comfortably as possible.
Image Analysis
The [11C]DASB PET emission data were reconstructed as 22 frames of increasing duration (4 × 30, 2 × 60, 2 × 90, 3 × 150, 3 × 210, 4 × 300, 3 × 600, and 1 × 900 s) using the 3-D ordinary Poisson ordered‐subset expectation maximization algorithm based on the symmetry and single‐instruction multiple data-based projection and back-projection.32 All reconstructed PET frames were normalized for detector efficiency and corrected for scatter, randoms, attenuation, decay, and dead time according to the procedures installed by the HRRT-PET manufacturer. These frames had a matrix size of 256 × 256 × 207 and an iso-voxel resolution of 1.22 × 1.22 × 1.22 mm3.
For reconstructed PET frames, the preprocessing steps were performed using Statistical Parametric Mapping 8 (SPM8; Wellcome Department of Imaging Neuroscience, London, UK)33 implemented in MATLAB R2014b (The MathWorks, Natick, MA, USA). For motion correction of the reconstructed PET frames, realignment was conducted according to a two-pass procedure. In the first pass realignment, each reconstructed PET frame was registered to the first frame, a reference frame with high signal-to-noise ratio in the series, after which a mean PET image was computed. In the second pass realignment, each frame from the first pass alignment was registered to the mean PET image. Next, the mean PET image was co-registered to the structural MRI image using a 12-parameter affine transformation, and the estimated transformation was applied to the corresponding reconstructed PET frames. To estimate SERT availability, the binding potential with respect to non-displaceable compartment (BPND) of [11C]DASB was calculated in the kinetic modeling tool of the PMOD software v3.2 (PMOD Technologies Ltd., Zürich, Switzerland) using the simplified reference tissue model 2 (SRTM2)34 with the cerebellum serving as the reference region, as previously used for this tracer.22,35–38 In the [11C]DASB BPND calculation process, the time-activity curves were obtained from reconstructed dynamic PET images by averaging all the voxels within each region of interest (ROI). The [11C]DASB BPND images were spatially normalized to the Montreal Neurological Institute (MNI) standard template using SPM8. The anatomical locations of the ROIs, ie, the prefrontal cortex and its subregions, were determined based on the Brodmann areas in the Talairach atlas,39 which include dorsolateral prefrontal, ventromedial prefrontal, ventrolateral prefrontal, and orbitofrontal subregions.40–42 The left and right regions were analyzed separately, given the lateralized cognitive processes and their deficits in schizophrenia.43,44
Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 23.0 (IBM Corp., Armonk, NY, USA). The relationship between the ITAQ scores and the [11C]DASB BPND values was examined using the a priori ROI-based correlation analysis with relevant variables as covariates. The statistical significance was set at a threshold of two-tailed p < 0.05.
A voxel-based analysis was also performed with the same data using SPM8 to supplement and corroborate the ROI-based results on the relationship between the ITAQ total scores and the [11C]DASB BPND values in the prefrontal cortex. The significance threshold was set at false discovery rate (FDR)-corrected p < 0.05. However, considering the exploratory perspective of the voxel-based analysis in our study, when no significant correlations were found at the FDR-corrected threshold, significant results were further investigated at an uncorrected p < 0.005 with an extent threshold of 50 voxels, which was considered statistically significant in previous PET imaging studies.45,46
Results
The mean ITAQ total score was 13.6 ± 6.6. The mean scores of the illness insight and treatment insight dimensions were 6.0 ± 3.2 and 7.6 ± 3.6, respectively. The ITAQ scores did not significantly correlate with age (r = 0.12 to 0.20, p > 0.05), gender (t = 0.65 to 1.35, p > 0.05), or the duration of antipsychotic-free period (r = 0.17 to 0.28, p > 0.05). The ITAQ scores had significant positive correlations with the duration of illness (illness insight dimension score: r = 0.48, p = 0.04; treatment insight dimension score: r = 0.64, p = 0.003; total score: r = 0.59, p = 0.008). Additionally, the ITAQ illness insight dimension score had a significant positive correlation with the PANSS positive score (r = 0.47, p = 0.045), and both the ITAQ treatment insight dimension score and ITAQ total score had significant positive correlations with the PANSS total score (treatment insight dimension score: r = 0.50, p = 0.029; total score: r = 0.49, p = 0.033).
The ROI-based correlation analysis with relevant PANSS scores and duration of illness as covariates revealed that the ITAQ total score had significant negative correlations with the [11C]DASB BPND values in the bilateral prefrontal cortex (left: p = 0.012; right: p = 0.035), dorsolateral prefrontal cortex (left: p = 0.015; right: p = 0.036), orbitofrontal cortex (left: p = 0.006; right: p = 0.039), and ventrolateral prefrontal cortex (left: p = 0.001; right: p = 0.008) (Table 2). The ITAQ illness insight dimension score had significant negative correlations with the [11C]DASB BPND values in the left prefrontal cortex (p = 0.034), left dorsolateral prefrontal cortex (p = 0.041), left orbitofrontal cortex (p = 0.021), and bilateral ventrolateral prefrontal cortex (left: p = 0.005; right: p = 0.034) (Table 2). The ITAQ treatment insight dimension score had significant negative correlations with the [11C]DASB BPND values in the left prefrontal cortex (p = 0.028), bilateral dorsolateral prefrontal cortex (left: p = 0.028; right: p = 0.048), left orbitofrontal cortex (p = 0.013), and bilateral ventrolateral prefrontal cortex (left: p = 0.002; right: p = 0.020) (Table 2). Representative scatter plots showing the relationship between the ITAQ scores and the [11C]DASB BPND values in the prefrontal cortex and its subregions are presented in Figure 1.
 |
Table 2 Mean [11C]DASB BPND Values and Their Correlation Coefficients with the ITAQ Scores in the Prefrontal Cortex and Its Subregions |
In addition, voxel-based correlation analysis with the PANSS total score and duration of illness as covariates revealed significant negative correlations between the ITAQ total score and [11C]DASB BPND values in the right orbitofrontal cortex (p = 0.0004), left ventromedial prefrontal cortex (p = 0.001), left dorsolateral prefrontal cortex (p = 0.002), and right ventrolateral prefrontal cortex (p = 0.003), which did not survive FDR correction for multiple correlations (FDR-corrected p = 0.320). These results are shown in Figure 2.
Discussion
In this study, we identified significant negative correlations between the level of insight measured by the ITAQ and the prefrontal SERT availability quantified by the [11C]DASB BPND in antipsychotic-free patients with schizophrenia, suggesting a significant involvement of serotonergic neurotransmission in insight deficits in schizophrenia. To the best of our knowledge, this is the first molecular PET study reporting the relationship between in vivo serotonin parameter and insight in schizophrenia.
It is unclear whether there is a simple competition model between SERT radiotracer and extracellular serotonin for SERT binding sites; however, it has been reported that lower SERT functioning is associated with greater extracellular serotonin.24 Ginovart et al47 reported that significantly decreased [11C]DASB binding was observed in cat brains under conditions in which tranylcypromine, a monoamine oxidase inhibitor, was administered to increase serotonin levels. In addition, Yamamoto et al48 also reported that increased endogenous serotonin using 5-hydroxy-L-tryptophan significantly decreased the binding potential of [11C]DASB in several brain regions in the conscious nonhuman primate. In contrast, Milak et al49 reported a decreased [11C]DASB binding under the rapid tryptophan depletion paradigm in the brains of baboons, suggesting the internalization of SERT. The effects of anesthesia on SERT and [11C]DASB radioligands and the interval between tryptophan depletion and [11C]DASB PET scan may be factors involved in these discrepancies.
Since a higher ITAQ score is indicative of good insight, we suppose that a low endogenous extracellular serotonin levels in the prefrontal cortex may be associated with low levels of insight in schizophrenia. The higher density of SERT could also increase serotonin clearance24 and the conditions of higher SERT density may lead to greater extracellular serotonin decrease, thereby accounting for an inverse association between SERT functioning and extracellular serotonin levels. Thus, based on this additional model as well as the model noted above,24,47,48 we may explain our results as being an association between more severe lack of insight assessed by the ITAQ and lower endogenous extracellular serotonin levels in the prefrontal cortex. Further studies with pharmacological challenge paradigms may be required. Moreover, since we cannot directly measure in vivo extracellular serotonin levels in the human brain,50 the interpretation regarding the relationship between insight deficits and SERT levels should be validated using a multitracer imaging method, combining both SERT and postsynaptic serotonergic markers.
Previous studies using clinical assessments including cognitive tests have implicated the prefrontal cortex in insight deficits in schizophrenia.10,11 In a structural MRI study, the left frontal pole volume was a significant predictor of the self-reflectiveness in schizophrenia.51 In a functional MRI study, the activation in the bilateral ventrolateral prefrontal cortex was significantly correlated with the self-reflective capacity in schizophrenia.6 The prefrontal cortex is also a critical brain region for self-reflective processes in the healthy human brain.52 It is also involved in critical functions, such as metacognition, which is associated with identifying and correcting erroneous beliefs and misinterpretations and is impaired in schizophrenia.53,54 Our study corroborates the importance of the prefrontal cortex underlying the lack of insight in schizophrenia and suggests the prefrontal SERT as the important neurochemical correlate.
In our study, the duration of illness was significantly positively associated with insight, particularly, treatment insight. This finding is in line with previous reports that insight impairment modestly improves over the course of the illness,55 and that patients in the chronic phase of schizophrenia have better insight than those in the first episode of psychosis or with a shorter duration of illness.56,57 Our finding along with previous reports may reflect the acceptance of the illness, more knowledge of the illness, or effects of a longer treatment period in patients with a longer duration than in those with a shorter duration of illness.55
The strengths of our study include the use of high-resolution PET imaging techniques. Compared with conventional PET systems, the HRRT-PET system used in our study has been reported to improve the quantification of monoamine neurotransmission parameters owing to reduced partial volume effects by enhanced spatial resolution.58,59 This enabled more accurate measurements of prefrontal SERT availability in our study. Another strength of this study is that all patients were antipsychotic-free and had not been taking psychotropic medications for at least 14 days before the PET scans. In addition, all patients had no history of taking antidepressant medications. All these aspects evidently minimized the confounding effects of psychotropic medications on the quantification of SERT availability.
The interpretation of the results of the present study should be considered in light of some limitations. The [11C]DASB BPND was quantified using the reference tissue model34,60 rather than using the metabolite-corrected arterial input function model. However, quantification using a metabolite-corrected arterial input function is invasive due to the requirement for cannulation of a radial artery, and consequent discomfort might be a confounding factor. Moreover, imprecision in determining the metabolite-corrected input function can be a source of variance in estimation of the endpoint.61 In our study, we used the [11C]DASB radiotracer. Additional research using other SERT tracers, such as [11C]MADAM62 and [11C]AFM63 may be required to confirm our findings. In addition, our study is considered preliminary as the results did not survive FDR correction for multiple correlations.
Conclusion
Our study provides in vivo evidence of significant negative correlations between insight deficits and prefrontal SERT availability in antipsychotic-free patients with schizophrenia, suggesting a significant involvement of cortical serotonergic signaling in impaired insight, one of the core symptoms of schizophrenia.
Acknowledgments
The authors are grateful to all the participants who took part in this study. The authors thank the staff of the cyclotron facility and PET technologist at Gachon University Neuroscience Research Institute. Finally, the authors express our gratitude to the anonymous reviewers for their many suggestions that helped improve this paper.
Funding
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2016M3C7A1914451), and by the NRF grant funded by the Korea government (MSIT) (NRF-2020R1A4A1019623).
Disclosure
The authors declare no conflicts of interest or financial disclosures for this work.
References
1. Lysaker PH, Pattison ML, Leonhardt BL, Phelps S, Vohs JL. Insight in schizophrenia spectrum disorders: relationship with behavior, mood and perceived quality of life, underlying causes and emerging treatments. World Psychiatry. 2018;17(1):12–23. doi:10.1002/wps.20508
2. Shad MU, Keshavan MS, Tamminga CA, Cullum CM, David A. Neurobiological underpinnings of insight deficits in schizophrenia. Int Rev Psychiatry. 2007;19(4):437–446. doi:10.1080/09540260701486324
3. Bedford NJ, Surguladze S, Giampietro V, Brammer MJ, David AS. Self-evaluation in schizophrenia: an fMRI study with implications for the understanding of insight. BMC Psychiatry. 2012;12(1):106. doi:10.1186/1471-244X-12-106
4. Bergé D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O. Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand. 2011;123(6):431–439. doi:10.1111/j.1600-0447.2010.01635.x
5. Buchy L, Ad-Dab’bagh Y, Lepage C, et al. Symptom Attribution in first episode psychosis: a cortical thickness study. Psychiatry Res. 2012;203(1):6–13. doi:10.1016/j.pscychresns.2011.09.009
6. Buchy L, Hawco C, Joober R, Malla A, Lepage M. Cognitive insight in first-episode schizophrenia: further evidence for a role of the ventrolateral prefrontal cortex. Schizophr Res. 2015;166(1–3):65–68. doi:10.1016/j.schres.2015.05.009
7. Ćurčić-blake B, van der Meer L, Pijnenborg GH, David AS, Aleman A. Insight and psychosis: functional and anatomical brain connectivity and self-reflection in Schizophrenia. Hum Brain Mapp. 2015;36(12):4859–4868. doi:10.1002/hbm.22955
8. Orfei MD, Piras F, Macci E, Caltagirone C, Spalletta G. The neuroanatomical correlates of cognitive insight in schizophrenia. Soc Cogn Affect Neurosci. 2013;8(4):418–423. doi:10.1093/scan/nss016
9. Shad MU, Keshavan MS. Neurobiology of insight deficits in schizophrenia: an fMRI study. Schizophr Res. 2015;165(2–3):220–226. doi:10.1016/j.schres.2015.04.021
10. Aleman A, Agrawal N, Morgan KD, David AS. Insight in psychosis and neuropsychological function: meta-analysis. Br J Psychiatry. 2006;189(3):204–212. doi:10.1192/bjp.189.3.204
11. Nair A, Palmer EC, Aleman A, David AS. Relationship between cognition, clinical and cognitive insight in psychotic disorders: a review and meta-analysis. Schizophr Res. 2014;152(1):191–200. doi:10.1016/j.schres.2013.11.033
12. Jackman H, Luchins D, Meltzer HY. Platelet serotonin levels in schizophrenia: relationship to race and psychopathology. Biol Psychiatry. 1983;18(8):887–902.
13. Bosia M, Anselmetti S, Bechi M, et al. Effect of 5-HT1A-receptor functional polymorphism on Theory of Mind performances in schizophrenia. Psychiatry Res. 2011;188(2):187–190. doi:10.1016/j.psychres.2010.11.014
14. Bora E. Relationship between insight and theory of mind in schizophrenia: a meta-analysis. Schizophr Res. 2017;190:11–17. doi:10.1016/j.schres.2017.03.029
15. Lin C-H, Tseng Y-L, Huang C-L, Chang Y-C, Tsai GE, Lane H-Y. Synergistic effects of COMT and TPH2 on social cognition. Psychiatry. 2013;76(3):273–294. doi:10.1521/psyc.2013.76.3.273
16. Lo C-H, Tsai GE, Liao C-H, et al. Emotional management and 5-HT2A receptor gene variance in patients with schizophrenia. Biol Psychol. 2010;83(2):79–83. doi:10.1016/j.biopsycho.2009.11.002
17. Quee PJ, van der Meer L, Bruggeman R, et al. Insight in psychosis: relationship with neurocognition, social cognition and clinical symptoms depends on phase of illness. Schizophr Bull. 2011;37(1):29–37. doi:10.1093/schbul/sbq133
18. Fénelon K, Mukai J, Xu B, et al. Deficiency of Dgcr8, a gene disrupted by the 22q11.2 microdeletion, results in altered short-term plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108(11):4447–4452. doi:10.1073/pnas.1101219108
19. Stephan KE, Friston KJ, Frith CD. Dysconnection in Schizophrenia: from Abnormal Synaptic Plasticity to Failures of Self-monitoring. Schizophr Bull. 2009;35(3):509–527. doi:10.1093/schbul/sbn176
20. Wilson AA, Ginovart N, Schmidt M, Meyer JH, Threlkeld PG, Houle S. Novel Radiotracers for Imaging the Serotonin Transporter by Positron Emission Tomography: synthesis, Radiosynthesis, and in Vitro and ex Vivo Evaluation of 11C-Labeled 2-(Phenylthio)araalkylamines. J Med Chem. 2000;43(16):3103–3110. doi:10.1021/jm000079i
21. Wilson AA, Ginovart N, Hussey D, Meyer J, Houle S. In vitro and in vivo characterisation of [11C]-DASB: a probe for in vivo measurements of the serotonin transporter by positron emission tomography. Nucl Med Biol. 2002;29(5):509–515. doi:10.1016/S0969-8051(02)00316-5
22. Ginovart N, Wilson AA, Meyer JH, Hussey D, Houle S. Positron Emission Tomography Quantification of [11C]-DASB Binding to the Human Serotonin Transporter: modeling Strategies. J Cereb Blood Flow Metab. 2001;21(11):1342–1353. doi:10.1097/00004647-200111000-00010
23. Meyer JH, Wilson AA, Ginovart N, et al. Occupancy of Serotonin Transporters by Paroxetine and Citalopram During Treatment of Depression: a [11C]DASB PET Imaging Study. Am J Psychiatry. 2001;158(11):1843–1849. doi:10.1176/appi.ajp.158.11.1843
24. Meyer JH. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci. 2007;32(2):86–102.
25. van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34(6):935–946. doi:10.1016/j.neubiorev.2009.12.004
26. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV.
27. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders Research Version (SCID-I). New York: Biometrics Research. New York State Psychiatric Institute; 1996.
28. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi:10.1093/schbul/13.2.261
29. McEvoy JP, Apperson LJ, Appelbaum PS, et al. Insight in schizophrenia: its relationship to acute psychopathology. J Nerv Ment Dis. 1989;177(1):43–47. doi:10.1097/00005053-198901000-00007
30. Kim BY, Lee CW, Park CW. The relationship among insight, psychopathology and drug compliance in the schizophrenic patient. J Korean Neuropsychiatr Assoc. 1993;32(3):373–380.
31. Knoess C, Rist J, Michel C, et al. Evaluation of single photon transmission for the HRRT.
32. Hong IK, Chung ST, Kim HK, Kim YB, Son YD, Cho ZH. Ultra Fast Symmetry and SIMD-Based Projection-Backprojection (SSP) Algorithm for 3-D PET Image Reconstruction. IEEE Trans Med Imaging. 2007;26(6):789–803. doi:10.1109/TMI.2007.892644
33. Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2(4):189–210. doi:10.1002/hbm.460020402
34. Wu Y, Carson RE. Noise Reduction in the Simplified Reference Tissue Model for Neuroreceptor Functional Imaging. J Cereb Blood Flow Metab. 2002;22(12):1440–1452. doi:10.1097/01.WCB.0000033967.83623.34
35. Bhagwagar Z, Murthy N, Selvaraj S, et al. 5-HTT Binding in Recovered Depressed Patients and Healthy Volunteers: a Positron Emission Tomography Study With [11C]DASB. Am J Psychiatry. 2007;164(12):1858–1865. doi:10.1176/appi.ajp.2007.06111933
36. Talbot PS, Bradley S, Clarke CP, et al. Brain Serotonin Transporter Occupancy by Oral Sibutramine Dosed to Steady State: a PET Study Using 11C-DASB in Healthy Humans. Neuropsychopharmacology. 2010;35(3):741–751. doi:10.1038/npp.2009.182
37. Reimold M, Knobel A, Rapp MA, et al. Central serotonin transporter levels are associated with stress hormone response and anxiety. Psychopharmacology. 2011;213(2–3):563–572. doi:10.1007/s00213-010-1903-y
38. Miller JM, Hesselgrave N, Ogden RT, et al. Positron Emission Tomography Quantification of Serotonin Transporter in Suicide Attempters with Major Depressive Disorder. Biol Psychiatry. 2013;74(4):287–295. doi:10.1016/j.biopsych.2013.01.024
39. Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–131. doi:10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8
40. Teffer K, Semendeferi K. Chapter 9 - Human prefrontal cortex: evolution, development, and pathology. In: Hofman MA, Falk D editors. Progress in Brain Research. Elsevier; 2012:191–218. doi:10.1016/B978-0-444-53860-4.00009-X
41. Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi:10.1016/j.neuropsychologia.2007.06.015
42. Murray EA, Wise SP, Graham KS. The Evolution of Memory Systems: Ancestors. Anatomy, and Adaptations. Oxford University Press; 2017.
43. Chance SA. The cortical microstructural basis of lateralized cognition: a review. Front Psychol. 2014;5:820. doi:10.3389/fpsyg.2014.00820
44. Fallgatter AJ, Strik WK. Reduced Frontal Functional Asymmetry in Schizophrenia During a Cued Continuous Performance Test Assessed With Near-Infrared Spectroscopy. Schizophr Bull. 2000;26(4):913–919. doi:10.1093/oxfordjournals.schbul.a033505
45. Sampedro F, Vilaplana E, de Leon MJ, et al. APOE-by-sex interactions on brain structure and metabolism in healthy elderly controls. Oncotarget. 2015;6(29):26663–26674. doi:10.18632/oncotarget.5185
46. Lin KJ, Hsiao IT, Hsu JL, et al. Imaging characteristic of dual-phase (18) F-florbetapir(AV-45/Amyvid) PET for the concomitant detection of perfusion deficits and beta-amyloid deposition in Alzheimer’s disease and mild cognitive impairment. Eur J Nucl Med Mol Imaging. 2016;43(7):1304–1314. doi:10.1007/s00259-016-3359-8
47. Ginovart N, Wilson AA, Meyer JH, Hussey D, Houle S. [11C]-DASB, a tool for in vivo measurement of SSRI-induced occupancy of the serotonin transporter: PET characterization and evaluation in cats. Synapse. 2003;47(2):123–133. doi:10.1002/syn.10155
48. Yamamoto S, Onoe H, Tsukada H, Watanabe Y. Effects of increased endogenous serotonin on the in vivo binding of [11C]DASB to serotonin transporters in conscious monkey brain. Synapse. 2007;61(9):724–731. doi:10.1002/syn.20422
49. Milak MS, Ogden RT, Vinocur DN, et al. Effects of tryptophan depletion on the binding of [11C]-DASB to the serotonin transporter in baboons: response to acute serotonin deficiency. Biol Psychiatry. 2005;57(1):102–106. doi:10.1016/j.biopsych.2004.09.026
50. Erritzoe D, Frokjaer VG, Haahr MT, et al. Cerebral serotonin transporter binding is inversely related to body mass index. Neuroimage. 2010;52(1):284–289. doi:10.1016/j.neuroimage.2010.03.086
51. Raju VB, Shukla A, Jacob A, et al. The frontal pole and cognitive insight in schizophrenia. Psychiatry Res Neuroimaging. 2021;308:111236. doi:10.1016/j.pscychresns.2020.111236
52. Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi:10.1038/nrn1884
53. Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2):319–329. doi:10.1016/S0920-9964(03)00189-0
54. Palmer EC, Gilleen J, David AS. The relationship between cognitive insight and depression in psychosis and schizophrenia: a review and meta-analysis. Schizophr Res. 2015;166(1):261–268. doi:10.1016/j.schres.2015.05.032
55. Gerretsen P, Plitman E, Rajji TK, Graff-Guerrero A. The effects of aging on insight into illness in schizophrenia: a review. Int J Geriatr Psychiatry. 2014;29(11):1145–1161. doi:10.1002/gps.4154
56. Schennach R, Meyer S, Seemüller F, et al. Insight in schizophrenia–course and predictors during the acute treatment phase of patients suffering from a schizophrenia spectrum disorder. Eur Psychiatry. 2012;27(8):625–633. doi:10.1016/j.eurpsy.2012.01.001
57. Li W, Zhang H-H, Wang Y, et al. Poor Insight in Schizophrenia Patients in China: a Meta-Analysis of Observational Studies. Psychiatr Q. 2020;91(4):1017–1031. doi:10.1007/s11126-020-09786-7
58. Leroy C, Comtat C, Trébossen R, Syrota A, Martinot J-L, Ribeiro M-J. Assessment of 11C-PE2I Binding to the Neuronal Dopamine Transporter in Humans with the High-Spatial-Resolution PET Scanner HRRT. J Nucl Med. 2007;48(4):538–546. doi:10.2967/jnumed.106.037283
59. Varrone A, Sjöholm N, Eriksson L, Gulyás B, Halldin C, Farde L. Advancement in PET quantification using 3D-OP-OSEM point spread function reconstruction with the HRRT. Eur J Nucl Med Mol Imaging. 2009;36(10):1639–1650. doi:10.1007/s00259-009-1156-3
60. Son Y-D, Cho Z-H, Choi E-J, et al. Individually Differentiated Serotonergic Raphe Nuclei Measured with Brain PET/MR Imaging. Radiology. 2014;272(2):541–548. doi:10.1148/radiol.14131547
61. Slifstein M, Laruelle M. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl Med Biol. 2001;28(5):595–608. doi:10.1016/S0969-8051(01)00214-1
62. Lundberg J, Odano I, Olsson H, Halldin C, Farde L. Quantification of 11C-MADAM Binding to the Serotonin Transporter in the Human Brain. J Nucl Med. 2005;46(9):1505–1515.
63. Huang Y, Hwang D-R, Narendran R, et al. Comparative Evaluation in Nonhuman Primates of Five PET Radiotracers for Imaging the Serotonin Transporters: [11C]McN 5652, [11C]ADAM, [11C]DASB, [11C]DAPA, and [11C]AFM. J Cereb Blood Flow Metab. 2002;22(11):1377–1398. doi:10.1097/01.WCB.0000040948.67415.05
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.