Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 15
Association Between Indoxyl Sulfate and Dialysis Initiation and Cardiac Outcomes in Chronic Kidney Disease Patients
Authors Takkavatakarn K , Phannajit J, Udomkarnjananun S , Tangchitthavorngul S, Chariyavilaskul P, Sitticharoenchai P, Praditpornsilpa K, Eiam-Ong S, Susantitaphong P
Received 17 December 2021
Accepted for publication 10 March 2022
Published 26 March 2022 Volume 2022:15 Pages 115—126
DOI https://doi.org/10.2147/IJNRD.S354658
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Kullaya Takkavatakarn,1 Jeerath Phannajit,1 Suwasin Udomkarnjananun,1 Suri Tangchitthavorngul,1 Pajaree Chariyavilaskul,2,3 Patita Sitticharoenchai,4 Kearkiat Praditpornsilpa,1 Somchai Eiam-Ong,1 Paweena Susantitaphong1,5
1Division of Nephrology, Department of Medicine, Faculty of Medicine, King Chulalongkorn Memorial Hospital, Chulalongkorn University, Bangkok, Thailand; 2Clinical Pharmacokinetics and Pharmacogenomics Research Unit, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 3Department of Pharmacology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 4Division of Cardiology, Department of Medicine, Faculty of Medicine, King Chulalongkorn Memorial Hospital, Chulalongkorn University, Bangkok, Thailand; 5Research Unit for Metabolic Bone Disease in CKD Patients, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
Correspondence: Paweena Susantitaphong, Division of Nephrology, Department of Medicine, Faculty of Medicine, King Chulalongkorn Memorial Hospital, Chulalongkorn University, 1873 RAMA IV, Bangkok, 10330, Thailand, Tel +662 256-4251, Fax +662 256-4560, Email [email protected]
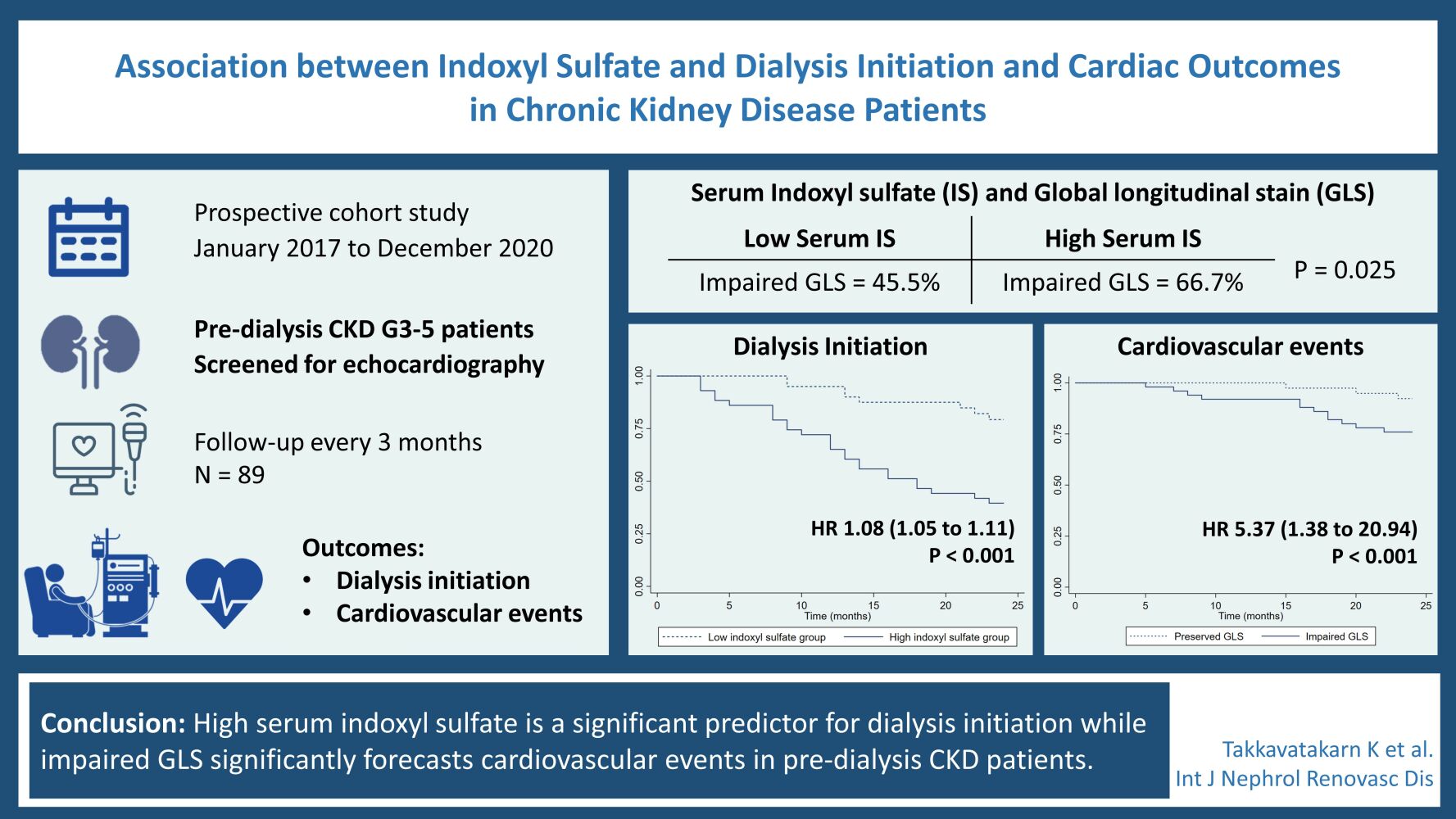
Introduction: Indoxyl sulfate, a protein-bound uremic toxin, has been reported as an atherosclerosis and fibrosis accelerator. This study aimed to determine whether serum indoxyl sulfate is associated with cardiac abnormalities, cardiovascular events, and renal progression to dialysis in patients with chronic kidney disease (CKD).
Methods: The prospective study enrolled 89 patients with CKD stage 3 to 5 patients. Serum biochemistry data and indoxyl sulfate were measured. All patients underwent echocardiographic examination. Global longitudinal strain (GLS) was calculated using two-dimensional speckle tracking. The clinical outcomes including cardiovascular event and dialysis initiation were recorded during a 2-year follow-up.
Results: Patients were divided into 2 groups based on the median value of serum indoxyl sulfate (low and high indoxyl sulfate groups). Kaplan–Meier analysis revealed that patients with higher indoxyl sulfate (≥ 6.124 mg/L) were significantly associated with renal progression to dialysis (p < 0.001). There was no significant difference in cardiovascular events between 2 groups (p = 0.082). In addition, serum indoxyl sulfate level was independently associated with GLS (r = 0.62; p = 0.01). The risk of cardiovascular events was significantly higher in patients with impaired GLS (>− 16%) (p = 0.015).
Conclusion: Serum indoxyl sulfate level was a significant predictor for CKD progression to dialysis and was correlated with GLS, a speckle tracking echocardiography parameter representing early LV systolic dysfunction. Furthermore, GLS was associated with cardiovascular events in CKD patients. Serum indoxyl sulfate measurement may help to identify the high dialysis and cardiovascular risk CKD patients beyond traditional risk factors.
Keywords: indoxyl sulfate, chronic kidney disease, echocardiographic parameters, renal outcomes, cardiovascular events
Graphical Abstract:
Introduction
Chronic kidney disease (CKD) is one of the most important global health problems and significantly increases cardiovascular disease (CVD).1 In CKD patients, the prevalence of heart failure and acute myocardial infarction was about four times higher than normal population.2 Moreover, cardiovascular events and mortality risks are significantly increasing along with the degree of declining renal function.3 The development of cardiomyopathy and vasculopathy in CKD patients could not be explained only by traditional risk factors, such as hypertension, dyslipidemia, diabetes, and smoking. Additional risk factors including oxidative stress, inflammation, endothelial dysfunction, and uremic-specific risk factors such as uremic toxin accumulation, anemia, and mineral bone disorder (CKD-MBD), are also proposed.4,5
Indoxyl sulfate is an important protein-bound uremic toxin derived from indole, a by-product of tryptophan metabolism of the gut microbiome. Indoxyl sulfate cannot be efficiently eliminated in patients with renal insufficiency, resulting in accumulation in the body and detrimental consequences on multiple organs.6 In vitro and animal studies previously revealed an association between indoxyl sulfate and renal tubular cell damage, tubulointerstitial fibrosis, cardiac fibrosis, vascular calcification, and atherosclerosis.6–9 A rising number of clinical studies suggests that indoxyl sulfate may also contribute to CVD and mortality in CKD patients.10,11 However, the association of serum indoxyl sulfate levels and renal progression especially dialysis initiation as well as structural cardiac abnormalities in CKD patients still requires more evidence.
In this prospective 2-year follow-up cohort study, we aimed to determine whether the indoxyl sulfate level is associated with renal progression and cardiac abnormalities represented by echocardiographic parameters. In addition, this study also investigated whether indoxyl sulfate and echocardiography-related cardiac abnormalities are potential predictors of cardiovascular (CV) events in pre-dialysis CKD stage 3–5 patients.
Methods
Study Population
We enrolled pre-dialysis CKD stage 3–5 patients who attended at outpatient clinic in the Nephrology Department of King Chulalongkorn Memorial Hospital, Bangkok, Thailand, from January 2017 to December 2020. The inclusion criteria were patients aged > 18 years old with estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2 defined by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation12 for at least 3 months. The exclusion criteria were patients with CVD (acute coronary artery disease, congestive heart failure, cerebrovascular disease) in the past 3 months.
Data Collection
Demographic data, including age, sex, causes of CKD, and medical history were obtained. All patients underwent baseline laboratory measurement, echocardiography, ankle-brachial index (ABI), and cardio-ankle vascular index (CAVI) measurement. Serum indoxyl sulfate was measured by high-performance liquid chromatography (HPLC) at the Pharmacology Department, Faculty of Medicine, Chulalongkorn University.
All echocardiographic parameters were performed and measured by one experienced cardiologist who was blind to patient data. Two-Dimensional (2D) and 2D guided M-mode images were recorded from the standardized views (4-chamber, 2-chamber, and apical long-axis) using gray-scale harmonic imaging and saved in raw data format (Epiq CVx, Philips Medical System, Bothell, Washington). Images were obtained at a frame rate of 50 to 70 per second and digital loops were saved onto an optical disc for offline analysis (Qlab version 10.2, Philips Healthcare). The echocardiographic measurements included left atrial (LA) diameter, LA volume index (LAVI), early transmitral filling wave velocity (E), late transmitral filling wave velocity (A), early diastolic mitral annular velocity (e’), E/A and E/e’ ratios, left ventricular end-diastolic diameter (LVDd), and left ventricular end-systolic diameter (LVDs). Left atrial enlargement was defined as LAVI > 34 m2. Left ventricular (LV) mass was calculated with the formula: LV mass = 0.8 × {1.04[([LV internal dimension + septal wall thickness + posterior wall thickness]3 − LV internal dimension3)] + 0.6 g. Left ventricular mass index (LVMI) was calculated by dividing left ventricular mass by body surface area.13 Left ventricular hypertrophy (LVH) was defined as LVMI > 95 g/m2 in women and > 115 g/m2 in men. End-diastolic and end-systolic volumes were used to calculate left ventricular ejection fraction (LVEF) using the Simpson biplane method from the apical 4- and 2-chamber views. Preserved LVEF was defined as ≥ 50%.14
LV systolic dysfunction was diagnosed by using global longitudinal strain (GLS) which is a parameter that expresses LV longitudinal shortening as a percentage (change in length as a proportion to baseline length). GLS measurements were performed offline utilizing commercially available dedicated automated software. The endocardial borders were traced in the end-systolic frame of the 2D images from the 3 apical views, assessed tracking quality and integration. Frame by frame tissue speckle tracking of the LV endocardium was performed and endocardial borders were readjusted manually until satisfactory tracking was achieved.15 GLS was calculated as the mean strain of 17 segments which the American Heart Association recommended.16 GLS varies typically with age, sex, and LV loading condition. Therefore, defining abnormal GLS is not straightforward. Previous studies have demonstrated that healthy individuals have GLS ranging from −16 to −20%.17 In our study, impaired GLS was defined as >-16% (a less negative value reflects a more impaired GLS).
ABI and CAVI were used to determine vascular stiffness at baseline by using a portable ultrasonography-based machine (VaSera VS-200; Fukuda-Denshi Company, Tokyo, Japan). ABI is determined by the highest systolic pressure on foot of that side/ average of the highest pressure from both arms, while CAVI score was calculated by the machine. The ABI score < 0.9 and 0.91–0.99 are peripheral arterial disease and borderline, respectively, while ABI at 1–1.4 and >1.4 are normal and non-compressible arteries, respectively.18 The CAVI score < 8 and 8–9 are normal and at risk for atherosclerosis, respectively, while CAVI > 9 is possible atherosclerosis.19
Study Follow-Up
Participants were followed-up every 3 months for 24 months. Complete clinical evaluation and renal function measurements were recorded. Patients received CKD care from multidisciplinary team consisting of nephrologists, nephrology nurses, and renal dietitians. Concomitant pharmacological and non-pharmacological therapies such as renin-angiotensin-aldosterone system (RAAS) inhibitors, anemia and CKD-MBD managements, and blood pressure control were prescribed according to the therapeutic target of standard guidelines. Dietary advice was organized by dietitians and nephrologists throughout the study period.
Definition of Outcomes
The primary outcome was CKD progression, defined as the initiation of renal replacement therapy (initiation of maintenance dialysis or kidney transplantation). The commencement of dialysis was made based on laboratory data, nutrition status, and uremic symptoms by nephrologist in the outpatient or inpatient consultation. The secondary outcome was cardiovascular (CV) events defined as fatal or non-fatal myocardial infarction (MI), angina pectoris, congestive heart failure, sudden death, cerebrovascular disorder, or peripheral arterial disease.
Statistical Analysis
Data were presented as mean ± (standard deviation; SD) for normally distributed or median (interquartile range; IQR) for non-normally distributed continuous variables. Categorical data were described as numbers and percentages. Statistical significance between the groups at baseline was evaluated by the One-way ANOVA or Kruskal–Wallis test. For analytical purposes, patients were divided according to the median serum indoxyl sulfate levels into 2 groups (low and high indoxyl sulfate groups). Comparison between the low and high indoxyl sulfate groups was evaluated by chi-square, unpaired t-tests, and Mann–Whitney U-test as appropriate.
The relationship between indoxyl sulfate levels and variables of interest and echocardiographic parameters were assessed using univariate and multivariate linear regression analyses for continuous variables. The multivariate model included all valuables that had a significant correlation with indoxyl sulfate in the univariate analysis.
Cumulative cardiovascular event-free survival estimates were calculated by utilizing the Kaplan–Meier method. The Log rank test was used to compare survival curves. Cox proportional hazards regression analysis was performed to calculate the hazard ratio (HR) and 95% confidence interval (95% CI) for cardiovascular events. Multivariate Cox proportional hazard regression analyses were performed to adjust various factors. A two-tailed p-value < 0.05 was considered significant. The participants with any missing data were excluded from analyses. Data were analyzed using the Stata Statistical Software version 15.0 (StataCorp LLC, College Station, TX).
Results
Patient Characteristics
This study cohort consists of 89 pre-dialysis CKD stage 3 to 5 patients. The mean age was 63.5±15 years. The most common cause of CKD was diabetic nephropathy (44.9%) followed by hypertensive nephropathy (16.8%), and chronic glomerulonephritis (12.4%). The percentage of CKD stage 3, 4, and 5 was 29.2%, 32.6%, and 38.2%, respectively. Serum indoxyl sulfate levels were significantly higher in patients with worsening renal function. The mean serum indoxyl sulfate levels were 2.73 ± 1.97, 5.83 ± 3.35, and 18.44 ± 10.31 mg/L in stages 3, 4, and 5 CKD, respectively.
Patients with worse renal function had a higher prevalence of left atrial (LA) enlargement (19% in CKD stage 3, 46.1% in CKD stage 4, and 57.6% in CKD stage 5; p=0.02), left ventricular hypertrophy (LVH) (48% in CKD stage 3, 75.8% in CKD stage 4, and 82.3% in CKD stage 5; p=0.01), and impaired global longitudinal strain (GLS) (43.3%, 51.7%, and 70.6% in CKD stage 3,4, and 5, respectively; p=0.04). However, there were no significant differences in the prevalence of the remaining echocardiographic parameters, including left ventricular ejection fraction (LVEF), among pre-dialysis CKD stage 3 to 5 patients (Data not shown).
Comparison Between Low and High Indoxyl Sulfate Groups
Patients were divided into 2 groups on the basis of the median serum indoxyl sulfate levels (low indoxyl sulfate group, < 6.124 mg/L and high indoxyl sulfate group, ≥ 6.124 mg/L) (Table 1). The high indoxyl sulfate group showed significantly lower eGFR, higher phosphate levels, and greater CAVI than the low indoxyl sulfate group. Patients with high indoxyl sulfate had a higher prevalence of LA enlargement, LVH, and impaired GLS) than patients in the low indoxyl sulfate group.
 |
Table 1 Clinical Characteristics Stratified According to Indoxyl Sulfate Levels |
Correlation Between Indoxyl Sulfate Levels and Clinical and Echocardiographic Parameters
The univariate correlations between serum indoxyl sulfate levels and clinical and echocardiographic parameters of the study population are shown in Table 2. A significant positive correlation was detected for creatinine, phosphate, and GLS, while a significant negative correlation was observed for hemoglobin. Figure 1 depicts a positive linear relationship between serum indoxyl sulfate levels and GLS (r=0.62, p = 0.01).
 |
Table 2 Univariate and Multivariate Linear Regression Analyses for Indoxyl Sulfate |
 |
Figure 1 Linear regression curve. Relationship between serum levels of indoxyl sulfate and global longitudinal strain. |
In multivariate linear regression analysis, creatinine (r = 1.49, p < 0.001), hemoglobin (r = −1.76, p = 0.002), and GLS (r= 0.42, p = 0.033) were independently correlated with indoxyl sulfate levels.
Predictor of Renal Outcomes
There were 34 patients who initiated renal replacement therapy during 24 months of follow-up. As detailed in Table 3, unadjusted predictors of progression to dialysis were age (p=0.005), creatinine (p<0.001), using of renin-angiotensin-aldosterone system (RAAS) inhibitors (p=0.001), serum indoxyl sulfate levels (p<0.001), LVEF (p=0.035), and GLS (p=0.010). Kaplan–Meier curve of the cumulative event-free survival for the dialysis dichotomized according to indoxyl sulfate levels (low and high) is reported in Figure 2. The high indoxyl sulfate group was associated with dialysis initiation when compared with low indoxyl sulfate group (log-rank p < 0.001). After adjustment for age, using RAAS inhibitors, creatinine, and GLS; indoxyl sulfate and LVEF remained independent predictors for progression to dialysis (HR 1.04; 95% CI 1.01–1.08; p=0.022) (Table 3).
 |
Table 3 Univariate and Multivariate Cox Proportional Hazards Models of Dialysis Initiation During Follow-Up |
 |
Figure 2 Kaplan–Meier curves of cumulative event-free survival for dialysis according to low- and high- indoxyl sulfate group. |
Predictors of Cardiovascular Events
There were 19 cardiovascular events during 24 months of follow-up. On univariate Cox proportional hazard analysis, LVEF and GLS were significantly associated with cardiovascular events together with age and CAVI. In contrast, there was no significant association between serum indoxyl sulfate levels and cardiovascular events (Table 4). Following adjustment with multivariate analysis, only GLS remained a significant predictor of cardiovascular events (HR 1.26; 95% CI 1.01–1.59; p=0.045) (Table 4). The cardiovascular events in impaired GLS group was significantly higher than the preserved GLS group (p=0.015) (Figure 3). There was no significant difference in cardiovascular events between low and high indoxyl sulfate groups (p=0.082).
 |
Table 4 Cox Univariate and Multivariate Regression Analyses for Predictors of Cardiovascular Events |
 |
Figure 3 Kaplan–Meier curve of the cumulative cardiovascular event-free survival stratified by GLS (preserved and impaired GLS groups). |
Discussion
In pre-dialysis CKD stage 3 to 5 patients, the results in the present prospective 2-year follow-up cohort study demonstrated that high serum indoxyl sulfate could predict progression to dialysis. In addition, indoxyl sulfate was significantly associated with GLS, an emerging echocardiography technique for measuring more subtle disturbances in LV systolic function that could forecast CVD events in pre-dialysis CKD patients.
Indoxyl sulfate has been reported as an atherosclerosis accelerator by increasing proinflammatory cytokines and oxidative stress, promoting endothelial dysfunction, inhibiting bone turnover, and inducing vascular calcification.6,7,20 Furthermore, a line of evidence illustrates that indoxyl sulfate could contribute to CKD progression. In in vitro and animal models of CKD, indoxyl sulfate was demonstrated to have direct toxic effects on tubular cells, leading to increased oxidative stress, inflammation, and fibrosis.21–23 Direct renal injury and vascular toxicity of indoxyl sulfate might accelerate renal progression. However, only a few prospective studies have evaluated the impact of serum indoxyl sulfate levels on CKD progression. The only one prospective cohort in 269 adult CKD G1-5 patients, revealed a significant association between serum indoxyl sulfate levels and renal progression, defined as a reduction of eGFR by 50% or end stage kidney disease requiring dialysis, after 21 months follow-up period.24 In the present study, we provided the result of a significant correlation between indoxyl sulfate and the commencement of dialysis which was independent of patients’ cardiovascular status, adjusted with CAVI, LVMI, and LVEF (Table 3). These findings suggest the temporal relationship between nephrotoxicity of indoxyl sulfate and CKD progression.
Regarding cardiovascular changes, hemodynamic and metabolic alterations in CKD result in cardiac remodeling which occurs early and is significantly worse than non-CKD.25 Hypervolemia and renal anemia contribute to volume load while hypertension, vascular calcifications, and increased sympathetic activation in CKD result in pressure load. Both volume and pressure overloads can cause LV geometric changes. In our cohort, LV mass and the prevalence of LVH increased with deteriorating kidney function, whereas there was no significant difference in LVEF among CKD stages 3, 4, and 5. The LVH enhancing effect observed in the present study was also detected in a previous study in patients with more advanced CKD26,27 while the prevalence of LV systolic dysfunction (reduced LVEF) in CKD patients varied according to different methodologies.28 Although the reduction in LVEF is good predictive of CV events and mortality, there were some limitations in utilizing LVEF as a predictor. The methods of LVEF measurement involve inaccuracies associated with unclear delineation of endocardial borders and the assumption of geometric uniformity.29 Furthermore, LVEF may be insufficiently sensitive to identify mild or subclinical degrees of LV systolic dysfunction. These limitations have led to an interest in new echocardiographic techniques that provide more objective and sensitive measures of LV myocardial function. GLS is a relatively new echocardiographic application used increasingly in the general population. This technique is an offline application that follows the motion of myocardial tissue throughout the cardiac cycle by tracking acoustic reflections, known as speckles. As GLS quantifies longitudinal contraction, especially in the subendocardial fibers, the wall layer most susceptible to ischemia, reductions in longitudinal strain may be found prior to a reduction in LVEF.30 GLS is an effective tool for detecting early subtle disturbances of LV systolic function when LVEF is normal. In the present study, patients with more advanced CKD had a higher prevalence of impaired GLS but had no significant differences in reduced LVEF.
Indoxyl sulfate has strong proinflammatory and pro-hypertrophic effects on cardiac cells. In addition, this protein-bound uremic toxin contributed to cardiac fibrosis in in vitro by stimulating synthesis of transforming growth factor-beta, and encouraging the NF-kB pathway activation.8 Therefore, accumulation of indoxyl sulfate in CKD patients might directly induce detrimental effects on the cardiac cells and cause adverse cardiac remodeling. Indoxyl sulfate also induces proinflammatory cytokines and oxidative stress, resulting in endothelial dysfunction and atherosclerosis.31 We demonstrated that patients with a high indoxyl sulfate group had a higher prevalence of LVH and impaired GLS than the low indoxyl sulfate group (Table 1). Of interest, there was a significant association between serum indoxyl sulfate levels and GLS in pre-dialysis CKD stage 3 to 5 patients (Table 2 and Figure 1). This finding was supported by a previous study in patients with CKD stages 3 and 4 by Krishnasamy et al who revealed that GLS was independently associated with free indoxyl sulfate level, body mass index, and arterial stiffness.32
In terms of cardiac events, we revealed a significant association between impaired GLS and cardiovascular events (Table 4). Although certain previous studies reported a correlation between GLS and cardiovascular events in non-CKD patients with heart failure,33,34 there were sparse data assessing the prognostic value of GLS in pre-dialysis CKD. In a cross-sectional study of 106 CKD patients stage 1–5 with preserved LVEF, Panoulas et al found that patients with impaired GLS (more than −16%) had an increased risk of major adverse cardiac events.35 However, this significant association was lost after adjustment for age, gender, diabetes, hypertension, and eGFR. In another prospective cohort in 106 pre-dialysis CKD patients with preserved LVEF, Sulemane et al demonstrated that GLS was a significant independent predictor of the composite endpoint of all-cause mortality, acute coronary syndrome, stable angina requiring revascularization, Hospitalization for heart failure, and stroke.36
As indoxyl sulfate is associated with GLS, serum indoxyl sulfate is supposed to be a good predictor of cardiac events in CKD patients. However, we did not find the significant association between serum indoxyl sulfate and cardiovascular events during the follow-up period (Table 4). On the contrary, Shimazu et al revealed that serum indoxyl sulfate was a significant predictor of cardiac events in patients with dilated cardiomyopathy.37 Moreover, a previous 3-year prospective cohort in CKD patients showed an association between indoxyl sulfate and major adverse cardiac events.38 The different outcomes might be explained by the disparities in populations, low incidence of cardiac events, and shorter follow-up time in our study.
There are some limitations in this study. First, the numbers of participants and the occurrence of cardiovascular events were relatively small. Therefore, the power to demonstrate the relationship between indoxyl sulfate and cardiovascular events might be limited. Second, the follow-up time is relatively short for detecting cardiovascular outcomes. Further studies with a larger sample size and longer follow-up time are still needed.
Conclusions
The persistently heightened serum indoxyl sulfate in CKD is associated with early impaired left ventricular systolic function detected by GLS. In addition, high serum indoxyl sulfate and reduced LVEF are significant predictors for dialysis initiation while impaired GLS significantly forecasts cardiovascular events in CKD patients.
Data Statement
The datasets generated and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Statement of Ethics
The study complies with the principles of the Helsinki Declaration 2013. The whole protocol has been reviewed and approved by the Ethical Committee, Chulalongkorn University, Bangkok, Thailand (IRB number 503/60; 7 August 2017). Written informed consent was obtained from all patients included in the study.
Acknowledgment
The authors would like to thank all staffs from the nephrology Research Unit.
Funding
This study is supported by the Royal College of Physicians of Thailand.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Carney EF. The impact of chronic kidney disease on global health. Nat Rev Nephrol. 2020;16(5):251. doi:10.1038/s41581-020-0268-7
2. System USRD. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020.
3. Jankowski J, Floege J, Fliser D, Bohm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–1172. doi:10.1161/CIRCULATIONAHA.120.050686
4. Valdivielso JM, Rodriguez-Puyol D, Pascual J, et al. Atherosclerosis in chronic kidney disease: more, less, or just different? Arterioscler Thromb Vasc Biol. 2019;39(10):1938–1966. doi:10.1161/ATVBAHA.119.312705
5. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi:10.1056/NEJMoa041031
6. Leong SC, Sirich TL. Indoxyl sulfate-review of toxicity and therapeutic strategies. Toxins. 2016;8(12):358. doi:10.3390/toxins8120358
7. Fujii H, Nakai K, Fukagawa M. Role of oxidative stress and indoxyl sulfate in progression of cardiovascular disease in chronic kidney disease. Ther Apher Dial. 2011;15(2):125–128. doi:10.1111/j.1744-9987.2010.00883.x
8. Liu S, Wang BH, Kompa AR, Lekawanvijit S, Krum H. Antagonists of organic anion transporters 1 and 3 ameliorate adverse cardiac remodelling induced by uremic toxin indoxyl sulfate. Int J Cardiol. 2012;158(3):457–458. doi:10.1016/j.ijcard.2012.05.022
9. Enomoto A, Takeda M, Tojo A, et al. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J Am Soc Nephrol. 2002;13(7):1711–1720. doi:10.1097/01.ASN.0000022017.96399.B2
10. Yamamoto S, Fuller DS, Komaba H, et al. Serum total indoxyl sulfate and clinical outcomes in hemodialysis patients: results from the Japan Dialysis Outcomes and Practice Patterns Study. Clin Kidney J. 2021;14(4):1236–1243. doi:10.1093/ckj/sfaa121
11. Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(10):1551. doi:10.2215/CJN.03980609
12. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
13. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi:10.1016/j.echo.2014.10.003
14. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi:10.1016/j.echo.2016.01.011
15. Leitman M, Lysyansky P, Sidenko S, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17(10):1021–1029. doi:10.1016/j.echo.2004.06.019
16. Bansal M, Cho GY, Chan J, Leano R, Haluska BA, Marwick TH. Feasibility and accuracy of different techniques of two-dimensional speckle based strain and validation with harmonic phase magnetic resonance imaging. J Am Soc Echocardiogr. 2008;21(12):1318–1325. doi:10.1016/j.echo.2008.09.021
17. Marwick TH, Leano RL, Brown J, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009;2(1):80–84. doi:10.1016/j.jcmg.2007.12.007
18. Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–2909. doi:10.1161/CIR.0b013e318276fbcb
19. Takahashi K, Yamamoto T, Tsuda S, Maruyama M, Shirai K. The background of calculating CAVI: lesson from the discrepancy between cavi and cavi0. Vasc Health Risk Manag. 2020;16:193–201. doi:10.2147/VHRM.S223330
20. Watanabe K, Tominari T, Hirata M, et al. Indoxyl sulfate, a uremic toxin in chronic kidney disease, suppresses both bone formation and bone resorption. FEBS Open Bio. 2017;7(8):1178–1185. doi:10.1002/2211-5463.12258
21. Milanesi S, Garibaldi S, Saio M, et al. Indoxyl sulfate induces renal fibroblast activation through a targetable heat shock protein 90-dependent pathway. Oxid Med Cell Longev. 2019;2019:2050183. doi:10.1155/2019/2050183
22. Miyazaki T, Ise M, Seo H, Niwa T. Indoxyl sulfate increases the gene expressions of TGF-beta 1, TIMP-1 and pro-alpha 1(I) collagen in uremic rat kidneys. Kidney Int Suppl. 1997;62:S15–22.
23. Chen CN, Chou CC, Tsai PSJ, Lee YJ. Plasma indoxyl sulfate concentration predicts progression of chronic kidney disease in dogs and cats. Vet J. 2018;232:33–39. doi:10.1016/j.tvjl.2017.12.011
24. Wu IW, Hsu KH, Lee CC, et al. P-cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26(3):938–947. doi:10.1093/ndt/gfq580
25. Kaesler N, Babler A, Floege J, Kramann R. Cardiac remodeling in chronic kidney disease. Toxins. 2020;12(3):161. doi:10.3390/toxins12030161
26. Nitta K, Iimuro S, Imai E, et al. Risk factors for increased left ventricular hypertrophy in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17(5):730–742. doi:10.1007/s10157-012-0758-4
27. London GM, Pannier B, Guerin AP, et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol. 2001;12(12):2759–2767. doi:10.1681/ASN.V12122759
28. Sulemane S, Panoulas VF, Nihoyannopoulos P. Echocardiographic assessment in patients with chronic kidney disease: current update. Echocardiography. 2017;34(4):594–602. doi:10.1111/echo.13495
29. Pecoits-Filho R, Barberato SH. Echocardiography in chronic kidney disease: diagnostic and prognostic implications. Nephron Clin Pract. 2010;114(4):c242–7. doi:10.1159/000276575
30. Amzulescu MS, De Craene M, Langet H, et al. Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging. 2019;20(6):605–619. doi:10.1093/ehjci/jez041
31. Hung SC, Kuo KL, Wu CC, Tarng DC. Indoxyl sulfate: a novel cardiovascular risk factor in chronic kidney disease. J Am Heart Assoc. 2017;6:2. doi:10.1161/JAHA.116.005022
32. Krishnasamy R, Isbel NM, Hawley CM, et al. Left ventricular global longitudinal strain (GLS) is a superior predictor of all-cause and cardiovascular mortality when compared to ejection fraction in advanced chronic kidney disease. PLoS One. 2015;10(5):e0127044. doi:10.1371/journal.pone.0127044
33. Otani K, Higa Y, Kitano T, Nabeshima Y, Takeuchi M. Prediction of cardiac events using fully automated GLS and BNP titers in patients with known or suspected heart failure. PLoS One. 2020;15(6):e0234294. doi:10.1371/journal.pone.0234294
34. Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71(18):1947–1957. doi:10.1016/j.jacc.2018.02.064
35. Panoulas VF, Sulemane S, Konstantinou K, et al. Early detection of subclinical left ventricular myocardial dysfunction in patients with chronic kidney disease. Eur Heart J Cardiovasc Imaging. 2015;16(5):539–548. doi:10.1093/ehjci/jeu229
36. Sulemane S, Panoulas VF, Bratsas A, Grapsa J, Brown EA, Nihoyannopoulos P. Subclinical markers of cardiovascular disease predict adverse outcomes in chronic kidney disease patients with normal left ventricular ejection fraction. Int J Cardiovasc Imaging. 2017;33(5):687–698. doi:10.1007/s10554-016-1059-x
37. Shimazu S, Hirashiki A, Okumura T, et al. Association between indoxyl sulfate and cardiac dysfunction and prognosis in patients with dilated cardiomyopathy. Circ J. 2013;77(2):390–396. doi:10.1253/circj.CJ-12-0715
38. Fan PC, Chang JC, Lin CN, et al. Serum indoxyl sulfate predicts adverse cardiovascular events in patients with chronic kidney disease. J Formos Med Assoc. 2019;118(7):1099–1106. doi:10.1016/j.jfma.2019.03.005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
