Back to Journals » OncoTargets and Therapy » Volume 9
Association between GSTP1 Ile105Val polymorphism and urinary system cancer risk: evidence from 51 studies
Authors Zhang Y, Yuan Y, Chen Y, Wang Z , Li F, Zhao Q
Received 16 February 2016
Accepted for publication 31 March 2016
Published 15 June 2016 Volume 2016:9 Pages 3565—3569
DOI https://doi.org/10.2147/OTT.S106527
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr William C. Cho
Yixiang Zhang,1 Yeqing Yuan,1 Yufang Chen,2 Zhao Wang,3 Fangfang Li,3 Qingsong Zhao4
1Department of Urology, The Second Affiliated Hospital of Jinan University, Shenzhen People’s Hospital, Shenzhen, Guangdong, 2Department of Pharmacology, School of Basic Medicine, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 3Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 4Department of Urology, The First People’s Hospital of Jining, Jining, Shandong, People’s Republic of China
Abstract: The GSTP1 gene plays an important role in detoxification of carcinogens. GSTP1 gene polymorphisms may alter the susceptibility of urinary system cancer. Numerous studies have been performed to investigate the association between GSTP1 Ile105Val (rs1695 A>G) polymorphism and urinary system cancer risk. Nevertheless, the results remain controversial and only prostate cancer and bladder cancer are covered. We identified eligible studies from PubMed, Elsevier, and three equivalent Chinese databases including the Chinese National Knowledge Infrastructure, Wanfang, and Weipu. Pooled odds ratios and 95% confidence intervals were used to assess the strength of the association between GSTP1 Ile105Val polymorphism and urinary system cancer risk. In total, 11,762 cases and 15,150 controls from 51 studies were included in the final meta-analysis. The pooled results from all included studies showed a statistically significant association between GSTP1 Ile105Val polymorphism and urinary system cancer. In the subgroup analyses, the GSTP1 Ile105Val polymorphism was found to be significantly associated with prostate cancer risk and also a risk factor for urinary system cancer among Asians. In conclusion, our meta-analysis indicated that GSTP1 Ile105Val polymorphism was associated with urinary system cancer susceptibility, which needs to be validated by more rigorous data from further large-scale population studies with different ethnicities.
Keywords: GSTP1, polymorphism, urinary system cancer, susceptibility, meta-analysis
Introduction
Urinary system cancer such as prostate cancer (PCa) and bladder cancer (BC) constitutes an enormous burden on the society in many countries.1 PCa is the fifth leading cause of cancer death worldwide, with an estimated 1.1 million new cases reported in 2012.1 Although BC incidence rates, on the other hand, have been declining or stable in some Western countries, the death rates still remain very high, eg, an estimated 429,800 new cases of BC and 165,100 deaths occurred in 2012 worldwide.1 In addition to the environmental factors such as smoking, being overweight, and physical inactivity,2,3 genetic factors also play an important role in the carcinogenesis of urinary system cancer.4,5
Glutathione S-transferases (GSTs) are members of a super family of genes the gene products of which are the most important Phase II metabolizing enzymes.6,7 These enzymes catalyze a variety of potentially carcinogenic electrophonic compounds.8,9 Therefore, GST genes and enzymes are considered to be involved in susceptibility to tumor formation. In humans, the GSTs super family has been categorized into eight distinct gene families: α, μ, κ, π, σ, ω, θ, and ζ.10 The GSTP1 gene, located on chromosome 11q13, belongs to the π gene family.11 The GSTP1 Ile105Val (rs1695 A>G) polymorphism may cause a substitution of isoleucine for valine at codon 105 in the protein, leading to substantial reduction in GSTP1 enzyme activity and its capability of detoxification of carcinogens.12
Previous published studies investigating the association between GSTP1 Ile105Val polymorphism and susceptibility to urinary system cancer reported controversial results. For instance, some studies suggest that the association between GSTP1 Ile105Val polymorphism and PCa or BC susceptibility is significant,13–15 whereas others suggest little or no association.16,17 Moreover, no review on other types of urinary system cancer such as renal cell carcinoma (RCC) has been published. Hence, we performed a meta-analysis of 51 publications covering 11,762 cases and 15,150 controls to get a clearer overall picture of the effect of GSTP1 Ile105Val polymorphism on the risk of urinary system cancer.
Materials and methods
Search strategies
We conducted a comprehensive literature search on PubMed, Elsevier, and three Chinese equivalents, including the Chinese National Knowledge Infrastructure, Wanfang, and Weipu databases (1999 to January 8, 2016), using the following keywords and free text words: “glutathione S-transferase P1 or GSTP1 or GSTP1|105V or Ile105Val or A313G”, and “polymorphism or variant or variation” and “prostate or bladder or urocyst or urotheli* or renal or urinary”. No language restriction was set. The reference lists of all eligible studies were searched manually to ascertain additional undetected published studies.
Inclusion and exclusion criteria
Studies were eligible for inclusion in this meta-analysis study when they met the following criteria: 1) studies evaluating the association between GSTP1 Ile105Val polymorphism and urinary system cancer, ie, PCa, BC, and RCC; 2) case–control studies; 3) studies with a 95% confidence interval (CI) for risk ratio or odds ratio (OR) or with sufficient data to calculate these numbers.
We excluded review articles, conference abstracts, case reports, and editorials. When two or more articles shared the same study data or were published in both English and Chinese sources, we excluded the ones published earlier or in Chinese.
Data extraction
Two authors (Yixiang Zhang and Yeqing Yuan) extracted the following information independently from all eligible studies: first author, publication year, the country of origin, ethnicity of the study population, cancer type, source of controls, genotyping methods, and number of cases and controls with different GSTP1 Ile105Val genotypes (AA, AG, and GG). Any disagreements were resolved by discussions between the two authors until consensus was reached.
Statistical analysis
Effect of heterogeneity was quantified with the I2 statistic that measures the degree of between-study inconsistency. I2 ranges between 0% and 100% with higher values indicating a greater degree of heterogeneity.18 Random-effects model was chosen when the P-value of the heterogeneity test was <0.1 or I2>25%, otherwise, the fixed-effects model was selected to calculate the pooled-effect estimates. Subgroup analyses were performed on the basis of cancer type, source of controls, and ethnicity. Publication bias was assessed by visual inspection of funnel plots, the Begg’s rank correlation method, and the Egger’s weighted regression method. The overall analysis and stratified analysis were performed with STATA software (Version 11, StataCorp LP, College Station, TX, USA). P<0.05 was considered statistically significant.
Results
Study characteristics
As shown in Figure 1, a total of 51 eligible studies met the inclusion criteria after abstract and full-text assessment. In total, 11,762 cases and 15,150 controls were included in the pooled analyses. Of all the 51 included studies, there was one study about two types of cancer and thus there were totally 52 data points: 27 about PCa, 19 about BC, and six about RCC. The characteristics of the included studies are summarized in Table S1.
  | Figure 1 Flowchart of 51 studies included in the meta-analysis. |
Meta-analyses results
The pooled results based on all the studies showed a statistically significant association between GSTP1 Ile105Val (rs1695 A>G) polymorphism and urinary system cancer (Table 1). The association was most apparent for the genotype GG (GG vs AA: OR =1.34, 95% CI =1.13–1.59; Figure S1). The tests for heterogeneity between eligible studies on different genotypes were significant (Table 1); thus, the random-effects model was performed for the data analysis of studies on a certain genotype.
For each genotype, we performed subgroup analysis. When stratifying by cancer type, the ORs for GSTP1 Ile105Val polymorphism were only significant in the analysis on PCa (GG vs AA: OR =1.39, 95% CI =1.10–1.76; GG vs AA + AG: OR =1.28, 95% CI =1.05–1.56; AG + GG vs AA: OR =1.24, 95% CI =1.04–1.49). When stratifying by the source of controls, we found a significant difference between GSTP1 Ile105Val and urinary system cancer risk in studies with hospital-based controls (Table 1). When stratifying by ethnicity, the significant association between GSTP1 Ile105Val polymorphism and urinary system cancer risk was only identifiable in the analysis among Asians, in which GSTP1 Ile105Val GG variant showed a marked increase in urinary system cancer risk compared to individuals carrying AA or AG genotype (Table 1).
Based on the results from Begg’s rank correlation method and Egger’s weighted regression method, we found that there was no evidence of publication bias in GSTP1 Ile105Val polymorphism studies (Table 2).
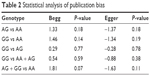  | Table 2 Statistical analysis of publication bias |
Discussion
The current meta-analysis, including 11,762 cases and 15,150 controls from 51 published studies, evaluated the association between GSTP1 Ile105Val polymorphism and urinary system cancer risk. To the best of our knowledge, this meta-analysis is the latest and largest one to explore GSTP1 Ile105Val (rs1695 A>G) polymorphism in the development of urinary system cancer. We found that GSTP1 Ile105Val polymorphism was significantly associated with the risk of urinary system cancer, and the association was even more evident for the genotype of GSTP1 Ile105Val GG genotype in studies with PCa and Asians.
Previous meta-analyses on the association between GSTP1 Ile105Val polymorphism and PCa risk were contradicting. Two studies found that GSTP1 Ile105Val polymorphism was significantly associated with PCa risk, with further subgroup analyses identifying significant association among Caucasians rather than Asians and African Americans.13,14 However, another meta-analysis with a total of 5,301 cases and 5,621 controls by Mo et al16 concluded no association between GSTP1 Ile105Val polymorphism and the risk of PCa. The results of the current study show that there is indeed significant association between GSTP1 Ile105Val polymorphism and PCa risk, but only among Asians, as suggested in the subgroup analyses of our study.
The results of the current study show that GSTP1 Ile105Val polymorphism is not significantly associated with BC risk, which is consistent with the finding of a meta-analysis by Kellen et al17 that GSTP1 Ile105Val polymorphism was not strongly associated with BC risk. However, Wu et al15 found that a specific genotype of GSTP1 313GG genotype was a strong predisposing risk factor for BC, while as for in our study, the specific genotype of GSTP1 313GG polymorphism is only associated with PCa.
As there are very few studies on the association between GSTP1 Ile105Val polymorphism and RCC, we only identified five studies in the current meta-analysis. Results of our meta-analysis show that GSTP1 Ile105Val polymorphism is not significantly associated with RCC risk, but the estimation may not be precise because of the paucity of data. More studies with large samples in different ethnicities are needed to provide more accurate estimation of the effect of GSTP1 Ile105Val polymorphism on RCC.
Before concluding this report, we should admit some limitations in this study. First, our results were based on unadjusted estimates due to the missing of individual data on lifestyles and environmental exposure such as smoking status and alcohol consumption. These lifestyle and environmental factors had been reported to play an important role in the development of urinary system cancer. Second, the sample size is not always large enough to provide precise estimation. For instance, the subgroup analyses regarding RCC might have insufficient statistical power to reveal the real association.
Conclusion
Despite the limitations, our meta-analysis indicated that GSTP1 Ile105Val polymorphism may be associated with urinary system cancer susceptibility. Further large-scale population studies with different ethnicities should be conducted to provide more rigorous data to validate our findings.
Acknowledgment
This work was supported by the Shenzhen Commission of Science and Innovation program (No JCYJ20150403101028172).
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Shiels MS, Gibson T, Sampson J, et al. Cigarette smoking prior to first cancer and risk of second smoking-associated cancers among survivors of bladder, kidney, head and neck, and stage I lung cancers. J Clin Oncol. 2014;32(35):3989–3995. | ||
Vermeulen SH, Hanum N, Grotenhuis AJ, et al. Recurrent urinary tract infection and risk of bladder cancer in the Nijmegen bladder cancer study. Br J Cancer. 2015;112(3):594–600. | ||
Chang WS, Liao CH, Miao CE, et al. The role of functional polymorphisms of cyclooxygenase 2 in renal cell carcinoma. Anticancer Res. 2014;34(10):5481–5486. | ||
Zhang Y, Yang D, Zhu JH, Chen MB, Shen WX, He J. The association between NQO1 Pro187Ser polymorphism and urinary system cancer susceptibility: a meta-analysis of 22 studies. Cancer Invest. 2015;33(2):39–40. | ||
Yang Q, Liu YJ, Zeng QY. Biochemical functions of the glutathione transferase supergene family of Larix kaempferi. Plant Physiol Biochem. 2014;77:99–107. | ||
Puglisi I, Lo Cicero L, Lo Piero AR. The glutathione S-transferase gene superfamily: an in silico approach to study the post translational regulation. Biodegradation. 2013;24(4):471–485. | ||
Kalinina EV, Chernov NN, Novichkova MD. Role of glutathione, glutathione transferase, and glutaredoxin in regulation of redox-dependent processes. Biochemistry. 2014;79(13):1562–1583. | ||
Buldak RJ, Buldak L, Kukla M, Gabriel A, Zwirska-Korczala K. Significance of selected antioxidant enzymes in cancer cell progression. Pol J Pathol. 2014;65(3):167–175. | ||
Pearson WR. Phylogenies of glutathione transferase families. Methods Enzymol. 2005;401:186–204. | ||
Liu X, An BH, Kim MJ, Park JH, Kang YS, Chang M. Human glutathione S-transferase P1-1 functions as an estrogen receptor alpha signaling modulator. Biochem Biophys Res Commun. 2014;452(3):840–844. | ||
Sawers L, Ferguson MJ, Ihrig BR, et al. Glutathione S-transferase P1 (GSTP1) directly influences platinum drug chemosensitivity in ovarian tumour cell lines. Br J Cancer. 2014;111(6):1150–1158. | ||
Cai Q, Wu T, Zhang W, et al. Genetic polymorphisms in glutathione S-transferases P1 (GSTP1) Ile105Val and prostate cancer risk: a systematic review and meta-analysis. Tumour Biol. 2013;34(6):3913–3922. | ||
Yu Z, Li Z, Cai B, et al. Association between the GSTP1 Ile105Val polymorphism and prostate cancer risk: a systematic review and meta-analysis. Tumour Biol. 2013;34(3):1855–1863. | ||
Wu K, Wang X, Xie Z, Liu Z, Lu Y. Glutathione S-transferase P1 gene polymorphism and bladder cancer susceptibility: an updated analysis. Mol Biol Rep. 2013;40(1):687–695. | ||
Mo Z, Gao Y, Cao Y, Gao F, Jian L. An updating meta-analysis of the GSTM1, GSTT1, and GSTP1 polymorphisms and prostate cancer: a HuGE review. Prostate. 2009;69(6):662–688. | ||
Kellen E, Hemelt M, Broberg K, et al. Pooled analysis and meta-analysis of the glutathione S-transferase P1 Ile 105Val polymorphism and bladder cancer: a HuGE-GSEC review. Am J Epidemiol. 2007;165(11):1221–1230. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

