Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Association Between Elevated Thyroid Peroxidase Antibody and Abdominal Fat Distribution in Patients with Type 2 Diabetes Mellitus
Authors Hu Y , Zheng J, Ye X, Song Y, Wu X
Received 30 October 2021
Accepted for publication 9 February 2022
Published 17 March 2022 Volume 2022:15 Pages 863—871
DOI https://doi.org/10.2147/DMSO.S345507
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Ye Hu,1 Jia Zheng,2 Xiao Ye,1 Yingxiang Song,1 Xiaohong Wu1
1Department of Health Management Center, Endocrinology, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, People’s Republic of China; 2The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Xiaohong Wu, Tel/Fax +86-579-85893937, Email [email protected]
Objective: Obesity and autoimmune thyroid disease (AITD) are both common disorders in the general population, which are major drivers for adverse medical conditions. While an interaction between thyroid function and visceral obesity is thought to exist, but very few studies have examined the relationship between AITD and visceral obesity, especially in the patients with type 2 diabetes mellitus (T2DM). In the present study, we investigated the association between elevated thyroid peroxidase antibody (TPOAb) titer and visceral fat area in T2DM patients.
Methods: A total of 390 T2DM patients who met the criteria for admission and joined the National Metabolic Management Center (MMC) in the Zhejiang Provincial People’s Hospital from April 2020 to December 2020 were enrolled in this study. The participants were divided into two groups based on visceral obesity. Thyroid function, thyroid associated antibody and other metabolic indicators were measured by blood tests. The visceral fat area (VFA) and the subcutaneous fat area (SFA) were measured by bioelectrical impedance analysis.
Results: There were 185 participants (47.4%) had visceral obesity. The positive rate of TPOAb was significantly higher in T2DM patients with visceral obesity (12.97% vs 5.37%, p < 0.01). Free triiodothyronine (FT3) and thyroid-stimulating hormone (TSH) were both significantly higher in T2DM patients with visceral obesity (p < 0.05). The increased TPOAb titer was significantly positively correlated with visceral fat area (r = 0.175, p < 0.01). Binary logistic analysis showed that the positive rate of TPOAb was associated with an increased risk of visceral obesity [(OR) 4.258, 95% confidence interval (CI) 1.594, 11.375, p = 0.004].
Conclusion: TPOAb-positive is more common in T2DM patients with visceral obesity, which has some effects on visceral obesity independent of thyroid function. This suggests that elevated TPOAb titer is a predictor of visceral obesity in T2DM patients.
Keywords: type 2 diabetes mellitus, visceral obesity, autoimmune thyroid disease, thyroid peroxidase antibody
Introduction
Obesity, especially visceral obesity, is closely related to many diseases, which pose serious health threats to the human.1 According to the distribution in the body, fat can be divided into visceral fat and subcutaneous fat, among which visceral fat is considered to be the main cause of metabolic diseases.2 It is common to see that type 2 diabetes mellitus (T2DM) with visceral obesity in clinical practice. Studies suggest that visceral obesity may induce adipose tissue dysfunction, adipocytokine secretion impaired, which causes the interaction between oxidative stress and inflammatory response, and ultimately induces insulin resistance and T2DM.3–5
Thyroid diseases are the main comorbidity of diabetes mellitus, which mainly include thyroid dysfunction and autoimmune thyroid diseases.6 Many studies have suggested that thyroid diseases are related to the fat distribution.7–12 It is generally believed that thyroid hormone can not only regulate basal metabolism and heat production in the body but also play an important role in lipid metabolism, glucose metabolism and fat oxidation, so it can affect the fat distribution in the body to a certain extent.10,13,14 Some previous studies have found that even in those euthyroid populations, the body mass index (BMI), waist circumference (WC), fat percentage (fat%), and visceral fat area (VFA) were significantly associated with changes in thyroid-stimulating hormone (TSH).12,15–17 Moreover, there was study pointed out that visceral obesity was not only associated with changes in thyroid hormone levels but may also be significantly associated with changes in AITD-related antibodies, such as TPOAb.18
Autoimmune thyroid diseases (AITDs) are characterized by autoantibodies against thyroid antigens, such as thyroid peroxidase antibody (TPOAb), thyroglobulin antibody (TGAb) and TSH receptor antibody (TRAb). Previous studies have shown that elevated thyroid autoantibodies are common in patients with T1DM and are generally considered to be possibly associated with autoimmune and inflammatory mechanisms.19,20 However, in recent years, studies have shown that the elevated levels of thyroid autoantibodies are more common in T2DM population, such as in patients with T2DM and NAFLD.21–24
Based on these conclusions, we hypothesized that thyroid autoimmunity is associated with type 2 diabetes mellitus complicated with visceral obesity, and may be a predictor of it. However, the relationship between autoimmune thyroid disease and visceral obesity in T2DM has not been reported. Therefore, we design this research to analyze the relationship between positive thyroid autoantibodies and the visceral fat area in T2DM patients to explore whether it is a predictor of the visceral obesity in patients with T2DM.
Methods
Study Design and Participants
This was a cross-sectional study designed to analyze the relationship between elevated TPOAb titer and visceral obesity. The study population eventually consisted of 390 patients with T2DM who met the criteria for admission as follows, and joined the National Metabolic Management Center (MMC) in the Zhejiang Provincial People’s Hospital from April 2020 to December 2020. All participants received complete questionnaires, physical examination and laboratory examination. According to the result of bioelectrical impedance analysis for visceral fat, the patients were divided into the visceral obesity group and the control group.
Inclusion criteria: 1. Age ≥ 18 years old; 2. The diagnosis of T2DM refers to the Standards of Medical Care in Diabetes issued by the American Diabetes association (ADA) in 2020. 3. Normal thyroid function.
Exclusion criteria: 1. Acute complications of diabetes, such as ketoacidosis, hyperosmolar hyperglycemia, acute infection; 2. History of thyroid disease using a thyroxine supplement or anti-thyroid therapy; 3. History of thyroid surgery; 4. Taking sex hormones, glucocorticoids, amiodarone or lithium; 5. Abnormal thyroid function; 6. History of malignant tumors; 7. Severe heart, liver, and kidney dysfunction; 8. Pregnant or lactating women.
Ethics Clearance
This study was conducted in accordance with the declaration of Helsinki. The Ethics Committee of Zhejiang Provincial People’s Hospital approved the study, and each patient gave informed written consent to participate in the study.
Data Collection
A standard questionnaire was used to obtain information on personal and family medical history. General data of the subjects were obtained in detail, such as the age, gender, duration of T2DM, risk factors in daily lives, previous medical history and medication history. Height, weight, waist circumference, hip circumference and arterial blood pressure were measured on the first day of admission by the same nurse. BMI was calculated as the body weight (kg) divided by the height squared (m2).
All subjects were submitted to venous blood after an 8-h fast in the morning. Thyroid antibodies and thyroid hormones including thyroid peroxidase antibody (TPOAb), thyroglobulin antibody (TGAb), free thyroxine (FT4), free triiodothyronine (FT3), thyroid stimulating hormone (TSH) were measured by chemiluminescent immunoassay (DXI800, Beckman-Coulter, USA). The total number of white blood cells (WBC), fasting plasma glucose (FPG), fasting insulin (FINS), glycosylated hemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), γ-glutamyltransferase (γGT), and aspartate aminotransferase (AST) were measured by standardized high-performance liquid chromatography assay (D100, Bio-Rad, USA) or chemiluminescence immunoassay (AU5800, Beckman-Coulter, USA). All assessments were done in the same lab. Homeostasis model assessment of insulin resistance (HOMA-IR) which was used to evaluate the individual level was calculated using the following formula: HOMA-IR = fasting serum insulin (mU/L) × fasting plasma glucose (mmol/L)/22.5.
The visceral fat area (VFA) and the subcutaneous fat area (SFA) were measured by bioelectrical impedance analysis using DUALSCAN HDS-2000. The measurement method is as follows: (1) The subjects were asked to fast at 20:00 the day before the examination. (2) Inform the subjects to lie down in supine position, bare their ankles, wrists and abdominal skin, and breathe calmly. At the end of calm expiratory breath, inform the patients to hold their breath, and then measure the horizontal umbilical and abdominal cross-sectional area. (3) Then install abdominal electrode belt and hand and foot electrode clamps to instruct subjects to breathe calmly. At the end of calm exhalation, tell the patients to hold their breath. At that time, the VFA and SFA were measured.
Definition
The normal reference range for TSH, FT3, FT4 were 0.34–5.60 mIU/L, 2.14–4.21 ng/L, 5.90–12.50 ng/L, respectively. Normal thyroid function was defined as serum TSH, FT3 and FT4 within the reference range. TPOAb positive (serum level ≥ 9.00 IU/mL) and/or TGAb positive (serum level ≥ 4.00 IU/mL) were considered as the presence of thyroid autoimmunity. VFA ≥ 100 cm2 was defined as visceral obesity.
Statistical Analysis
All analyses were performed using SPSS version 22.0 (IBM, USA) and the value of P < 0.05 was considered statistically significant. The distribution of the index was assessed by the Kolmogorov–Smirnov test. Normally distributed data were descriptive by mean and standard deviation (SD), median and quartile were used to descriptive for the non-normally distributed data. Student’s t-test or the nonparametric Mann–Whitney U-test was also performed to determine any difference between the two groups. Chi-square tests were used to compare differences in categorical variables, respectively. Pearson correlation analysis was used for correlating variables. Independent risk factors associated with visceral obesity were determined using a binary logistic regression model.
Results
Clinical Characteristics of the Subjects
An overview of the study characteristics of the two groups is shown in Table 1. Compared with the T2DM without visceral obesity group, indicators such as blood pressure, HOMA-IR, BMI, WC, TG, ALT, γGT and AST of patients were significantly increased in the T2DM with visceral obesity group (p < 0.05). Other clinical data such as age, duration of diabetes, HbA1c, TC and LDL-C were not significantly different in the two groups (p > 0.05).
 |
Table 1 Clinical Characteristics of T2DM Patients with and without Visceral Obesity |
Visceral Obesity and Thyroid Antibodies
In the group of T2DM with visceral obesity, 24 (12.97%) were TPOAb-positive and 12 (6.49%) were TGAb-positive. While in the group of T2DM without visceral obesity, 11 (5.37%) were TPOAb-positive and 9 (4.39%) were TGAb-positive. The positive rate of TPOAb was significantly different between the two groups (12.97% vs 5.37%, p < 0.01), while the positive rate of TGAb was not significantly different (6.49% vs 4.39%, p > 0.05). FT3 and TSH levels were significantly higher in T2DM patients with visceral obesity (p < 0.05) (shown in Table 2).
 |
Table 2 Comparison of Thyroid Indicators of T2DM Patients with and without Visceral Obesity |
Correlations Between Thyroid Indicators and Visceral Fat Area
To further assess the relationship between thyroid diseases and visceral obesity, the correlation between VFA and TPOAb titer was analyzed using both Pearson’s correlation analysis and linear correlation analysis. In this study, TSH, FT3 and FT4 levels were within the normal reference range for all participants in both groups. Pearson’s correlation analysis suggested that the increased TPOAb titer was significantly positively correlated with VFA (r = 0.175, p < 0.01) and SFA (r = 0.184, p < 0.01) (shown in Table 3). Besides, we can see from Table 3, FT3 and TSH levels were also significantly positively correlated with VFA (r = 0.179, p < 0.01; r = 0.145, p < 0.01). Therefore, when we adjusted for the effect of TSH, the increased TPOAb titer was still significantly associated with visceral fat area. Linear correlation analysis also suggested the same results (shown in Figures 1 and Figure 2).
 |
Table 3 Association of TPOAb and VFA with Other Indicators in T2DM Patients |
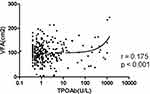 |
Figure 1 Association between TPOAb titer and VFA. Linear correlation analysis showed an obviously positive correlation of VFA with TPOAb titer in T2DM patients (r = 0.175, p < 0.001). |
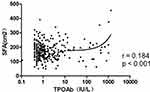 |
Figure 2 Association between TPOAb titer and SFA. Linear correlation analysis showed an obviously positive correlation of SFA with TPOAb titer in T2DM patients (r = 0.184, p < 0.001). |
Association of the TPOAb and Visceral Fat Area in T2DM Patients
We performed a binary logistic regression analysis of the factors that may affect visceral fat area in T2DM patients. After full adjustment for age, duration, TSH, FT4, FT3, hyperglycemia and hyperlipidemia, binary logistic analysis showed that TPOAb-positive was associated with an increased risk of visceral obesity in T2DM patients [odds ratio (OR) 4.258, 95% confidence interval (CI) 1.594, 11.375, p = 0.004] (shown in Table 4).
 |
Table 4 Association of the TPOAb with Visceral Fat Area: Binary Logistic Regression |
Discussion
The high prevalence of autoimmune thyroid disease in T1DM patients has been reported before.19 However, this in T2DM is a less explored field, especially in T2DM with obesity. The current study is the first research to evaluate the direct relationship between visceral obesity and thyroid autoimmunity in a Chinese population with T2DM. We demonstrated an independently positive association between the visceral obesity and thyroid autoimmunity among the subjects with T2DM and normal thyroid function.
Obesity and thyroid diseases are two common conditions of the endocrine system. Previous studies suggest that the prevalence of thyroid diseases is significantly higher in the T2DM patients.21,25 We all know that T1DM patients are more likely to have autoimmune thyroid diseases than T2DM patients due to the associated autoimmunity and common genetic basis.20,26 However, it has also been found that there are more T2DM patients complicated with autoimmune thyroid disease, not just thyroid dysfunction, and the pathogenesis may be more complex. Some studies have reported that obesity was significantly related to Hashimoto thyroiditis (HT) and positive-TPOAb in normal population.22,27 Our results also support this opinion in T2DM patients.
Our results suggest that the positive rate of TPOAb in the T2DM combined visceral obesity group was higher compared with the T2DM only group. This conclusion is consistent with the previous studies in healthy people. Tamer et al found that levels of anti-thyroid peroxidase antibody correlated with triglyceride levels and waist circumference.18 In addition, studies showed that elevated TPOAb is indeed predominant in T2DM patients with thyroid autoimmunity disease.25,28 These conclusions may confirm the involvement of autoimmunity in the pathogenesis of diabetes with visceral obesity. We hypothesize that the TPOAb-positive is a risk factor for T2DM patients with visceral obesity.
The result showed that TPOAb titer was significantly positively correlated with visceral fat area and subcutaneous fat area. Based on the result, we make two assumptions. On the one hand, we consider that patients with high TPOAb, whose thyroid function is susceptible to such effects as hypothyroidism, which increases the risk of T2DM and visceral obesity. We did find that FT3 and TSH levels were significantly higher in the patients with T2DM and visceral obesity, which were also significantly positively correlated with visceral fat area, which was consistent with previous studies.8,9,29 The hypothesis for correlation is that TSH is involved in the differentiation of pre-adipocytes and induced adipogenesis. The previous study has found that TSH may infect the visceral fat through TSH receptor, which can be expressed in adipocyte precursor cells and participate in adipocyte differentiation and adipogenesis.30 Another hypothesis is the leptin hypothesis. And a positive correlation between leptin and TSH has been demonstrated.11 On the other hand, we suppose that the elevated TPOAb may cause increased visceral fat through autoimmunity itself, not only through affecting thyroid function. Therefore, we adjusted for the effect of TSH, and the result told us that even if we controlled for TSH, TPOAb titer was still significantly correlated with visceral fat area. Moreover, the result of binary logistic regression analysis showed that when the patient had TPOAb-positive, the risk of visceral obesity will be 4 times higher (OR 4.258). This result also confirmed that TPOAb-positive was a risk factor for visceral obesity independent of thyroid function in T2DM patients.
The mechanisms of AITD are based on the reaction to the deregulated immune system, but the exact mechanisms are not completely clear. There are some opinions that antibodies secreted by B cells are deposited in the basal membrane of the thyroid follicle, activate complement system, and thereby induce necrosis. Increased levels of the cytokines IFN-γ and IL-12 (Th1 cytokines) induce TNF-α, IL-1β, IL-2, and CD40L, which cause apoptosis of thyrocytes.31 In HT it is possible that increased IFN-γ and TNF-α may cause obesity without elevated TSH levels.18 It is also believed that insulin resistance will arise when B cells and other immune cells react against the self-antigens. TPOAb is an inductor of oxidative stress evidenced by decreased antioxidant potential, advanced glycosylation products, and oxygen metabolites in the blood.32 This has a similar pathophysiological basis in T2DM and obesity as we know it. Immune cells are important factors in the regulation of adipose tissue function, and the dysfunction of adipose tissue caused by the imbalance of immune homeostasis is an important pathological and physiological mechanism of obesity.5 Therefore, we hypothesized that obesity, T2DM and AITDs might share some common pathways. There is also further work we will do to explore how thyroid autoimmunity affects obesity and visceral obesity.
There are some limitations to our study. First, magnetic resonance imaging (MRI) and computerized tomography are precise methods to measure visceral fat area and are internationally recognized.33 However, its application is limited due to the defects of exposure to radiation, long testing time and high testing cost. Study has shown that the bioelectrical impedance analysis, which is simpler, has a high correlation with the detection of CT, and is convenient for large-scale clinical applications.34 Moreover, for the definition of visceral obesity, there is no unified definition in China at present. Our study refers to the standard of Japan.35 Secondly, patients included in this paper were from Zhejiang province in China, which was influenced by race, living habits and other factors. Finally, due to the cross-sectional study design, we are still unable to determine the causality between thyroid autoimmunity and T2DM, visceral obesity. It requires prospective studies to further explore the deeper relationships and related mechanisms.
Conclusion
Elevated TPOAb was positively associated with the visceral fat area, which may be independent of thyroid function, suggesting that thyroid autoimmunity may effect the autoimmune development in T2DM patients with visceral obesity. Therefore, we suggest that routine screening for thyroid disease in patients with T2DM, especially the patients with obesity, for early detection and reasonable intervention, which may be beneficial in improving insulin resistance and reducing related cardiovascular events.
Abbreviations
T2DM, type 2 diabetes mellitus; VFA, visceral fat area; SFA, subcutaneous fat area; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; HOMA-IR, homeostasis model assessment of insulin resistance; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Hcy, homocysteine; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γGT, γ-glutamyltransferase; TPOAb, thyroid peroxidase antibody; TGAb, thyroglobulin antibody; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable requests.
Acknowledgments
The authors gratefully acknowledge the participation and cooperation of all patients in this study as well as the Department of Endocrinology of the Zhejiang Provincial People’s Hospital. Moreover, thanks for the support of the Key Laboratory of endocrine gland diseases in Zhejiang Province.
Funding
This study was supported by the Medical health Science and Technology project of Zhejiang Province, China (No. 2020KY412).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an endocrine society scientific statement. Endocr Rev. 2018;39(2):79–132. doi:10.1210/er.2017-00253
2. Fang L, Guo F, Zhou L, Stahl R, Grams J. The cell size and distribution of adipocytes from subcutaneous and visceral fat is associated with type 2 diabetes mellitus in humans. Adipocyte. 2015;4(4):273–279. doi:10.1080/21623945.2015.1034920
3. González N, Moreno-Villegas Z, González-Bris A, Egido J, Lorenzo Ó. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc Diabetol. 2017;16(1):44. doi:10.1186/s12933-017-0528-4
4. Huang JW, Yang CY, Wu HY, Liu KL, Hung KY. Metabolic syndrome and abdominal fat are associated with inflammation, but not with clinical outcomes, in peritoneal dialysis patients. Cardiovasc Diabetol. 2013;12(1):86. doi:10.1186/1475-2840-12-86
5. Ruiz HH, Díez RL, Arivazahagan L, Ramasamy R, Schmidt AM. Metabolism, obesity, and diabetes mellitus. Arterioscler Thromb Vasc Biol. 2019;39(7):e166–e174. doi:10.1161/ATVBAHA.119.312005
6. Chistiakov DA. Immunogenetics of Hashimoto’s thyroiditis. J Autoimmune Dis. 2005;2(1):1–21. doi:10.1186/1740-2557-2-1
7. García-Solís P, García OP, Hernández-Puga G, et al. Thyroid hormones and obesity: a known but poorly understood relationship. Endokrynol Pol. 2018;69(3):292–303. doi:10.5603/EP.2018.0032
8. Chang YC, Hua SC, Chang C-H. High TSH level within normal range is associated with obesity, dyslipidemia, hypertension, inflammation, hypercoagulability, and the metabolic syndrome: a novel cardiometabolic marker. J Clin Med. 2019;8(6):817. doi:10.3390/jcm8060817
9. Chen Y, Chen Y, Wang N, et al. Thyroid stimulating hormone within the reference range is associated with visceral adiposity index and lipid accumulation product: a population-based study of SPECT-China. Horm Metab Res. 2018;50(1):29–36. doi:10.1055/s-0043-122235
10. Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12(4):287–293. doi:10.1089/10507250252949405
11. Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clin Endocrinol. 2005;62(4):487–491. doi:10.1111/j.1365-2265.2005.02247.x
12. Kitahara CM, Platz EA, Ladenson PW, Mondul AM, Menke A, Berrington de González A. Body fatness and markers of thyroid function among U.S. men and women. PLoS One. 2012;7(4):e34979. doi:10.1371/journal.pone.0034979
13. Asvold BO, Vatten LJ, Nilsen TI, Bjøro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol. 2007;156(2):181–186. doi:10.1530/eje.1.02333
14. De Pergola G, Ciampolillo A, Paolotti S, Trerotoli P, Giorgino R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clin Endocrinol. 2007;67(2):265–269. doi:10.1111/j.1365-2265.2007.02874.x
15. Pearce EN. Thyroid hormone and obesity. Curr Opin Endocrinol Diabetes Obes. 2012;19(5):408–413. doi:10.1097/MED.0b013e328355cd6c
16. Ren R, Jiang X, Zhang X, et al. Association between thyroid hormones and body fat in euthyroid subjects. Clin Endocrinol. 2014;80(4):585–590. doi:10.1111/cen.12311
17. Roef GL, Rietzschel ER, Van Daele CM, et al. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid. 2014;24(2):223–231. doi:10.1089/thy.2013.0314
18. Tamer G, Mert M, Tamer I, Mesci B, Kilic D, Arik S. Effects of thyroid autoimmunity on abdominal obesity and hyperlipidaemia. Endokrynol Pol. 2011;62(5):421–428.
19. Dosi RV, Tandon N. A study on prevalence of thyroid auto-immunity in type 1 diabetes mellitus. J Indian Med Assoc. 2010;108(6):
20. Shun CB, Donaghue KC, Phelan H, Twigg SM, Craig ME. Thyroid autoimmunity in Type 1 diabetes: systematic review and meta-analysis. Diabet Med. 2014;31(2):126–135. doi:10.1111/dme.12318
21. Elebrashy IN, El Meligi A, Rashed L, Salam RF, Youssef E, Fathy SA. Thyroid dysfunction among type 2 diabetic female Egyptian subjects. Ther Clin Risk Manag. 2016;12:1757–1762.
22. Sarfo-Kantanka O, Sarfo FS, Ansah EO, et al. Frequency and determinants of thyroid autoimmunity in Ghanaian type 2 diabetes patients: a case-control study. BMC Endocr Disord. 2017;17(1):2. doi:10.1186/s12902-016-0152-4
23. Chen Y, Wang N, Chen Y, et al. The association of non-alcoholic fatty liver disease with thyroid peroxidase and thyroglobulin antibody: a new insight from SPECT-China study. Autoimmunity. 2018;51(5):238–244. doi:10.1080/08916934.2018.1488968
24. Wang C, Niu Q, Lv H. Elevated TPOAb is a strong predictor of autoimmune development in patients of type 2 diabetes mellitus and non-alcoholic fatty liver disease: a case-control study. Diabetes Metab Syndr Obes. 2020;13:4369–4378. doi:10.2147/DMSO.S280231
25. Sotak S, Felsoci M, Lazurova I. Type 2 diabetes mellitus and thyroid disease: a two-sided analysis. Bratisl Lek Listy. 2018;119(6):361–365. doi:10.4149/BLL_2018_067
26. American Diabetes Association. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S37–S47. doi:10.2337/dc20-S004
27. Song RH, Wang B, Yao QM, Li Q, Jia X, Zhang JA. The impact of obesity on thyroid autoimmunity and dysfunction: a systematic review and meta-analysis. Front Immunol. 2019;10:2349. doi:10.3389/fimmu.2019.02349
28. Fleiner HF, Bjøro T, Midthjell K, Grill V, Åsvold BO. Prevalence of thyroid dysfunction in autoimmune and type 2 diabetes: the population-based HUNT study in Norway. J Clin Endocrinol Metab. 2016;101(2):669–677. doi:10.1210/jc.2015-3235
29. Mousa U, Bozkuş Y, Kut A, Demir CC, Tutuncu NB. Fat distribution and metabolic profile in subjects with Hashimoto’s thyroiditis. Acta Endocrinol. 2018;14(1):105–112. doi:10.4183/aeb.2018.105
30. Lu S, Guan Q, Liu Y, et al. Role of extrathyroidal TSHR expression in adipocyte differentiation and its association with obesity. Lipids Health Dis. 2012;11:17. doi:10.1186/1476-511X-11-17
31. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521. doi:10.3389/fimmu.2017.00521
32. Ruggeri RM, Vicchio TM, Cristani M, et al. Oxidative stress and advanced glycation end products in Hashimoto’s thyroiditis. Thyroid. 2016;26(4):504–511. doi:10.1089/thy.2015.0592
33. Lukaski HC, Johnson PE, Bolonchuk WW, Lykken GI. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr. 1985;41(4):810–817. doi:10.1093/ajcn/41.4.810
34. Park KS, Lee DH, Lee J, et al. Comparison between two methods of bioelectrical impedance analyses for accuracy in measuring abdominal visceral fat area. J Diabetes Complications. 2016;30(2):343–349. doi:10.1016/j.jdiacomp.2015.10.014
35. Yuji M. New criteria for “obesity disease” in Japan: the examination committee of criteria for ‘obesity disease’in Japan, Japan society for the study of obesity. New criteria for ‘obesity disease’in Japan. Circ J. 2002;66(11):987–992. doi:10.1253/circj.66.987
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
