Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 13
Association Between Dyslipidemia and Serum Level of 25-Hydroxyvitamin-D in Early Chronic Kidney Disease, Not on Dialysis: An Observational Cross-Sectional Study from the Himalayan Country
Authors Sah SK , Adhikary LP
Received 11 June 2020
Accepted for publication 24 August 2020
Published 24 September 2020 Volume 2020:13 Pages 211—218
DOI https://doi.org/10.2147/IJNRD.S267252
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Shiv Kumar Sah,1,2 Laxman Prasad Adhikary3
1Department of Pharmacy, Purbanchal University, Little Buddha College of Health Science, Kathmandu, Nepal; 2Gastro and Liver Foundation, Kathmandu, Nepal; 3Nephrology Unit, Department of Medicine, Kathmandu Medical Hospital Teaching Hospital, Kathmandu, Nepal
Correspondence: Shiv Kumar Sah Email [email protected]
Background: Patients with CKD have a higher prevalence of dyslipidemia and hypovitaminosis than the normal population. Recent studies in the general population have shown a potential link between 25(OH)D and dyslipidemia. However, such evidence in the early CKD population, especially in the Nepalese setting, is lacking. Thus, the present study aimed at investigating the status of 25(OH)D and dyslipidemia in the early CKD patients, and further to establish an association between 25(OH)D and lipid profile.
Patients and Methods: In this cross-sectional study, we analyzed 136 clinically stable non-dialyzed CKD patients. 25(OH)D and lipid profile were evaluated as a core variable, and their direction and magnitude of a relationship were evaluated.
Results: The estimated prevalence of dyslipidemia was 49.3%, and 63.2% population had a deficiency of 25(OH)D level. Compared with the patient with normal 25(OH)D level, the patient with deficient 25(OH)D level had a significantly higher level of LDL-c (P=0.04) and lower level of HDL-C (P=0.048). Serum 25(OH)D level was significantly lower in dyslipidemic patients than non-dyslipidemic patients (P=0.015). Regression analysis demonstrated a significant inverse relationship between serum 25(OH)D levels and LDL-c (β=− 1.5; P=< 0.001), and TC levels (β=− 1.4;P=0.003), and the association remained unchanged with further adjustment for age, sex, HTN, DM, serum albumin and eGFR.
Conclusion: Our study unveiled a high rate of dyslipidemia and hypovitaminosis in a considerable number of early CKD patients. Low serum level of 25(OH)D was significantly correlated with a higher rate of dyslipidemia. These findings indicate some evidence for 25(OH)D level as a marker of dyslipidemia prediction, and that decrease in serum level of 25(OH)D is associated with increased serum level of LDL and TC; it could increase the risk of cardiovascular disease. Therefore, early recognition and timely management of hypovitaminosis and dyslipidemia is vital to prevent an inevitable cardiovascular event.
Keywords: chronic kidney disease, dyslipidemia, 25(OH)D
Introduction
Chronic kidney disease is an increasing worldwide and becoming a major public health threat to public health.1,2 The prevalence of chronic kidney disease (CKD) is high in developing countries, and the prevalence is estimated to be 10.6% in the Nepalese population.3 The patients with CKD exhibit significant alteration in lipoprotein metabolism,4,5 with a higher prevalence of dyslipidemia than the normal population, and are estimated to be over 40% in patients with kidney failure.6–8 Serum 25 – hydroxyvitamin-D level is a sensitive measure of vitamin D status of an individual.9 And 25(OH)D deficiency is very frequently reported in non-dialyzed CKD patients, affecting more than 80% of patients in pre-dialysis patients,10 with a significant increased risk of mortality.11
Recent epidemiological studies suggest that low serum 25(OH)D level is associated with atherosclerosis,12 obesity,13 DM,14 hypertension,15 myocardial infarction,16 and stroke.17 In the general population, several studies have been reported regarding the association between the low 25(OH)D and dyslipidemia. Recent studies in the general population have shown that a low 25(OH)D level is associated with hyperlipidemia.18,19 However, in non-dialyzed patients, the relationship between serum 25(OH)D and lipid profile is obscure. Moreover, the status of dyslipidemia and its potential link to 25(OH)D level in resource-limiting country, especially in the Nepalese setting is lacking. Therefore, the present study aims to determine the prevalence of dyslipidemia in early CKD patients, and further to examine the relationship between 25(OH)D and lipid panel.
Patients and Methods
Study Design and Duration
This cross-sectional study was conducted at the nephrology department, Kathmandu medical college and teaching hospital, Nepal from June 2016 to March 2017.
Study Population and Patient’s Selection
In this cross-sectional study, a total 136 patients eligible for the inclusion criteria were included. To be eligible for the study, the patients who were ages 18 years or above, clinical stable for 3 months preceding the study with a confirmed diagnosis of CKD stage 2–5 not on dialysis were included for analysis. Patients with renal replacement therapy were excluded from the study.
Outcome Variables
The primary outcome variables in this study were serum level of 25(OH)D, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), total cholesterol (TC) and triglyceride (TG). The covariates included in this study were age, sex, co-morbidities (Hypertension, Diabetes mellitus) biochemical laboratory test including serum creatinine (SCr), estimated glomerular filtration rate (eGFR), serum albumin and duration of disease.
Data Collection
Demographics status including age, sex, and relevant clinical information such as CKD stage, CKD diagnosis, hypertension history, diabetes mellitus history were approached for analysis. Laboratory data corresponding to lipid profile including LDL-C, HDL-c, TG, and total cholesterol were assessed.
Estimation of GFR
GFR were estimated by using MDRD-4 equation20
GFR= 175x (SCr)−1.154 x(age)−0.203 x(0.742 if female)
GFR units are mL/min/1.73 m2.
Classification of the stage of CKD21
CKD was classified into five stages defined by the GFR and/or evidence of kidney damage, as recommended by the National Kidney Foundation (Table 1).
 |
Table 1 Classification of Stage of CKD |
Assessment of 25(OH)D and Dyslipidemia
Electrochemiluminescence (ECLIA) assay was used to measure the level of 25(OH)D. According to the lab manual and recent opinion, we considered 25(OH) deficiency if the value <20 ng/mL and normal if the level were ≥20 ng/mL.9,22 Hyperlipidemia was defined as: 1) a TG level of >150 mg/dL; 2) an LDL of >160 mg/dL; or 3) an HDL <50 mg/dL for women or <40 mg/dL for men, as previously described.23
Statistical Analysis
Continuous data were presented as mean ± SD or median ± interquartile range for skewed data. The significance tests for differences among continuous variables were performed by One-way Analysis of Variance (ANOVA), and the significance of association for categorical data by Chi-square (χ2). Univariate linear regression analysis was performed to assess the relationship between each 25(OH)D metabolite and lipid profile including LDL-c, HDL-c, TG, and TC. Multivariate regression analysis was performed to examine the combined effects of clinical parameters on the serum level of each 25(OH)D metabolite. Variables were unadjusted in Model 1, whereas model 2 for age and sex, and model 3 was adjusted for age, sex, Hypertension, Diabetes mellitus, and eGFR. A p-value <0.05 was considered at a level of significance for all statistical analyses.
Ethical Issue
Patients were well informed about the nature of the study, and all participants signed informed consent before participating in the study. The study was conducted in accordance with the Declaration of Helsinki. The study protocols were approved by the institutional review board (IRB) of Kathmandu medical college teaching hospital Sinamangal, Kathmandu, Nepal.
Results
Baseline characteristics and the lipid profiles of the enrolled patients are illustrated in Table 2. Mean ages of the patients were 51.97±14.82 years, and male subjects were 101 (74.3%). Mean serum creatinine (mg/dl) and CrCl (mL/min/1/73m2) of the enrolled patients were 2.22±1.6 and 51.96 ±25.99, respectively.
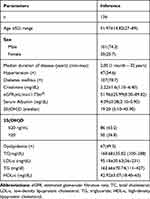 |
Table 2 Baseline Characteristics of the Population |
Figure 1 demonstrates the association between lipid panel and 25(OH)D statuses. Compared to the patients with normal 25(OH)D level, the patients with deficient serum 25(OH)D had insignificantly higher level serum Triglyceride (p=0.32), and total cholesterol (P=0.16). However, compared with the patients with normal 25(OH)D level, the patients with deficient 25(OH)D level had a significantly higher level of LDL-c (P=0.04). A significantly higher level of HDL-C was found in the patients with normal 25(OH)D level, compared to those who had a deficiency of 25(OH)D level (P=0.048). The estimated prevalence of dyslipidemia was 49.3% (95%CI: 40.59-57.40) (Table 3).
 |
Figure 1 Association between 25(OH) D and lipid panel. |
Table 4 presents the association between dyslipidemia and demographic/clinical parameters of the enrolled patients. Age, gender, systolic blood pressure, diastolic blood pressure, duration of disease, diabetes mellitus, stages of chronic kidney disease, serum albumin were not significantly correlated with dyslipidemia (P>0.05). Hypertension reported being significantly higher in dyslipidemic patients, compared to non-dyslipidemic patients (P<0.05). Serum level of 25(OH)D (strata), defined as <20 ng/mL and ≥20 ng/mL was insignificantly linked to dyslipidemia (P=0.09). However, 25(OH)D level (as a continuous) reported being lower in dyslipidemic patients compared with those in non-dyslipidemic patients (19.73±4.68 vs 22.34±7.29; P=0.015)
 |
Table 3 Prevalence of Dyslipidemia |
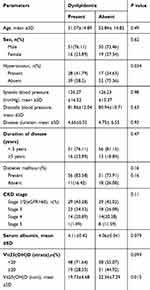 |
Table 4 Association Between Dyslipidemia and Demographic/Clinical Parameters |
Table 5 presents the association between serum 25(OH)D and lipid panel. Regression analysis demonstrated a significant inverse relationship between 25(OH)D levels and LDL-c (β=−1.5; P=<0.001), and TC levels (β=−1.4;P=0.003). The association of 25(OH)D levels with LDL-c (β=−0.19;P=0.025) and total cholesterol (β=−0.16;P=0.045) remained significant even after adjusting for age, sex, HTN, DM, serum albumin and eGFR.
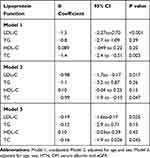 |
Table 5 Association Between 25(OH)D and Natural Log Transformed LDL, HDL, TC and TG Level (Multivariable Linear Regression Model) |
Figures 2–5 depict the correlation between 25(OH)D and LDL-C, HDL-C, TG and total cholesterol. Pearson’s correlation revealed a significant inverse correlation between 25(OH)D and LDL-C (r=−0.30;P=<0.001), 25(OH)D, and total cholesterol (r=−0.25;P=0.003). An insignificant inverse correlation was observed between 25(OH)D and TG (r=−0.079; P=0.39) and insignificant positive correlation was observed between 25(OH)D and HDL-C (r=0.11; P=0.20)
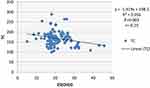 |
Figure 2 Correlation between 25(OH)D and TC level. |
 |
Figure 3 Correlation between 25(OH)D and LDL-c level. |
 |
Figure 4 Correlation between 25(OH)D and TG level. |
 |
Figure 5 Correlation between 25(OH)D and HDL-c level. |
Discussion
Recent data in the general population from epidemiological studies have suggested the potential link between 25(OH)D and lipid profile. However, data on early CKD patients have not been documented to date, particularly in lower-income middle country like Nepal. This cross-sectional analysis of baseline data of 25(OH)D and lipid profile in patients with early CKD population demonstrates that hypovitaminosis and dyslipidemia are common and that there is a significant and reciprocal connection between serum lipids such as LDL-C, TC and 25(OH)D, while an insignificant but positive correlation between HDL-c and 25(OH)D, and an insignificant but positive correlation between TG and 25(OH)D. In particular, our study demonstrated a high prevalence of vitamin D deficiency (63.2%), which corresponds well with the data from earlier studies, where vitamin D deficiency was frequently seen in patients with early chronic kidney disease, ranges from 41.7%24 up to 86%.25,26
Although lipid abnormalities were primarily considered as complications of end-stage renal disease (ESRD), these changes can be present in the early stage of chronic kidney disease as well. In the present study, 49.3% study population had dyslipidemia, which conforms to the results of an earlier study in children with chronic kidney disease, where the prevalence dyslipidemia was reported to be 45%.27 The alteration of lipid profile in CKD patients are multi-factorial, and the anticipated mechanisms of dyslipidemia in chronic kidney disease are hypothesized to involve impaired Triglyceride lipolysis, associated with elevated apolipoprotein C-III (an inhibitor of lipoprotein lipase) and decreased insulin sensitivity in the vascular endothelium of skeletal muscle and other major sites of Triglyceride (fatty acid) energy utilization.28
In the present study, the mean serum level of 25(OH)D was significantly lower in dyslipidemic patients, compared with those in non-dyslipidemic patients (19.73±4.68 vs 22.34±7.29). Although there is no documented evidence of this association in early CKD patients, in the general population, it is suggested that low serum 25(OH)D is linked to metabolic derangement, including abnormal lipoprotein,29,30 which may also present in early CKD population. Data regarding the association between 25(OH)D and lipid profile have been published in different populations across the nations, and illustrated divergent results in both cross-sectional and intervention trials.18,31,32
In these early CKD patients, the patients with 25(OH)D deficiency (<20 ng/mL) were significantly associated with elevated serum level of LDL cholesterol (P=0.04), and insignificantly associated with the increased serum level of total cholesterol, and this reiterates the finding of various parts of the country: Chaudhuri et al33 demonstrated a significant increased mean level of total cholesterol in asymptomatic Indian subjects with deficiency of 25-hydroxyvitamin D compared to those with normal 25-hydroxyvitamin D subjects. Similarly, Karhapaa et al in his study showed that hypovitaminosis D was associated with high total cholesterol in Belgian men.18 Auwerx et al in his study detected similar findings in Finnish people.19 A meta-analysis of randomized controlled trial by Wang Hao et al observed a slight effect of vitamin D supplementation on LDL, but no effect on TC, HDL, or TG.34 In another meta-analysis, Jorde R et al30 included 10 placebo-controlled double-blind intervention studies with vitamin D, and in his interventional studies, divergent results, with some showing positive and some negative effects of vitamin D supplementation were noted. Only one study demonstrated a significant effect with an 8% (0.28 mmol/L) increase in serum LDL-C and a 16% (0.22 mmol/L) decrease in serum TG in those given vitamin D as compared to the placebo group. However, it is crucial to note that none of the interventional studies in this meta-analysis were specifically designed for evaluating the relation between vitamin D and lipids, none had hyperlipemia as an inclusion criterion, and none were sufficiently powered.
In the present study, the trend of association between 25(OH) and LDL cholesterol was reverse and highly significant (β=−1.5; P=<0.001). Importantly, this outcome remained unchanged (β=–0.19;P=0.025) with further adjustment for demographic (age, sex) and disease-specific (HTN, DM, eGFR) and serum albumin. And for total cholesterol, the trend of association with 25(OH) was significant and reverse (r=−0.25, P=0.003), and remained significant (β =−0.16; P=0.045) in multivariate analysis after adjusting for age, sex, HTN, DM, serum albumin and eGFR. This trend is also supported by the results of earlier reports in patients with rheumatoid arthritis.29
In the present early CKD patients, elevated serum 25(OH)D (>20ng/mL) was insignificantly associated with reduced mean serum level of TG (P>0.05). However, we noted an insignificant but reciprocal association (P=0.39, r=−0.079) between 25(OH)D and TG level. This finding is supported by Jorde R et al30 where in his review of 22 cross-sectional studies, serum level of 25(OH)D was appeared to have an inverse association between serum level of 25(OH) and triglyceride in all studies. However, in the review of 10 placebo-controlled interventional studies, there was no uniform agreement on the effects of 25(OH)D on serum levels of TG, with some showed positive and some negative effect of 25(OH)D supplementation.30 With respect to the connection between 25(OH)D and HDL-c, we noted a positive correlation, though the tendency towards the significance was null. Thus, the inverse trends of 25(OH)D with TG and positive trend with HDL-c indicate a protective role of Vitamin D on cardiovascular health, as an increment of serum level of 25(OH)D linked to decrease in TG level and increased HDL-c. However, this trend should be justified in an interventional study in a large-scale reprehensive population.
Limitations
We acknowledge that the present study has been accomplished with some limitations. The sample size the study is relatively small which may affect the association secondly, the present study includes its cross-sectional nature, and therefore, the study only allows evaluating the association at a given point but could not draw any inference about the causal relationship over the time. Some inherent factors such as protein loss through urine, individuals with habits of physical exercise outside routinely, food pattern that would elevate 25(OH)D levels and may have other healthy habits which could favorably affect lipid profiles. Proteinuria, which may be responsible for hyperlipidemia and hypovitaminosis in our study population, was not taken into consideration for analysis. Therefore, an interventional study addressing all the aforementioned parameters in a large population should explore the causal relationship between 25(OH)D and lipid profile.
Conclusion
Our study unveiled a high prevalence of dyslipidemia and hypovitaminosis in early CKD patients, not on dialysis. Low serum levels of 25(OH)D were significantly correlated with a higher prevalence of dyslipidemia. We noted a significant inverse correlation between serum 25(OH)D and LDL, and TC, an insignificant inverse correlation with TG, a positive correlation with HDL-c. These findings indicate some evidence for 25(OH)D level as a marker of dyslipidemia prediction, and that decrease in serum level of 25(OH)D is associated with increased serum level of LDL and TC, it could increase the risk of cardiovascular disease. Thus, early recognition and timely management of hypovitaminosis and dyslipidemia is crucial to prevent the inevitable cardiovascular event. However, further interventional studies in large populations in these early CKD populations are needed to examine whether the correction of 25(OH)D could improve lipid profile.
Acknowledgment
We thank IRB, Nepal Medical College, Teaching Hospital Sinamangal, Kathmandu for ethical approval. Also, we thank the nephrology unit, department of medicine, KMC for all kinds of support during data collection.
Funding
There was no funding source involved in this study.
Disclosure
Disclosure
The authors declare that they do not have conflicts of interest.
References
1. Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi:10.1016/S0140-6736(12)60033-6
2. Singh AK, Farag YM, Mittal BV, et al. Epidemiology and risk factors of chronic kidney disease in India–results from the SEEK (screening and early evaluation of kidney disease) study. BMC Nephrol. 2013;14(1):114. doi:10.1186/1471-2369-14-114
3. Sharma SK, Dhakal S, Thapa L, et al. Community-Based Screening for Chronic Kidney Disease, Hypertension and Diabetes in Dharan. 2013.
4. Tsimihodimos V, Dounousi E, Siamopoulos KC. Dyslipidemia in chronic kidney disease: an approach to pathogenesis and treatment. Am J Nephrol. 2008;28(6):958–973. doi:10.1159/000144024
5. Tsimihodimos V, Mitrogianni Z, Elisaf M. Dyslipidemia associated with chronic kidney disease. Open Cardiovasc Med J. 2011;5(1):41. doi:10.2174/1874192401105010041
6. Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56(6):2214–2219. doi:10.1046/j.1523-1755.1999.00773.x
7. Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis. 1998;32(5):S142–S56. doi:10.1053/ajkd.1998.v32.pm9820472
8. Coresh J, Longenecker JC, Young H, Klag M. Epidemiology of cardiovascular risk factors in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S24–30.
9. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi:10.1056/NEJMra070553
10. Caravaca-Fontán F, Gonzales-Candia B, Luna E, Caravaca F. Relative importance of the determinants of serum levels of 25-hydroxy vitamin D in patients with chronic kidney disease. Nefrol. 2016;36(5):510–516. doi:10.1016/j.nefroe.2016.11.010
11. Navaneethan SD, Schold JD, Arrigain S, et al. Low 25-hydroxyvitamin D levels and mortality in non–dialysis-dependent CKD. Am J Kidney Dis. 2011;58(4):536–543. doi:10.1053/j.ajkd.2011.04.028
12. De Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. Clin J Am Soc Nephrol. 2009;20(8):1805–1812. doi:10.1681/ASN.2008111157
13. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi:10.1093/ajcn/72.3.690
14. Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the third national health and nutrition examination survey. Diabetes Care. 2004;27(12):2813–2818. doi:10.2337/diacare.27.12.2813
15. Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–1069. doi:10.1161/HYPERTENSIONAHA.107.087288
16. Scragg R, Jackson R, Holdaway IM, Lim T, BEAGLEHOLE R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19(3):559–563. doi:10.1093/ije/19.3.559
17. Poole KE, Loveridge N, Barker PJ, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37(1):243–245. doi:10.1161/01.STR.0000195184.24297.c1
18. Karhapää P, Pihlajamäki J, Pörsti I, et al. Diverse associations of 25‐hydroxyvitamin D and 1, 25‐dihydroxy‐vitamin D with dyslipidaemias. J Intern Med. 2010;268(6):604–610. doi:10.1111/j.1365-2796.2010.02279.x
19. Auwerx J, Bouillon R, Kesteloot H. Relation between 25-hydroxyvitamin D3, apolipoprotein AI, and high density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol. 1992;12(6):671–674. doi:10.1161/01.ATV.12.6.671
20. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi:10.7326/0003-4819-145-4-200608150-00004
21. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi:10.7326/0003-4819-139-2-200307150-00013
22. Malabanan A, Veronikis I, Holick M. Redefining vitamin D insufficiency. Lancet. 1998;351(9105):805–806. doi:10.1016/S0140-6736(05)78933-9
23. Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2011;123(20):2292–2333.
24. Kim YL, Kim H, Kwon YE, et al. Association between vitamin D deficiency and anemia in patients with end-stage renal disease: a cross-sectional study. Yonsei Med J. 2016;57(5):1159–1164. doi:10.3349/ymj.2016.57.5.1159
25. González EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. Am J Nephrol. 2004;24(5):503–510. doi:10.1159/000081023
26. Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxyvitamin D deficiency and inflammation and their association with hemoglobin levels in chronic kidney disease. Am J Nephrol. 2009;30(1):64–72. doi:10.1159/000202632
27. Saland JM, Pierce CB, Mitsnefes MM, et al. Dyslipidemia in children with chronic kidney disease. Kidney Int. 2010;78(11):1154–1163. doi:10.1038/ki.2010.311
28. Saland JM, Ginsberg HN. Lipoprotein metabolism in chronic renal insufficiency. Pediatr Nephrol. 2007;22(8):1095–1112.
29. Baker JF, Mehta NN, Baker DG, et al. Vitamin D, metabolic dyslipidemia, and metabolic syndrome in rheumatoid arthritis. Am J Med. 2012;125(10):
30. Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res. 2011;50(4):303–312. doi:10.1016/j.plipres.2011.05.001
31. Saedisomeolia A, Taheri E, Djalali M, Moghadam AM, Qorbani M. Association between serum level of vitamin D and lipid profiles in type 2 diabetic patients in Iran. J Diabetes Metab Disord. 2014;13(1):7. doi:10.1186/2251-6581-13-7
32. Ponda MP, Huang X, Odeh MA, Breslow JL, Kaufman HW. Vitamin D may not improve lipid levels: a serial clinical laboratory data study. Circulation. 2012;126(3):270–277. doi:10.1161/CIRCULATIONAHA.111.077875
33. Chaudhuri JR, Mridula KR, Anamika A, et al. Deficiency of 25-hydroxyvitamin d and dyslipidemia in Indian subjects. J Lipids. 2013;2013.
34. Wang H, Xia N, Yang Y, Peng D-Q. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids Health Dis. 2012;11(1):42. doi:10.1186/1476-511X-11-42
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
