Back to Journals » Journal of Inflammation Research » Volume 15
Association Between Depression or Anxiety and the Risk of Hepatitis B Flares: A Nationwide Population-Based Cohort Study
Authors Tsai MK , Sytwu HK, Hsieh TY, Chien WC, Lai CH, Chen HC
Received 9 January 2022
Accepted for publication 30 April 2022
Published 19 May 2022 Volume 2022:15 Pages 2983—2993
DOI https://doi.org/10.2147/JIR.S355314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zili You
Meng-Ko Tsai,1– 3 Huey-Kang Sytwu,4,5 Tsai-Yuan Hsieh,6 Wu-Chien Chien,7,8 Chao-Hung Lai,9,* Hsiang-Cheng Chen2,10,*
1Division of Allergy, Immunology, and Rheumatology, Department of Internal Medicine, Taichung Armed Forces General Hospital, Taichung, Taiwan; 2Division of Rheumatology, Immunology, and Allergy, Department of Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan; 3Institute of Medical Sciences, National Defense Medical Center, Taipei, 114, Taiwan; 4National Institute of Infectious Diseases and Vaccinology, National Health Research Institutes, Zhunan, Taiwan; 5Department and Graduate Institute of Microbiology and Immunology, National Defense Medical Center, Taipei, Taiwan; 6Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan; 7School of Public Health, National Defense Medical Center, Taipei, Taiwan; 8Department of Medical Research, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan; 9Division of Cardiology, Department of Internal Medicine, Taichung Armed Forces General Hospital, Taichung, Taiwan; 10Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei, Taiwan
*These authors contributed equally to this work
Correspondence: Hsiang-Cheng Chen, Division of Rheumatology, Immunology and Allergy, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan, Tel +886-2-87927135, Fax +886-2-87927136, Email [email protected]
Purpose: Depression and anxiety have been reported to increase the risk of infectious diseases and reactivation of latent infection. We conducted a nationwide population-based retrospective cohort study to determine the relationship between hepatitis B flares and depression or anxiety, utilizing outpatient and inpatient data from the Taiwan National Health Insurance Research database collected from 2000 to 2015.
Patients and Methods: A total of 12,992 patients with chronic hepatitis B and newly diagnosed anxiety/depression, without advanced liver disease, were propensity score-matched for age, sex, and comorbidities in a 1:4 ratio to 51,968 controls with chronic hepatitis B without depression/anxiety or advanced liver disease. Both groups were followed-up until December 31, 2015. Cox proportional hazards regression was used to determine the risk factors for hepatitis B flares. The Log rank test and Kaplan-Meier analysis were performed to assess differences in the cumulative incidence of hepatitis B flares according to anxiety/depression status.
Results: The incidence of hepatitis B flares was higher in the depression/anxiety cohort than in the control cohort (log-rank; p < 0.001). Patients with depression/anxiety had a significantly higher incidence rate of hepatitis B flares than those without depression/anxiety (3017 per 105 person-years versus 2042 per 105 person-years, p = 0.003). After adjusting for age and comorbidities, anxiety/depression was independently associated with an increased risk of hepatitis B flares (hazard ratio, 1.173; 95% confidence interval, 1.033– 1.277; p = 0.003).
Conclusion: This analysis suggests that in patients with chronic hepatitis B without advanced liver disease, those with concomitant depression or anxiety may be at higher risk of hepatitis B flares.
Keywords: chronic hepatitis B, hepatitis B virus, hepatitis B virus reactivation, mental disorder, mood disorder
Introduction
Hepatitis B virus (HBV) infection is a major cause of liver cirrhosis and hepatocellular carcinoma.1 As curative treatment for HBV is not currently available, there are many people with chronic hepatitis B (CHB) who are at risk of hepatitis B flares. Flares in viremic patients with cirrhosis may increase mortality and the risk of hepatic decompensation;2 therefore, the prevention of hepatitis B flares is of prime importance.
A hepatitis B flare is defined as a 2-fold or greater elevation in the serum alanine aminotransferase (ALT) level above the upper limit of normal or the patient’s baseline value3,4 in individuals with CHB. Hepatitis B flares may occur during the hepatitis B e antigen (HBeAg)-negative reactive immune clearance phase and during the HBeAg-positive immune clearance phase. Hepatitis B flares are considered to be the result of complex dynamic changes of the innate and adaptive immune responses, including the cytotoxic T lymphocyte-mediated immune response against HBV antigen-expressing hepatocytes and its downstream mechanisms.5
Mental disorders, especially depression and anxiety, are among the most common illnesses seen in primary care.6,7 The coexistence of depression and anxiety with other medical disorders is a topic of clinical interest. Many medical disorders, including obesity, myocardial infarction, and infections, are more common in individuals with severe mental disorders.8
Markers of impairment of cellular immunity (such as decreased natural killer [NK] cell cytotoxicity) have been associated with depression.9 Depression-induced immune dysregulation has a strong effect on the peripheral immune system,10 primarily due to its effect on the function and number of immune cells, particularly lymphocytes and monocytes/macrophages. Thus, immune dysregulation may play a critical role in the relationship between depression and medical disorders such as infections.11 Psychological stress and the concomitant negative emotions such as anxiety also have an impact on the B- and T-cell-mediated immune system.12 Both depression and anxiety are associated with an increased risk of acquiring infectious diseases8,13 and reactivation of latent viral infections such as herpesvirus infections.14
However, the concept of depression/anxiety-induced immune dysregulation as a potential contributor to hepatitis B flares is not widely recognized. To address the paucity of knowledge regarding the natural history of CHB in patients with depression and anxiety, we conducted a longitudinal nationwide population-based cohort study in Taiwan to explore the role of depression/anxiety in determining the long-term risk of hepatitis B flares.
Materials and Methods
Data Sources
The study data were extracted from the Taiwan National Health Insurance Research Database (NHIRD). National Health Insurance is a government-operated single-payer system that covers approximately 99% of the 23 million residents in Taiwan. The NHIRD includes records of inpatient, outpatient, and emergency consultations of the general population of Taiwan. Diagnoses are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD −9-CM).15 To protect the privacy of registered individuals, the NHIRD converts the identification numbers of all NHIRD records. The study protocol was approved by the Institutional Review Board of Tri-Service General Hospital (no. A202005009).
Study Participants
This study used outpatient and inpatient data on 1,936,512 patients in the NHIRD, collected between 2000 and 2015. A total of 292,754 patients aged ≥18 years who were diagnosed with HBV infection (ICD-9-CM codes: 70.2–70.23, 70.3–70.33, v02.61) (Table S1) as outpatients and inpatients were included for analysis (Figure 1). The data of patients with newly diagnosed depression and anxiety were used as the index data. Anxiety was identified using the ICD-9-CM code 300.00, and depressive disorder was identified using the ICD-9-CM codes 296.2–296.3 and 300.4.
 |
Figure 1 The flowchart of study sample selection. |
Hepatitis B flares were defined as present when a patient showed a 2-fold or greater elevation in their serum ALT levels3,4 and were prescribed nucleoside/nucleotide analog (NA) therapy, including adefovir, entecavir, lamivudine, telbivudine, or tenofovir. The National Health Insurance (NHI) in Taiwan follows the European Association for the Study of the Liver guidelines16 for starting antiviral therapy (Table S2). Interferon therapy is one of the treatments used for treating hepatitis B flares; however, it may cause depression. Therefore, patients who received interferon as treatment for hepatitis B flares were excluded.
Patients who did not have any follow-up visits, and those who were already using NA therapy and interferon-α therapy, were of unknown sex, or were coinfected with hepatitis C virus (HCV) (ICD-9-CM codes: 70.41, 70.44, 70.51, 70.54, and v02.62) or human immunodeficiency virus (ICD-9-CM codes: 042, 795.71, and V08) were excluded. Patients who received medication that may induce hepatitis B flares, such as steroids (betamethasone, cortisone, hydrocortisone, dexamethasone, fludrocortisone, methylprednisolone, prednisolone, triamcinolone), specific immunotherapy (azathioprine, infliximab, and methotrexate), or chemotherapy (ICD-9-CM codes: 37038B-37040B) were also excluded.
To control for the effect of confounders, each patient with depression/anxiety was matched with four controls with CHB but without depression/anxiety, by index date, age (10-year age category), and comorbidities to form the control cohort. To determine the propensity score, logistic regression was used to calculate the probability of hepatitis B flares after adjusting for comorbidities and demographic characteristics. The baseline comparison of the characteristics of the two groups included common comorbidities such as hyperlipidemia (ICD-9-CM code: 272), hypertension (ICD-9-CM codes: 401–405), chronic kidney disease (CKD; ICD-9-CM code: 585), ischemic heart disease (ICD-9-CM codes: 410–414), type 2 diabetes mellitus (DM; ICD-9-CM code: 250), congestive heart failure (ICD-9-CM code: 428), stroke (ICD-9-CM codes: 430–438), chronic obstructive pulmonary disease (ICD-9-CM codes: 490–496), and cancer (ICD-9-CM codes: 140–238).
Statistical Analysis
The demographic traits and common comorbidities in the depression/anxiety cohort and the control cohort were compared using chi-squared tests. The median ages of the cohorts were compared using Student’s t-test. The incidence rate (per 105 person-years) of hepatitis B flares was calculated according to the sex, age, and comorbidities of each cohort. Sex, age, and concomitant comorbidities were included in the multivariable Cox proportional hazards regression model. The cumulative incidence of hepatitis B flares according to depression/anxiety status was calculated using Kaplan-Meier analysis and compared using Log rank tests. All analyses were conducted using SPSS software, version 20 (IBM Corp., Armonk, NY, USA), with a two-tailed p-value < 0.05 considered as statistically significant.
Results
Participants
A total of 264,682 adults with CHB and without any of the exclusion factors were identified. A total of 12,992 patients with CHB, without advanced liver disease, were included in the depression/anxiety cohort, and 251,760 patients with CHB, without depression/anxiety or advanced liver disease, were included in the control cohort (Figure 1). In the depression/anxiety cohort, 7625 (58.7%) patients were male with median age of 49.2 years.
The demographic characteristics of patients in both cohorts are shown in Table 1. The median time to hepatitis B flares was 1.3 years in the depression/anxiety cohort and 1.5 years in the control cohort. By the end of follow-up, 3922 participants in the depression/anxiety cohort and 10,920 participants in the control cohort had developed hepatitis B flares (p < 0.001). At the end of follow-up, the median age was 51.7 and 52.9 years in the depression/anxiety cohort and the control cohort, respectively (p < 0.001) (Table S3).
 |
Table 1 Baseline Characteristics of the Depression/Anxiety Cohort and the Control Cohort |
Cumulative Incidence of Hepatitis B Flares
The cumulative incidence of hepatitis B flares is shown in Figure 2. In the depression/anxiety cohort, the median duration of follow-up and the time to hepatitis B flares was 5.3 and 1.3 years, respectively. The Kaplan-Meier analysis indicated that in the first year, the incidence of hepatitis B flares was higher in the depression/anxiety cohort than in the control cohort, and this increased incidence persisted throughout the follow-up period (log-rank p < 0.001; Figure 2).
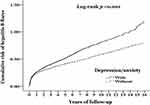 |
Figure 2 Kaplan-Meier analysis of the cumulative risk of hepatitis B flares according to depression/anxiety status. |
Multivariable Analysis of Risk Factors
In the unadjusted Cox proportional hazards regression analysis, the patients in the depression/anxiety cohort had a higher risk of hepatitis B flares than the patients in the control cohort. After adjusting for age and comorbidities, the hazard ratio (HR) for hepatitis B flares in the depression/anxiety cohort increased. Among the comorbidities, DM was the most strongly associated with hepatitis B flares, followed by stroke (Table 2). In subgroup analyses, patients with depression only (HR, 1.155; 95% CI, 1.019–1.261; p = 0.019) and anxiety only (HR, 1.167; 95% CI, 1.031–1.275; p = 0.008), and both depression and anxiety all had a higher risk of hepatitis B flares than patients in the control cohort (Table 3). Moreover, patients with both depression and anxiety had a higher risk of flares (HR, 1.506; 95% CI, 1.321–1.639; p = 0.003) than patients with either depression or anxiety alone.
 |
Table 2 Risk of Hepatitis B Flares According to Selected Patient Characteristics |
 |
Table 3 Factors of HBV Flares Stratified by Variables Listed in the Table by Using Cox Regression |
Multivariable Stratified Analysis for Depression/Anxiety
Multivariable stratified analyses were performed for subgroups of patients to determine the association of depression/anxiety with increased hepatitis B flare risk within subgroups (Table 4). In the subgroup analyses, the risk of hepatitis B flares was higher in the patients with depression/anxiety than that in the control patients in the 18–29-years age group, the 30–39-years age group, males, females, patients with DM, patients without DM, patients with hypertension, patients without hypertension, patients with a history of stroke, patients without a history of stroke, patients with CKD, patients without CKD, and patients without underlying osteoporosis. Although patients with depression/anxiety had a higher risk of developing hepatitis B flares than patients without depression/anxiety in all of the subgroups analyzed, the HR was not statistically significant in several subgroups, including those with underlying osteoporosis, and in patients aged 40–49 years, 50–59 years, or 60 years and older.
 |
Table 4 Influence of Demographic and Clinical Variables on the Risk of Hepatitis B Flares Determined by Cox Regression |
Incidence of Hepatitis B Flares According to Depression and Anxiety Status
The incidence rates of hepatitis B flares according to anxiety and depression status are shown in Table 3. The incidence rate of hepatitis B flares in the depression/anxiety group was higher than that in the control group. The incidence rate of hepatitis B flares was the highest in patients with both depression and anxiety, followed by that of patients with anxiety only, and was least increased in patients with depression only.
Discussion
This population-based cohort study revealed that the long-term risk of hepatitis B flares was significantly higher in patients with depression/anxiety than in those without these psychological comorbidities. This study represents the largest population-based study of the incidence of hepatitis B flares, with the longest follow-up period, reported to date. Patients with depression/anxiety had a 1.189-fold increased risk of hepatitis B flares compared with the control cohort during a 16-year follow-up. The Kaplan-Meier analysis showed that in the first year of follow-up, the incidence of hepatitis B flares was higher in the depression/anxiety cohort than in the control cohort, and this difference persisted throughout the follow-up period. Depression/anxiety was independently associated with an increased risk of hepatitis B flares after adjusting for age and comorbidities.
The mechanism by which depression/anxiety increase the risk of hepatitis B flares is unclear. T helper type 1 (Th1) lymphocytes secrete interleukin (IL)-2, interferon (IFN)-γ, and stimulate type 1 immunity, which is characterized by intense phagocytic activity. Conversely, Th2 cells secrete IL-4, IL-5, IL-9, IL-10, and IL-13, and stimulate type 2 immunity, which is characterized by high antibody titers. In most infections, type 1 immunity is protective, whereas type 2 immunity assists with the resolution of cell-mediated inflammation.17
IL-10 is an immunosuppressive cytokine. During infection, it inhibits the activity of Th1 cells, CD8+ T cells, NK cells, and macrophages, all of which are required for optimal pathogen clearance but also contribute to tissue damage. IL-10 can both impede pathogen clearance and ameliorate immunopathology. Many different types of cells can produce IL-10, with the major source of IL-10 varying in different tissues, and during the acute and chronic stages of the same infection.18 In patients with CHB, IL-10 is produced by regulatory B and T cells, intrahepatic T cells, and monocytes.19
CHB is commonly divided into four phases: the immune tolerant phase, the immune clearance phase, the inactive residual phase, and the reactivation phase.20 The key difference between effective and ineffective immune clearance may rely on whether the Th1 response has been activated.2 IL-10 is elevated in patients with hepatitis B flares and is thought to trigger a feedback mechanism that dampens liver inflammation, but it also results in suppression of HBV-specific CD8 T cell responses.21 Both depression and anxiety have been shown to increase IL-10 production,22–25 which is consistent with our study findings that the incidence of hepatitis B flares was higher in patients with depression only, anxiety only, and both depression and anxiety, compared with that in the control group.
Furthermore, antidepressant treatment may have an immunomodulatory effect.26 IFN-γ-producing T cells may be the major target for the immunomodulatory actions of antidepressants.27 Antidepressants decrease the production of IFN-γ,27,28 and may also increase IL-10 production28,29 and reduce the IFN-γ/IL-10 ratio.30–32 A higher level of HBV-specific IFN-γ-producing T cells is associated with more rapid HBV viral clearance, and IFN-γ is thought to be associated with HBV viral clearance in patients with hepatitis B flares.33 The antidepressant-induced immunomodulatory effect is one possible mechanism that could explain the increased risk of hepatitis B flares among patients with depression in our cohort. However, we did not have data on antidepressant use, and therefore we were unable to determine whether there was an association between hepatitis B flares and antidepressant use.
We hypothesize that both depression and anxiety have an impact on hepatitis B flares through a shift in the Th1–Th2 balance toward a Th2 response. This effect (a state of relative immunosuppression) is also seen in pregnant woman with hepatitis B flares after delivery.34 Furthermore, the anxiety- and stress-induced shift in the Th1–Th2 cytokine balance toward a Th2 response is thought to play a role in the immune response to HBV infection.35
The immunomodulatory effect of antidepressants may have a direct effect on increasing the risk of hepatitis B flares. However, further studies are needed to evaluate the underlying pathogenic mechanisms by which depression and anxiety cause hepatitis B flares.
The male dominance and the annual incidence of hepatitis B flares of 2% in the patients without depression or anxiety in this study are consistent with the findings of previous studies conducted in Southeast Asia, which have reported an annual incidence of 1.5–2.2%;36–38 however, the annual incidence in this study was lower than that of 4.3% reported in a European population.39 The higher incidence of hepatitis B flares observed in the European study might be due to the use of different viral assays or to referral bias.
Hepatitis B reactivation is defined by the level of changes in ALT and HBV DNA, and is usually associated with a hepatitis B flare several weeks later. HBV reactivation can be anticipated with some immunosuppressive drugs, and the risk is classified as low (<1%), moderate (1–10%), or high (>10%) risk. Well-established high-risk drugs include rituximab and corticosteroid therapy.3 One study showed that in 150 patients with newly diagnosed lymphoma who received rituximab-based chemotherapy, the incidence of hepatitis B flares was 6400 per 105 person-years.40 Another study found an incidence of HBV reactivation of 1930 per 105 person-years in patients with rheumatic disease and resolved HBV infection who were treated with immunosuppressive drugs.41 The incidence of hepatitis B flares in the patients with anxiety/depression in our study was 3016 per 105 person-years, which is lower than the reported risk associated with the use of high-risk drugs (rituximab) and higher than the reported risk associated with combined immunosuppressive drugs, suggesting that depression/anxiety has a moderate immunosuppressant effect. HBV reactivation induced by immunosuppressive agents or cytotoxic chemotherapy is a well-recognized complication in cancer patients with pre-existing HBV infection.42 The current American Gastroenterological Association recommendations (published in 2015) suggest antiviral prophylaxis for patients who are categorized as at moderate or high risk of developing hepatitis B flares.43 Patients with depression/anxiety may have an increased risk of HBV flares and might need antiviral drugs for prophylaxis. However, further studies are needed for confirmation.
Our finding that depression/anxiety increased the risk of hepatitis B flares is consistent with previous studies.8,12–14 Thus, CHB patients with depression/anxiety should be closely monitored and should have regular follow-up visits.
This study has several limitations. First, although many potential confounders were taken into consideration, a causal relationship between depression/anxiety and hepatitis B flares risk cannot be directly inferred owing to the observational nature of the study. The causal relationship needs to be confirmed by a prospective proof-of-concept study. Second, detailed laboratory data on factors such as HBV viral load and HBeAg levels were not available. These are major risk factors for hepatitis B reactivation and may associated with hepatitis B flares.3 However, we excluded patients with major risk factors for hepatitis B flares, such as those using immunosuppressive therapy or cancer chemotherapy, organ transplant recipients, and patients coinfected with other viruses.4,44 Other independent risk factors for hepatitis B flares are pre-core mutations, male sex, and age at presentation.39 We adjusted the estimates for sex and age, but did not adjust for pre-core mutations. Third, none of the patients had received previous NA therapy; therefore, our results may not be generalizable to patients who have previously received NA therapy. Hepatitis B flares are not uncommon during and after withdrawal of NA therapy.2 Further studies are needed to determine whether depression/anxiety increases the risk of hepatitis B flares in patients on NA therapy. Fourth, patients who fulfilled the definition of a hepatitis B flare (ALT >2-fold the upper limit of normal) but did not meet the NHI medication criteria were not included (because they did not fulfill the HBV DNA level criterion and did not have a liver biopsy). However, the strict inclusion criteria for assessing hepatitis B flares are relatively reliable, and many of our patients also fulfilled the definition of HBV reactivation.
Conclusions
The results of this long-term cohort study suggest that depression and anxiety may be associated with an increased risk of hepatitis B flares in patients with CHB. Therefore, CHB patients with depression and anxiety should be closely monitored and should have regular follow-up visits.
Data Sharing Statement
Restrictions apply to the availability of these data. The data were obtained from the Taiwan National Health Insurance Research Database and are available from the authors with the permission of Taiwan National Health Insurance.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Tri-Service General Hospital (no. A202005009).
Informed Consent Statement
The requirement for patient consent was waived because the study was based on a retrospective analysis of an anonymized database.
Acknowledgments
We thank the staff of the Department of Medical Education and Research of the Taichung Armed Forces General Hospital for their assistance. We also thank Mr. Kai-Ti Chang and Mr. Hsin-Yi, Pan for their assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Taichung Armed Forces General Hospital (Grant no.: TCAFGH-E-110042), Ministry of National Defense Medical Affairs Bureau (MAB-108-084), Tri-Service General Hospital (Grant no.: TSGH-B-111018, TSGH-E-111213).
Disclosure
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
1. Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52(4):594–604. doi:10.1016/j.jhep.2009.10.033
2. Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: pathogenesis, natural course, and management. J Hepatol. 2014;61:1407–1417. doi:10.1016/j.jhep.2014.08.033
3. Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221–244. doi:10.1053/j.gastro.2014.10.038
4. Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120:1009–1022. doi:10.1053/gast.2001.22461
5. Liaw YF. Hepatitis B flare after cessation of nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B: to retreat or not to retreat. Hepatology. 2021;73:843–852. doi:10.1002/hep.31525
6. Charlson F, van Ommeren M, Flaxman A, Cornett J, Whiteford H, Saxena S. New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis. Lancet. 2019;394:240–248. doi:10.1016/S0140-6736(19)30934-1
7. Tiller JW. Depression and anxiety. Med J Aust. 2013;199:S28–S31. doi:10.5694/mja12.10628
8. Hert MDE, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. doi:10.1002/j.2051-5545.2011.tb00014.x
9. Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25:221–229. doi:10.1016/j.bbi.2010.10.008
10. Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17:497–511. doi:10.1038/nrn.2016.69
11. Kronfol Z. Immune dysregulation in major depression: a critical review of existing evidence. Int J Neuropsychopharmacol. 2002;5:333–343. doi:10.1017/S1461145702003024
12. Andersson NW, Goodwin RD, Okkels N, et al. Depression and the risk of severe infections: prospective analyses on a nationwide representative sample. Int J Epidemiol. 2016;45:131–139. doi:10.1093/ije/dyv333
13. Seminog OO, Goldacre MJ. Risk of pneumonia and pneumococcal disease in people with severe mental illness: English record linkage studies. Thorax. 2013;68:171–176. doi:10.1136/thoraxjnl-2012-202480
14. Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006;1:421–427. doi:10.1007/s11481-006-9036-0
15. Cheng TM. Taiwan’s national health insurance system: high value for the dollar. In: Six Countries, Six Reform Models: The Healthcare Reform Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan. World Scientific; 2009:171–204. doi:10.1142/9789814261593_000710.1142/9789814261593_0007
16. European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi:10.1016/j.jhep.2017.03.021
17. Spellberg B, Edwards JE
18. Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi:10.4049/jimmunol.180.9.5771
19. Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med. 2013;19:859–868. doi:10.1038/nm.3251
20. Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582–592. doi:10.1016/S0140-6736(09)60207-5
21. Das A, Ellis G, Pallant C, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–3935. doi:10.4049/jimmunol.1103139
22. Köhler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–387. doi:10.1111/acps.12698
23. Himmerich H, Patsalos O, Lichtblau N, Ibrahim MAA, Dalton B. Cytokine research in depression: principles, challenges, and open questions. Front Psychiatry. 2019;10:30. doi:10.3389/fpsyt.2019.00030
24. Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi:10.1002/da.20564
25. Guo M, Liu T, Guo JC, Jiang XL, Chen F, Gao YS. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac J Trop Med. 2012;5:323–325. doi:10.1016/S1995-7645(12)60048-0
26. Gibney SM, Drexhage HA. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J Neuroimmune Pharmacol. 2013;8:900–920. doi:10.1007/s11481-013-9462-8
27. Diamond M, Kelly JP, Connor TJ. Antidepressants suppress production of the Th1 cytokine interferon-gamma, independent of monoamine transporter blockade. Eur Neuropsychopharmacol. 2006;16:481–490. doi:10.1016/j.euroneuro.2005.11.011
28. Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5:401–412. doi:10.1017/S1461145702003164
29. Blatteau JE, de Maistre S, Lambrechts K, Abraini J, Risso JJ, Vallée N. Fluoxetine stimulates anti-inflammatory IL-10 cytokine production and attenuates sensory deficits in a rat model of decompression sickness. J Appl Physiol. 2015;119:1393–1399. doi:10.1152/japplphysiol.00602.2015
30. Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, Maes M. Anti-inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol. 2001;21:199–206. doi:10.1097/00004714-200104000-00012
31. Kubera M, Kenis G, Bosmans E, Scharpé S, Maes M. Effects of serotonin and serotonergic agonists and antagonists on the production of interferon-gamma and interleukin-10. Neuropsychopharmacology. 2000;23:89–98. doi:10.1016/S0893-133X(99)00150-5
32. Maes M, Kenis G, Kubera M, De Baets M, Steinbusch H, Bosmans E. The negative immunoregulatory effects of fluoxetine in relation to the cAMP-dependent PKA pathway. Int Immunopharmacol. 2005;5:609–618. doi:10.1016/j.intimp.2004.11.008
33. Wang H, Luo H, Wan X, et al. TNF-α/IFN-γ profile of HBV-specific CD4 T cells is associated with liver damage and viral clearance in chronic HBV infection. J Hepatol. 2020;72:45–56. doi:10.1016/j.jhep.2019.08.024
34. Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis. 2007;45:1192–1199. doi:10.1086/522182
35. He Y, Gao H, Li X, Zhao Y. Psychological stress exerts effects on pathogenesis of hepatitis B via type-1/type-2 cytokines shift toward type-2 cytokine response. PLoS One. 2014;9:e105530. doi:10.1371/journal.pone.0105530
36. Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116(12):829–834. doi:10.1016/j.amjmed.2003.12.040
37. Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow-up. Hepatology. 2007;45:1187–1192. doi:10.1002/hep.21612
38. Chu CM, Liaw YF. Spontaneous relapse of hepatitis in inactive HBsAg carriers. Hepatol Int. 2007;1:311–315. doi:10.1007/s12072-007-9002-9
39. Kumar M, Chauhan R, Gupta N, Hissar S, Sakhuja P, Sarin SK. Spontaneous increases in alanine aminotransferase levels in asymptomatic chronic hepatitis B virus-infected patients. Gastroenterology. 2009;136:1272–1280. doi:10.1053/j.gastro.2009.01.011
40. Hsu C, Tsou HH, Lin SJ, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology. 2014;59:2092–2100. doi:10.1002/hep.26718
41. Fukuda W, Hanyu T, Katayama M, et al. Incidence of hepatitis B virus reactivation in patients with resolved infection on immunosuppressive therapy for rheumatic disease: a multicentre, prospective, observational study in Japan. Ann Rheum Dis. 2017;76:1051–1056. doi:10.1136/annrheumdis-2016-209973
42. Zhang X, Zhou Y, Chen C, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer. 2019;7:322. doi:10.1186/s40425-019-0808-5
43. Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215–219. doi:10.1053/j.gastro.2014.10.039
44. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297–1309. doi:10.1053/j.gastro.2017.02.009
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
