Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 13
Association Between CYP17A1, CYB5A Polymorphisms and Efficacy of Abiraterone Acetate/Prednisone Treatment in Castration-Resistant Prostate Cancer Patients
Authors Wu X, Xu QJ, Chen PZ, Yu CB, Ye LF, Li T
Received 7 January 2020
Accepted for publication 13 April 2020
Published 4 June 2020 Volume 2020:13 Pages 181—188
DOI https://doi.org/10.2147/PGPM.S245086
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Xiang Wu,1,2 Qing-Jiang Xu,1,2 Ping-Zhou Chen,1,2 Chen-Bo Yu,1,2 Lie-Fu Ye,1,2 Tao Li1,2
1Provincial Clinical Medical College of Fujian Medical University, Fuzhou, 350001, People’s Republic of China; 2Department of Urology, Fujian Provincial Hospital, Fuzhou 350001, People’s Republic of China
Correspondence: Tao Li Tel +86 13705078075
Email [email protected]
Purpose: The purpose of this study was to investigate the association between single nucleotide polymorphisms (SNPs) of CYP17A1, CYB5A and the efficacy of abiraterone acetate treatment in patients with castration-resistant prostate cancer (CRPC).
Patients and Methods: Data were collected from 58 CRPC patients who had been treated with abiraterone acetate/prednisone (AA/P). The SNPs rs743572 and rs10883783 on CYP17A1 and SNPs rs1790834 and rs1790858 on CYB5A were assayed, and their relationship with prostate-specific antigen (PSA) response in patients after AA/P treatment, overall survival (OS) and progression-free survival (PFS) were analyzed by logistic regression, Cox regression, Kaplan–Meier and Log rank analyses.
Results: The SNP rs1790834 on CYB5A showed significant association with PSA response in CRPC patients treated with AA/P (P < 0.05), but rs743572, rs10883783 and rs1790858 did not. The rs1790834 variant significantly decreased both PFS and OS (P < 0.05).
Conclusion: The CYB5A rs790834 genotype is a novel SNP related to CRPC and may be used as a biomarker for CRPC treatment.
Keywords: abiraterone, androgens, castration-resistant prostate cancer, CYP17A1, CYB5A
Introduction
Prostate cancer (PCa) is one of the most common malignancy, which caused approximately 29,430 deaths in the United States in 2018.1 In China, the incidence of PCa is much lower than that in Western countries, but is increasing linearly every year due to unhealthy lifestyles. The growth of PCa requires androgens, which are detected by androgen receptor (AR). At early stage, PCa could be treated by radical prostatectomy, brachytherapy and radiotherapy. When PCa recurs, the androgen deprivation therapy (ADT), which inhibits the function of AR, displays good efficacy in most PCa patients. However, increased production of androgen in tumor cells, overexpression and mutation of AR, changes in coregulatory molecules and alteration of factors indirectly activating AR would invalidate ADT.2–4 Proliferation of cancer cells was then stimulated, causing castration-resistant prostate cancer (CRPC)5 and finally leading to a lethal outcome.6
Many studies suggest that recurrent PCa patients are neither hormone refractory nor androgen independent, but maintain a clinically reliance on the AR signaling axis.7–9 Due to high sensitivity to the residual level of exogenous prostaglandins, the AR signaling axis is vital to the understanding of steroid synthesis signaling pathways in PCa and is a promising target pathway for the treatment of CRPC. PCa studies have focused on candidate genes based on biological pathways relevant to prostate carcinogenesis, including cytochrome P450 17A1 (CYP17A1), 17β-hydroxysteroid dehydrogenase (HSD17B) family and 5α reductase (SRD5A).10–15 Some new drugs targeting on the AR signaling axis have been reported to successfully slowdown CRPC progression and improve survival. For example, abiraterone is an inhibitor of CYP17A1, presumably through blockade of multiple steroidogenic enzymes and antagonism of AR.16 Recent studies showed that abiraterone acetate and prednisone (AA/P) significantly increased overall survival (OS) in patients at castration-resistant stage.3,14,16
Androsterone is synthesized by the sequential action of several enzymes, particularly CYP17A1 and heme-containing protein cytochrome b5 (CYB5A). CYB5A is a membrane-bound cytochrome that reduces methemoglobin to ferrous hemoglobin and stimulates CYP17A1 activity.17,18 Since CYP17A1 and CYB5A are directly or indirectly involved in the biosynthesis of androgen, genetic variation of these two genes may affect prostate growth, development of PCa and its sensitivity to chemical treatment.19–21 Single nucleotide polymorphism (SNP) represents a difference in a single nucleotide in DNA sequence. It is the most common genetic variation in human. Binder et al22 reported that the SNP rs26486758 on CYP17A1 was associated with lower odds of experiencing a biochemical response and a shorter time to biochemical progression in CRPC patients. Besides, Wang et al20 revealed significant associations between rs619824, rs2486758 polymorphisms and PCa risk, as well as a significant association between rs743572 polymorphism and PCa risk in Black population but not in Caucasian or Asian populations. Salvi et al14 highlighted no significant associations between rs743572, rs10883783, rs17115100, rs284849 polymorphisms on CYP17A1 and clinical outcome of CRPC. However, individuals with the most common TT genotype for rs10883783 had a 3 months’ longer progression-free survival (PFS) than individuals with the TA + AA genotype.14 Wright et al23 found that men with the variant A allele in rs10883783 had a 56% risk reduction in PCa-specific mortality. Obviously, studies on the relationship between prostate cancer outcomes and CYP17 gene rs743572, rs10883783 variants showed conflicting results. More investigations are still required. In CYB5A, both rs1790834 and rs1790858 were associated with rheumatoid arthritis.24 Furthermore, the SNP rs1790834 may help to maintain androgen levels in women.25 However, their relationship with PCa risk has not been reported to the best of our knowledge.
In the present study, to clarify the association between CYB5A rs1790834, rs1790858 variants and responses to AA/P, as well as to validate the association between CYP17A1 rs743572, rs10883783 variants and responses to AA/P, 58 CRPC patients were examined. Moreover, the relationship between clinical efficacy of AA/P and these SNPs were also investigated. These results may contribute more information for the clinical treatment of CRPC.
Patients and Methods
Patients
This study was approved by the Ethics Committee of the Fujian Provincial Hospital. As a retrospective survey, preparation of informed consent was exempted by the Ethics Committee. The study was conducted according to the principles of the Declaration of Helsinki (2013). Records and/or information of all patients were anonymized and de-identified before analyses.
From January 2015 to December 2018, approximately 80 participants diagnosed as hormone-refractory prostate cancer (HRPC) and treated with AA/P without chemotherapy or second-line treatment were collected from the Fujian Provincial Hospital and the First Hospital of Peking University. The HRPC criteria as follows: (1) Androgen deprivation with serum testosterone <50 ng/dL; (2) Rising prostate-specific antigen (PSA) defined as three rises of PSA two weeks apart; (3) Anti-androgen withdrawal therapy for more than four weeks; (4) Progression of PSA during second-line treatment; (5) Progression of bone or soft tissue metastatic. Finally, 58 patients diagnosed as CRPC were included in the present study.
SNP Sequencing
Two SNPs on CYP17A1 (rs743572 and rs10883783) and two SNPs on CYB5A (rs1790834 and rs1790858) were included in the present study. Genomic DNA was isolated from whole blood using Biospin Whole Blood Genomic DNA Extraction Kit (Bioer, Hangzhou) and then stored at −20°C. Purified DNA was amplified for detection of the CYP17A1 and CYB5A variants using the TaqMan real-time quantitative polymerase chain reaction (RT-qPCR) assay.26 Primer sequences and TaqMan probes are shown in Table 1. For each 25 μL of PCR reaction, 5 μL extracted DNA was used. The reaction was initially carried out at 95 °C for 10 min, followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 42 °C for 90 s, and extension at 72 °C for 30 s, with a final extension at 72 °C for 5 min. All DNA samples were analyzed in duplicate using a TaqMan PCR Master Mix on a 7500 real-time PCR cycler according to the manufacturer’s instructions.
 |
Table 1 Primer Sequences and TaqMan Probes of rs743572, rs10883783, rs1790834 and rs1790858 |
Clinical Data
Treatment consisted of 28-day cycles of 1000 mg of abiraterone acetate daily on an empty stomach with 5 mg of prednisone twice per day. Treatment continued until there was evidence of disease progression and/or unacceptable toxicity. Before commencement of treatment, blood samples were collected from all patients to measure the level of PSA, and all patients underwent a computed tomography (CT) scan of the chest and abdomen. Patients were evaluated monthly to check the change of PSA level and to monitor any hepatic and renal toxicity. CT scan was performed every 3 months during treatment with abiraterone. Disease progression was defined according to the Prostate Cancer Working Group 2 (PCWG2) criteria.27 Compared with the PSA level before treatment, a 50% decline of PSA level, which could be repeatedly confirmed after at least three weeks, was considered as non-progression. Progression was defined as the increase in PSA. After therapied by AA/P, the data of PSA level, testosterone level, age, tumor node metastasis (TNM) stages, Gleason score and Eastern Cooperative Oncology Group (ECOG) for each patient were recorded.
Statistical Analysis
Tests for Hardy–Weinberg equilibrium were performed for each SNP. Fisher’s exact test and Chi-square test were used to compare biochemical responses to AA/P between various SNP groups. PFS was defined as the time from the starting date of abiraterone treatment to the first observation of progression, relapse or death (whichever came first). OS was defined as the time from the starting date of abiraterone treatment to the date of death resulted from any cause. The Kaplan–Meier method was used to estimate PFS and OS. The Log-rank test was carried out to compare the PFS and OS curves between different genotypes. Logistic regression was used for association analyses. Odds ratios (OR) and 95% confidential intervals (CI) were calculated. Cox proportional hazards regression was used to assess the association between genetic variation and time to biochemical progression. All statistical tests were two-sided, and P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 23.0.
Results
The clinical and pathological characteristics of the 58 patients included in the present study are shown in Table 2. At the time of initial PCa diagnosis, the median age, PSA level and testosterone level of patients were 69 years, 4.75 ng/mL and 0.95 nmol/L, respectively. Forty-eight patients (83%) had Gleason score of ≥8.
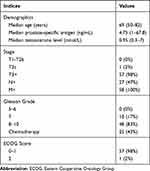 |
Table 2 Clinical Characteristics of the 58 Patients Involved in the Present Study. Demographics Were the Values at the Time of Initial Diagnosis |
All studied SNP polymorphisms were in Hard-Weinberg equilibrium (HWE). PSA level decreased for 50% was defined as effective PSA response. Statistical analyses showed a significant association between rs1790834 and PSA response (AA vs GG, P = 0.02; AG + AA vs GG, P = 0.047), but no significant association between rs743572, rs10883783, rs1790858 and PSA response (Tables 3 and 4).
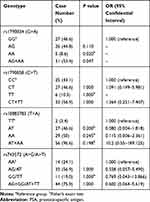 |
Table 3 Association of CYP17A1 and CYB5A Gene Polymorphisms with PSA Response. PSA Response Was Defined as 50% Reduction of PSA Level After Treatment with AA/P |
 |
Table 4 Logistic Regression Analyses Between rs1790834 Variants and PSA Response |
Based on the data of all CRPC patients, treatment with AA/P revealed the median PFS of 12 months (95% CI: 10.794–13.206) and the median OS of 28 months (95% CI: 24.526–31.474) (Figure 1). For rs1790834, significant differences in either PFS or OS were detected between patients with GG and AG + AA (P < 0.001). The PFS of patients with GG variant (14 months) was 4 months longer than those with AG+AA genotype (10 months) (Figure 2).
 |
Figure 1 Kaplan–Meier curves of progression-free survival (A) and overall survival (B) for all 58 patients. |
 |
Figure 2 Kaplan–Meier curves of progression-free survival (A) and overall survival (B) for patients with different CYP17A1 rs1790834 genotypes. |
The results of Cox proportional hazards analyses for PFS and OS are shown in Table 5. Cox regression analysis with adjustment for age, Gleason score and tumor stage at diagnosis showed that rs1790834 variation significantly affected PFS and OS of CRPC patients. The hazard ratios of AG genotype and AA genotype were greatly higher than those of GG genotype for both PFS and OS.
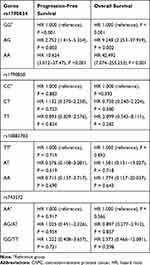 |
Table 5 Cox Proportional Hazards Model of Biochemical Progression for CRPC Patients |
Discussion
Endogenous hormones, especially androgens, are required for essential prostate functions, and affect the proliferation and differentiation of luminal epithelia.12 Since endogenous factors affecting the functional genome may differ among different populations, it is important to define the polymorphic spectrum of genes that are implicated in cancer causation. The biosynthesis of androgens depends on two key enzymes, 17α-hydroxylase and 17.20-lyase. Both of them link to CYP17A,28 and 17.20-lyase activity requires additional participation of P450 oxidoreductase and CYB5A.29 In addition, CYB5A is also involved in a number of other processes such as drug metabolism and fatty acid desaturation.30 Thus, CYP17A and CYB5A are greatly important to androgen synthesis and may influence prostatic carcinogenesis and its sensitivity to chemotherapy.
In previous studies, the rs743572 and rs10883783 polymorphism of CPY17A1 showed no significant association with PCa risk.31–34 A meta-analysis between 2404 PCa patients and 2755 health persons concluded that the rs743572 polymorphism was unlikely to alter PCa risk.35 In the present study, rs10883783 and rs743572 on CYP17A1 did not show significant association with PSA response, suggesting that mutation on these two sites might not contribute to efficacy and AA/P treatment in Chinese patients. These results were consistent with the results provided by Wang et al,20 Salvi et al,14 Han et al12 and Severi et al,31 but conflicted with the results from African-American and Japanese men.23,36,37 Salvi et al14 revealed that patients with TT genotype on rs10883783 showed better survival than TA + AA genotype, but Wright et al23 found that the variant A allele had a lower risk of PCa-specific mortality. In the present study, our data cannot support or deny these two conclusions, due to insufficient sample size. Only two patients had TT variant on rs10883783 in the present study. More samples are still required to test this hypothesis on Chinese population in future.
Compared with CYP17A1, association between efficacy of AA/P treatment and SNPs on CYB5A was less investigated. In the present study, two SNPs (rs1790858 and rs1790834) were investigated. SNPs rs1790858 showed no significant association with PSA response. However, rs1790834 was significantly correlated with PSA response. More importantly, the variant AG+AA showed significantly lower PFS and OS than GG, respectively. These results indicated that patients with AG + AA might be less effective to AA/P treatment than GG, which could be useful for selection of therapeutic plan. To the best of our knowledges, this is the first report for the relationship between rs1790834 and efficacy of AA/P treatment in PCa patients.
In humans, CYP17A1 catalyzes the 17α-hydroxylase and 17.20-lyase reactions to convert pregnenolone to 17α-hydroxypregnenolone and dehydroepiandrosterone (DHEA), and also catalyzes the 17.20 lyase reaction to produce androstenone.38,39 DHEA and androstenone are the precursors of androgen and testosterone.40 Meanwhile, CYB5A promotes the 17.20-lyase reaction by CYP17A1. Peacock et al41 identified a rare polymorphism on the pig CYB5A gene just upstream of the translational start site, which decreased levels of CYB5A and synthesis of androstenone. Similarly, the SNP rs1790834 is localized to the intron 1 of the CYB5A gene in humans. Genotype dependent gene expression in synovial fibroblasts, a cell type potentially responsible for androgen biosynthesis in the joint, showed an association between the rare allele of rs1790834 and increased CYB5A expression,24,25 which then should promote the biosynthesis of androgen. A high level of androgen would negatively affect the efficacy of ADT and then increase the risk of CRPC.
Associations between four SNPs and efficacy of ADT were analyzed in the present study. The results showed that the SNP rs1790834 might be a promising biomarker for selection of the best treatment method for CRPC patients. However, the present study only investigated 58 patients and all the patients were from the mainland of China. Investigations on more patients are still required to test the conclusions in different populations. Moreover, the present study demonstrated that CYB5A was an important gene for CRPC patients. The pharmacokinetics and pharmacodynamics of abiraterone on patients with different CYB5A genotypes should be further investigated.
Conclusion
This study found that the SNP rs1790834 is associated with the efficacy of AA/P in CRPC patients and decreases the sensitivity of patients to ADT. No associations were found between the SNPs rs743572, rs10883783, 1790858 and PSA response. There is a promising approach of primary genotyping to select the best treatment method in prostate cancer patients.
Acknowledgments
The authors thank all the staff of Fujian Provincial Hospital and all patients that were associated with this study. This work was supported by the Youth Innovation Project of Fujian Natural Science Foundation (No. 2018J05123) and the Young and Middle-aged Key Talents Training Project of Fujian Provincial Health Commission (No. 2017-ZQN-2).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflict of interests to declare.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;60(5):277–300.
2. Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253–8261. doi:10.1200/JCO.2005.03.4777
3. Lin DW. Commentary on Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. Urol Oncol Semin Ori. 2009;27(6):690–691.
4. Yu C, Clegg NJ, Scher HI. Anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target. Lancet Oncol. 2010;57(2):356–357.
5. Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. New Engl J Med. 1998;339(15):1036–1042. doi:10.1056/NEJM199810083391504
6. Aneja S, Pratiwadi RR, Yu JB. Hypofractionated radiation therapy for prostate cancer: risks and potential benefits in a fiscally conservative health care system. Oncology. 2012;26(6):512.
7. Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS. Secondary hormonal therapy for advanced prostate cancer. J Urol. 2006;175(1):27–34. doi:10.1016/S0022-5347(05)00034-0
8. Ryan CJ, Small EJ. Role of secondary hormonal therapy in the management of recurrent prostate cancer. Urology. 2003;62(6B):87–94. doi:10.1016/j.urology.2003.10.002
9. Kosaka T, Miyajima A, Nagata H, Maeda T, Kikuchi E, Oya M. Human castration resistant prostate cancer rather prefer to decreased 5alpha-reductase activity. Sci Rep. 2013;3:1268.
10. Ezzi AAE, Baker MT, Zaidan WR, Hraiki KM, Saidi MAE, Kuddus RH. Association of polymorphisms in the VDR, CYP17 and SRD5A2 genes and prostate cancer among lebanese men. Asian Pac J Cancer Prev. 2017;18(1):93–100. doi:10.22034/APJCP.2017.18.1.93
11. Kraft P, Pharoah P, Chanock SJ, et al. Genetic variation in the HSD17B1 gene and risk of prostate cancer. PLoS Genet. 2005;1(5):e68.
12. Han JH, Lee YS, Kim HJ, Lee SY, Myung SC. Association between cytochrome CYP17A1, CYP3A4, and CYP3A43 polymorphisms and prostate cancer risk and aggressiveness in a Korean study population. Asian J Androl. 2015;17(2):285–291. doi:10.4103/1008-682X.133320
13. Risio M, Venesio T, Kolomoets E, et al. Genetic polymorphisms of CYP17A1, vitamin D receptor and androgen receptor in Italian heredo-familial and sporadic prostate cancers. Cancer Epidemiol. 2011;35(4):e18–e24. doi:10.1016/j.canep.2010.10.003
14. Salvi S, Casadio V, Burgio SL, et al. CYP17A1 polymorphisms and clinical outcome of castration-resistant prostate cancer patients treated with abiraterone. Int J Biol Markers. 2016;31(3):e264–e269. doi:10.5301/jbm.5000197
15. Kanda S, Tsuchiya N, Narita S, et al. Effects of functional genetic polymorphisms in the CYP19A1 gene on prostate cancer risk and survival. Int J Cancer. 2015;136(1):74–82. doi:10.1002/ijc.28952
16. Zhenfei L, Bishop AC, Mohammad A, et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature. 2015;523(7560):347–351. doi:10.1038/nature14406
17. Akhtar MK, Kelly SL, Kaderbhai MA. Cytochrome b5 modulation of 17α hydroxylase and 17–20 lyase (CYP17) activities in steroidogenesis. J Endocrinol. 2005;187(2):267–274. doi:10.1677/joe.1.06375
18. Davis SM, Squires EJ. Association of cytochrome b5 with 16-androstene steroid synthesis in the testis and accumulation in the fat of male pigs. J Anim Sci. 1999;77(5):1230–1235. doi:10.2527/1999.7751230x
19. Attard G, Reid AH, Olmos D, de Bono JS. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009;69(12):4937–4940. doi:10.1158/0008-5472.CAN-08-4531
20. Wang F, Zou YF, Feng XL, Su H, Huang F. CYP17 gene polymorphisms and prostate cancer risk: a meta-analysis based on 38 independent studies. Prostate. 2011;71(11):1167–1177. doi:10.1002/pros.21332
21. Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17(18):5913–5925. doi:10.1158/1078-0432.CCR-11-0728
22. Binder M, Zhang BY, Hillman DW, et al. Common genetic variation in CYP17A1 and response to abiraterone acetate in patients with metastatic castration-resistant prostate cancer. Int J Mol Sci. 2016;17(7):1097.
23. Wright JL, Kwon EM, Lin DW, et al. CYP17 polymorphisms and prostate cancer outcomes. Prostate. 2010;70(10):1094–1101. doi:10.1002/pros.21143
24. Stark K, Schmidt M, Rovenský J, Blažičková S, Lowin T, Straub RH. Influence of CYB5A gene variants on risk of rheumatoid arthritis and local endocrine function in the joint. Brain Behav Immun. 2013;29:S12–S13.
25. Stark K, Straub RH, Rovenský J, Blažičková S, Eiselt G, Schmidt M. CYB5A polymorphism increases androgens and reduces risk of rheumatoid arthritis in women. Arthritis Res Ther. 2015;17(1):1–11. doi:10.1186/s13075-014-0514-0
26. Livak KJ. Allelic discrimination using fluorogenic probes and the 5ʹ nuclease assay. Genet Anal. 1999;14(5–6):143–149. doi:10.1016/s1050-3862(98)00019-9
27. Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26(7):1148–1159. doi:10.1200/JCO.2007.12.4487
28. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. doi:10.1210/er.2010-0013
29. Billen MJ, Squires EJ. The role of porcine cytochrome b5A and cytochrome b5B in the regulation of cytochrome P45017A1 activities. J Steroid Biochem Mol Biol. 2009;113(1–2):98–104. doi:10.1016/j.jsbmb.2008.11.012
30. Idkowiak J, Randell T, Dhir V, et al. A missense mutation in the human cytochrome b5 gene causes 46,XY disorder of sex development due to true isolated 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012;97(3):E465–E475. doi:10.1210/jc.2011-2413
31. Severi G, Hayes VM, Tesoriero AA, et al. The rs743572 common variant in the promoter of CYP17A1 is not associated with prostate cancer risk or circulating hormonal levels. BJU Int. 2008;101(4):492–496. doi:10.1111/j.1464-410X.2007.07272.x
32. Han QH, Shan ZJ, Hu JT, Zhang N, Zhang XP. Relationship between gene polymorphisms and prostate cancer risk. Asian Pac J Trop Med. 2015;8(7):569–573. doi:10.1016/j.apjtm.2015.06.005
33. Setiawan VW, Schumacher FR, Haiman CA, et al. CYP17 genetic variation and risk of breast and prostate cancer from the national cancer institute breast and prostate cancer cohort consortium (BPC3). Cancer Epidemiol Biomarkers Prev. 2007;16(11):2237–2246. doi:10.1158/1055-9965.EPI-07-0589
34. Sarma AV, Dunn RL, Lange LA, et al. Genetic polymorphisms in CYP17, CYP3A4, CYP19A1, SRD5A2, IGF-1, and IGFBP-3 and prostate cancer risk in African-American men: the flint men’s health study. Prostate. 2008;68(3):296–305. doi:10.1002/pros.20696
35. Ntais C, Polycarpou A, Ioannidis JP. Association of the CYP17 gene polymorphism with the risk of prostate cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2003;12(2):120–126.
36. Taioli E, Sears V, Watson A, et al. Polymorphisms in CYP17 and CYP3A4 and prostate cancer in men of African descent. Prostate. 2013;73(6):668–676. doi:10.1002/pros.22612
37. Yamada T, Nakayama M, Shimizu T, et al. Genetic polymorphisms of CYP17A1 in steroidogenesis pathway are associated with risk of progression to castration-resistant prostate cancer in Japanese men receiving androgen deprivation therapy. Int J Clin Oncol. 2013;18(4):711–717. doi:10.1007/s10147-012-0430-8
38. Yoshimoto FK, Auchus RJ. The diverse chemistry of cytochrome P450 17A1 (P450c17, CYP17A1). J Steroid Biochem Mol Biol. 2015;151:52–65. doi:10.1016/j.jsbmb.2014.11.026
39. Squires EJ, Gray MA, Lou Y. Effect of mutations in porcine CYB5A and CYP17A1 on the metabolism of pregnenolone. J Steroid Biochem Mol Biol. 2019;195:105469.
40. Barnard M, Mostaghel EA, Auchus RJ, Storbeck KH. The role of adrenal derived androgens in castration resistant prostate cancer. J Steroid Biochem Mol Biol. 2020;197:105506.
41. Peacock J, Lou Y, Lundström K, Squires EJ. The effect of a c.-8G>T polymorphism on the expression of cytochrome b5A and boar taint in pigs. Anim Genet. 2008;39(1):15–21. doi:10.1111/j.1365-2052.2007.01674.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
