Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Association Between Cognitive Impairment and Blood Pressure Among Patients with Type II Diabetes Mellitus in Southern Iran
Authors Jamalnia S, Javanmardifard S, Akbari H , Sadeghi E , Bijani M
Received 12 November 2019
Accepted for publication 15 January 2020
Published 5 February 2020 Volume 2020:13 Pages 289—296
DOI https://doi.org/10.2147/DMSO.S238247
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Sheida Jamalnia,1 Sorur Javanmardifard,2 Hamed Akbari,3,4 Erfan Sadeghi,5,6 Mostafa Bijani7
1Medical Journalism Department, Shiraz University of Medical Sciences, Shiraz, Iran; 2School of Nursing and Midwifery, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; 3Endocrinology and Metabolism Research Center, Institute of Basic and Clinical Physiology Sciences, Kerman University of Medical Sciences, Kerman, Iran; 4Department of Biochemistry, Afzalipur Faculty of Medicine, Kerman University of Medical Sciences, Kerman, Iran; 5Noncommunicable Diseases Research Center, Fasa University of Medical Sciences, Fasa, Iran; 6Department of Biostatistics and Epidemiology, Faculty of Health, Isfahan University of Medical Sciences, Isfahan, Iran; 7Department of Medical Surgical Nursing, Fasa University of Medical Sciences, Fasa, Iran
Correspondence: Mostafa Bijani
Department of Medical Surgical Nursing, Fasa University of Medical Sciences, Fasa 86688-74616, Iran
Tel +989173308451
Fax +98 7153357091
Email [email protected]
Purpose: Both type 2 diabetes mellitus (T2DM) and hypertension are regarded as life-threatening diseases known to be risk factors for vascular diseases. They may be associated with the increased risk of cognitive impairment (CI), although there are conflicting data relating hypertension to the risk of CI. Therefore, this study aimed to explore the probable association between hypertension and CI in patients with T2DM.
Patients and Methods: This cross-sectional study assessed the degree of CI of a total of 350 patients with T2DM using the Mini–Mental State Examination (MMSE). In clinical examinations, the mean of the first, second, and third measurements of systolic and diastolic blood pressure (SBP and DBP) was recorded.
Results: The mean of subjects’ MMSE scores was 25.48 ± 3.73. Additionally, the means of SBPSs and DBPs were found to be 118.50 ± 17.27 and 73.47 ± 10.25 mmHg, respectively. The Spearman correlation coefficient showed a mild, significant, negative correlation between MMSE scores and those of SBP (r = − 0.199, p < 0.001) and DBP (r = − 0.233, p < 0.001). Accordingly, a 1-unit increase in one’s SBP would lead to a significant rise in mild CI (2.8%) in comparison with subjects who have normal CIs. However, it was shown that if one’s DBP increased by 1 unit, the odds of mild CI occurring would increase significantly by 6.7% compared with those who have normal CIs.
Conclusion: The present findings revealed that hypertension might be related to the development of CI in people with a diabetic condition, thus emphasizing the fact that the prevention and treatment of these highly prevalent diseases assume the utmost significance.
Keywords: blood pressure, cognitive impairment, hypertension, type II diabetes mellitus
Introduction
Diabetes is considered to be a costly disease worldwide due to its many complications.1,2 The chronic nature of the disease and its associated complications impose heavy economic burdens and reduce the quality of life for patients and their families.3,4 According to the International Diabetes Federation (IDF), out of four diabetic people (352 million people), three are of working age (ie, between 20 and 64 years). It is expected that this figure will rise to 417 million and 486 million by 2030 and 2045, respectively. In 2019, an approximate 54.8 million adults aged 20–79 years, or 12.8% of the Middle East and North Africa regional population in the same age group, have diabetes, coupled with a total of 24.5 million adults whose diabetes are undiagnosed. Pakistan (19.4 million), Egypt (8.9 million), and Iran (5.4 million), respectively, account for the countries with the largest number of diabetic adults aged 20–79 years old. Iran’s national prevalence of diabetes in adults 20–79 years was 9.4 (7.4–12.3) in 2019.5
The effects of diabetes are not limited to early and late consequences, such as hyperglycemia and complications of small and large arteries.6 It may also lead to primary and secondary disorders as well as complications in the central nervous system (CNS) and its higher levels, ie, cognitive functions and processes.6 The primary effects of diabetes on CNS can be due to hyperglycemia, deficiency in insulin function, or both. Secondary effects, however, may be associated with diabetic vascular disorders, excessive insulin therapy, or brain damage caused by severe hypoglycemia.7,8 One of the lesser known complications of T2DM is cognitive impairment (CI). CI caused by T2DM involves reductions in information processing speed, attention, memory, learning, problem-solving power, visual intelligence, and mental flexibility.9 Biessels et al studied CI in patients with T2DM and concluded that this disease was associated with Alzheimer’s disease. Indeed, the glucose metabolism process and metabolic disorders could be effective in reducing cognitive function.10
Cognitive disorder can, directly or indirectly, interfere with cognitive and nervous system functions, causing disturbances in individuals’ awareness of themselves and the world around them. It can also create certain behavioral abnormalities that may greatly affect patients’ individual and social lives. Therefore, identifying the risk factors for CI is of utmost importance.11 The most predictable risk factor for CI is blood pressure (BP), because high BP and CI are quite common among elderly people.12 Nonetheless, the mechanisms associated with CI are complex.13 Recent evidence has suggested that hypertension might cause changes in brain structure and function by interfering with brain autoimmune regulation, therefore reducing brain perfusion and limiting the ability of the brain to eliminate potentially harmful proteins, such as β-amyloid.10 Reduced cerebral blood flow after aging can disrupt the mechanisms regulating the brain, and this influence is enhanced by high BP.14 In addition, it is likely that the severity of atherosclerosis of the arteries associates hypertension with CI.11 The Framingham study conducted on 1702 patients showed that cognitive function is correlated with the baseline BP. In addition, the patients’ CI worsened after 12–14 years.12 Previous studies conducted to determine the relationship between BP and cognitive function have come to controversial results.15 Some have reported that high BP (usually systolic blood pressure (SBP)) is associated with poor cognitive function,16 while others have shown a positive relationship between CI and low BP.17
A major risk factor for cerebrovascular disease, hypertension has been reported to result in a weaker performance in neuropsychological tests. Furthermore, it has been revealed that one of the major complications of diabetes is CI.18 However, the way in which diabetes, blood pressure, and cognitive performance are related to each other is not yet known unequivocally.19 Traditionally, it has been believed that CI is a primary neurodegenerative disorder and that it is not of a vascular origin. Nevertheless, cumulative evidence has come to suggest that CI is not dissociated from vascular factors and disorders as once considered.16,19,20 Whether hypertension is a risk factor for CI and decline or not has been the topic of much debate, such that some studies consider a positive association between these factors while others do not. Therefore, the present study aimed to evaluate the association between hypertension and CI in patients with T2DM.
Materials and Methods
This cross-sectional study was conducted on 350 patients with T2DM selected by random sampling in centers affiliated with Shiraz University of Medical Sciences, Shiraz, Iran (Imam Reza Clinic, Nader Kazemi Clinic, and Heart House). Inclusion criteria were age (30–60 years old), education, and diabetes for more than 1 year. The criteria for exclusion included a psychiatric or neurological disorder such as Alzheimer’s disease, anxiety, depression, schizophrenia, multiple sclerosis, dementia, severe somatic disease unrelated to diabetes and capable of disrupting cognitive functioning, history of alcohol or drug abuse, cerebrovascular accidents, history of stroke, transient ischemic attack, cardiovascular disease, or liver and kidney diseases. All participants gave written informed consent to participate in the study. The present study was conducted in accordance with the principles of the revised Declaration of Helsinki, a statement of ethical principles which directs physicians and other participants in medical research involving human subjects. Moreover, the study was approved by the local Ethics Committee of Fasa University of Medical Sciences, Fasa, Iran (IR.FUMS.REC.1398.040).
The study data were gathered using a demographic information form and the Mini Mental Status Examination (MMSE). MMSE is a brief examination of CI first used by Folstein et al as a functional method for categorizing cognitive function.21 The total score of this test is 30. Accordingly, scores 0–9, 10–20, 21–26, and 27–30 represent severe CI, moderate CI, mild CI, and normal CI, respectively. The reliability of the test was approved by Cronbach’s alpha which equaled 78%.
BP was measured on the basis of the criteria for the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure.22 Briefly, three blood pressure measurements were recorded at 2-min intervals after the subject had been in a sitting position for approximately 1 h using a standard mercury sphygmomanometer (Exacta-Riester 1350, Rudolf Riester GmbH, Jungingen, Germany). Then, the mean of the three BP measurements was recorded. Hypertension was defined as SBP ≥120 mmHg, DBP ≥80 mmHg, or current use of antihypertensive medication. BP levels were then classified as follows:22 SBP was graded as normal (90–119 mmHg), elevated (120–139 mmHg), stage I (140–159 mmHg), or stage II (>160 mmHg). DBP was classified as normal (60–79 mmHg), elevated (80–89 mmHg), stage I (90–99 mmHg), or stage II (>100 mmHg).
Statistical Analysis
All continuous variables were reported as mean ± standard deviation (M±SD), and categorical variables were presented as percentages. The Kolmogorov–Smirnov test was used to test for the normality assumption. The differences between subgroups of cognitive status were analyzed using Kruskal–Wallis with post hoc Mann–Whitney U-tests as well as Chi-square/Fisher’s exact tests (applying the Bonferroni correction). Correlations between continuous variables were evaluated using the Spearman’s rho correlation coefficient. The multinomial logistic regression model was also employed to assess the association between blood pressure and cognitive status. All statistical analyses were performed using SPSS statistical software version 24.0 for Windows (SPSS Inc.), and a p-value <0.05 was considered statistically significant.
Results
The descriptive characteristics of all subjects are summarized in Table 1. The study was conducted on a total of 350 patients suffering from T2DM (79 (22.6%) men and 271 (77.4%) women). The mean age of the patients was 52.156±8.11 years. The means of SBP and DBP were 118.50 ± 17.27 mmHg and 73.47±10.25 mmHg, respectively. The mean of the subjects’ MMSE scores was 25.48 ± 3.73. Of all the patients, 141 (40.3%), 157 (44.9%), 52 (14.9%) reported normal, mild and moderate CI, respectively, and no one was shown to suffer from severe CI. In this study, hypertension was seen in 141 (40.3%) of the patients, of which 87 (24.9%) had elevated SBP, 47 (13.4%) had stage I SBP, and 6 (1.7%) had stage II SBP. Moreover, 88 patients (25.1%) had elevated DBP, 14 (4.0%) had stage I DBP, and 6 (1.7%) had stage II DBP. The means of SBP and DBP were 118.50 ± 17.27 mmHg and 73.47 ± 10.25 mmHg, respectively (Table 1).
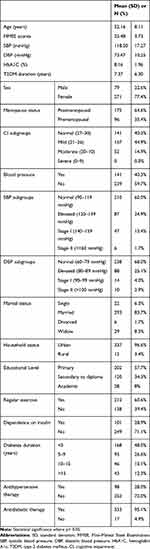 |
Table 1 Demographic, Anthropometric and Social Factors Among the Sample Study |
Table 2 presents the comparison of the proportion of subjects with and without BP among the different CI subgroups. Hypertensive patients showed, respectively, 53.2% and 17.7% mild and moderate CI compared with 29.1% hypertensive patients without CI. Normotensive diabetic patients showed 47.8%, 38.2%, and 12.9% normal, mild, and moderate CI, respectively.
 |
Table 2 Association of Blood Pressure with CI Subgroups |
Table 3 illustrates the comparison of SBP and DBP mean values with CI subgroups. The average of both SBP and DBP was significantly different within CI subgroups (p <0.001). Pairwise comparisons revealed that patients with mild and moderate CI had significantly higher SBP and DBP compared with patients without CI (Table 3).
 |
Table 3 Comparison of SBP and DBP Mean Values with CI Subgroups |
The Spearman correlation coefficient showed that MMSE scores had a mild, significant, negative correlation with SBP (r = −0.199, p <0.001) and DBP (r = −0.233, p <0.001). Although a negative correlation was found between MMSE scores and age, it was not statistically significant (r = −0.068, p =0.207) (data not shown).
The association between SBP and DBP subgroups and those of CI is presented in Table 4. The results of the chi-square test showed that most patients with normal SBP (71.6%) and normal DBP (80.9%) levels had normal CI. Hypertensive patients showed a higher percentage of CI, moderate level (30.8% in SBP and 34.6% in SBP). Patients with stage I hypertension showed more cases of mild CI in both SBP (3.2%) and DBP (5.7%) groups. In addition, the majority of stage II hypertensive patients reported mild CI (Table 4).
 |
Table 4 Association of SBP and DBP Subgroups with CI Subgroups |
Multinomial logistic regression was carried out in different models to determine the association of SBP and DBP with CI by adjusting the existing confounders. As shown in Table 5, in the model adjusted by all available confounders, the findings showed that if one’s SBP increased by 1 mmHg, the odds of having mild CI were significantly increased (by 2.8%) compared with subjects with normal CIs. Moreover, if one’s DBP increased by 1 mmHg, the odds of having mild CI were significantly increased (by 6.7%) compared with subjects with normal CIs. Although this trend between SBP, DBP, and moderated CI was the same, they did not show a significant association as shown by multinomial logistic regression. However, in the model adjusted by age and gender, both mild and moderate CIs showed a significant increase in the odds ratio compared with those with normal CIs (Table 5).
 |
Table 5 Crude and Adjusted Odds Ratios and 95% Confidence Intervals Derived from Multinomial Logistic Regression |
Discussion
Confirming the long-time prediction, a substantial increase in the occurrence of T2DM is currently prevailing, particularly in Iran. Despite the fact that the link between T2DM and CI is clinically relevant and obvious, on an individual level, whether there is an association between more mild to moderate CI and hypertension is not well known.23 Because few diabetes healthcare professionals conduct analyses of their elderly patients for evidence of CI, the odds of an under-diagnosis is high. Hence, in the present study, the presence of CI in patients with T2DM suffering from hypertension was assessed in a population from southern Iran. The present findings revealed an association between hypertension and CI in this Iranian adult population, such that hypertensive diabetic patients showed a cognitive decline.
The current findings obtained from correlation analyses showed an association between CI scores and SBP, DBP, and age in T2DM patients, which is in line with the hypothesis that the increased risk of CI in older T2DM patients is related to underlying vascular disease. The findings were in agreement with those of previous studies.24 Multinomial logistic regression results adjusted by existing confounders demonstrated that with a 1-unit increase in both SBP and DBP, the odds of having mild CI were significantly increased (by 2.8% and 6.7%, respectively) compared with subjects with normal CI. Although this trend occurred in the same way between SBP, DBP, and moderated CI, no significant association was reported between them. The findings indicated the important role of hypertension in CI among patients with diabetes. The findings of Cacciatore et al showed that in subjects aged 75 years and older who had no neurological disorders, DBP rather than SBP predicted CI independent of gender, age, education, and antihypertensive treatment; their findings are not in line with the present findings.
High DBP leads to weakness in small vessels of the brain, thus extending these small areas of brain damage and CI.16 In the current study, most patients with normal systolic and diastolic pressure levels had normal CI. However, previous studies have produced contradictory results regarding the relationship between BP and cognitive function. The potential link between T2DM, hypertension, and CI is intriguing. According to studies conducted by a specialist group under the supervision of the National Institutes of Health (NIH), the relationship between hypertension and CI is very weak due to heterogeneity in the definitions of mild CI and hypertension as well as differences in the hypertension detection methods (for example, measuring hypertension versus patients’ self-reporting).16 Alpérovitch et al examined changes in BP and the risk of dementia in the elderly and disclosed that changes in BP were associated with an increased risk of dementia, while the mean of BP was not associated with dementia.25 On the other hand, Hassing et al24 reported a statistically non-significant impact of hypertension on cognitive function despite the fact that the hypertensive patients in their study had a somewhat greater decline than the non-cases. Nevertheless, the present study showed that this trend was statistically significant. Moreover, according to Pandav et al,26 low BP may be the result of, or a risk factor for, degenerative brain disease. The discrepancies between the results of these investigations and those of the present one might be attributed to differences in sample size, sampling methods, patients’ cultures, patients’ diets, definitions of hypertension, and BP measurement methods. For example, CI has been defined as a disorder with pathological features. Thus, if CI subtypes are defined differently, it would lead to varying outcomes.
T2DM has been shown to have various complications. Not only does T2DM influence the microvessels of the eyes, kidneys, and peripheral nerves, but it is also considered to be a crucial risk factor for macrovascular disease. Moreover, it results in significant degrees of morbidity and mortality through ischemic heart disease and stroke. It should also be noted that patients with T2DM, and especially those with the combination of diabetes mellitus and hypertension, are most vulnerable and prone to cerebrovascular and cardiovascular diseases.27–30 Additionally, as dyslipidemia and obesity are conceived to be modifiable risk factors which, either directly or indirectly, make a contribution to the development of CIs in diabetes, it is strongly suggested that the risk of CI can be reduced by intensively managing the vascular risk factor. Thus, progression of vascular disease can be a factor upon which the relationship between cognitive changes and diabetes is based.13,31,32
Given that hypertension is clearly shown to be a risk factor for CI,33 it was predicted that hypertensive patients would experience a greater cognitive decline, for many findings have confirmed such a result,34–37 which is in keeping with the results of the present study in which high levels of cognitive decline were seen in hypertensive diabetic patients. According to previously conducted studies, if T2DM is accompanied by hypertension, the result would be a dramatic rise in the prevalence of CI.24,35 Also, according to findings obtained by Elias et al, hypertensive patients with non-insulin-dependent diabetes mellitus (NIDDM) are the main group susceptible to risks for poor performance on tests which evaluate visual organization and memory.35 On the other hand, the Frontal Assessment Battery (FAB) test has reportedly shown slight changes in normotensive diabetic patients.
Given that T2DM is a disorder with a metabolically complex nature, it can be suggested that the pathogenesis of diabetes-related CI is multifactorial.1 However, to date, no study has been conducted particularly to assess the cognitive function of people with T2DM to produce a profound and comprehensive clinical characterization in order to make it possible to examine the effect of numerous cognitive risk factors. Even though macrovascular disease and hypertension per se are considered as major possible risk factors, the odds are that genetic susceptibility plays an important role as well. The need is great for further clinical trials and longitudinal studies encompassing a higher sample number with a focus on genetic susceptibility factors; better clarifying the potential mechanisms of these diseases would be much more fruitful.
Strengths and Limitations
Considering the limitations of this study, we must be cautious in interpreting the results. The first limitation might be the relatively small sample size. In addition, a major limitation to this study was that the diagnosis of diabetes was based on self-report, HbA1C levels, or history of antidiabetic medication, and no objective measurements of fasting blood glucose were made. Moreover, another limitation of the present study was the comparatively higher number of women compared to men, which makes it difficult to easily generalize the current results. The main strength of the present study, however, is that unlike previous ones, patients with a history of stroke or transient ischemic attack were excluded. Therefore, the results indicated the effect of a vascular risk factor on cognitive changes in the absence of a potential clinical disease. Moreover, the present study addressed some methodological problems mentioned in recent studies, including dependence on self-reported BP or physicians’ measurement of BP only once. The results of studies that are based solely on self-reported BP may be misleading. Another strength of this study was the diagnosis of CI using standard criteria and the completion of clinical evaluations related to BP.
Conclusion
The findings obtained from the present study demonstrate that hypertension might be associated with increased odds of CI, particularly mild CI, in diabetic conditions, suggesting that it should be taken into account at the time of managing T2DM combined with hypertension.
Acknowledgments
This paper was extracted from a research project with the ethical code (IR.FUMS.REC.1398.040) in Fasa University of Medical Sciences, Fasa, Iran. The authors appreciate Fasa University of Medical Sciences for financially supporting this research.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Katharina K, Laxy M, Schneider U, Rogowski WH, Lhachimi SK, Holle R. Health care costs associated with incident complications in patients with type 2 diabetes in Germany. Diabetes Care. 2018;41(5):971–978. doi:10.2337/dc17-1763
2. Baxter M, Hudson R, Mahon J, et al. Estimating the impact of better management of glycaemic control in adults with type 1 and type 2 diabetes on the number of clinical complications and the associated financial benefit. Diabet Med. 2016;33(11):1575–1581. doi:10.1111/dme.2016.33.issue-11
3. Wändell PE. Quality of life of patients with diabetes mellitus an overview of research in primary health care in the Nordic countries. Scand J Prim Health Care. 2005;23(2):68–74. doi:10.1080/02813430510015296
4. Akbari H, Sarrafzadegan N, Aria H, Garaei AG, Zakeri H. Anxiety but not depression is associated with metabolic syndrome: the Isfahan healthy heart program. J Res Med Sci off J Isfahan Univ Med Sci. 2017;22:90.
5. International Diabetes Federation. IDF diabetes atlas - 8th edition; 2017. Available from: www.idf.org/diabetesatlas.
6. Dimas AS, Lagou V, Barker A, et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes. 2014;63(6):2158–2171. doi:10.2337/db13-0949
7. Sima AAF, Kamiya H, Li ZG. Insulin, C-peptide, hyperglycemia, and central nervous system complications in diabetes. Eur J Pharmacol. 2004;490(1–3):187–197. doi:10.1016/j.ejphar.2004.02.056
8. Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301(15):1565–1572. doi:10.1001/jama.2009.460
9. Whitmer RA. Type 2 diabetes and risk of cognitive impairment and dementia. Curr Neurol Neurosci Rep. 2007;7(5):373–380. doi:10.1007/s11910-007-0058-7
10. Biessels GJ, Strachan MWJ, Visseren FLJ, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2(3):246–255. doi:10.1016/S2213-8587(13)70088-3
11. Cechetto DF, Hachinski V, Whitehead SN. Vascular risk factors and Alzheimer’s disease. Expert Rev Neurother. 2008;8(5):743–750. doi:10.1586/14737175.8.5.743
12. Shang S, Li P, Deng M, Jiang Y, Chen C, Qu Q. The age-dependent relationship between blood pressure and cognitive impairment: a cross-sectional study in a rural area of Xi’an, China. PLoS One. 2016;11(7):e0159485. doi:10.1371/journal.pone.0159485
13. Ryan JP, Fine DF, Rosano C. Type 2 diabetes and cognitive impairment: contributions from neuroimaging. J Geriatr Psychiatry Neurol. 2014;27(1):47–55. doi:10.1177/0891988713516543
14. Zheng T, Qin L, Chen B, et al. Association of plasma DPP4 activity with mild cognitive impairment in elderly patients with type 2 diabetes: results from the GDMD study in China. Diabetes Care. 2016;39(9):1594–1601. doi:10.2337/dc16-0316
15. Hajjar I, Goldstein FC, Martin GS, Quyyumi AA. Roles of arterial stiffness and blood pressure in hypertension-associated cognitive decline in healthy adults. Hypertension. 2016;67(1):171–175. doi:10.1161/HYPERTENSIONAHA.115.06277
16. Goldstein FC, Levey AI, Steenland NK. High blood pressure and cognitive decline in mild cognitive impairment. J Am Geriatr Soc. 2013;61(1):67–73. doi:10.1111/jgs.2013.61.issue-1
17. Ogliari G, Sabayan B, Mari D, et al. Age-and functional status–dependent association between blood pressure and cognition: the milan geriatrics 75+ cohort study. J Am Geriatr Soc. 2015;63(9):1741–1748. doi:10.1111/jgs.13616
18. Huebner N, Lee Y-A, Kreutz R, Lindpaintner K, Ganten D. The stroke-prone spontaneously hypertensive rat and its role in the genetic dissection of cardiovascular disease. In: Guesry P, Hennerici M, Sitzer G, editors. Nestle nutrition workshop series. Rowen Press; 1997:87–100.
19. Cacciatore F, Abete P, Ferrara N, et al. The role of blood pressure in cognitive impairment in an elderly population. J Hypertens. 1997;15(2):135–142. doi:10.1097/00004872-199715020-00003
20. Johnson RJ, Nakagawa T, Sanchez-Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–3315. doi:10.2337/db12-1814
21. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6
22. A V C, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi:10.1161/01.HYP.0000107251.49515.c2
23. Luchsinger JA. Type 2 diabetes and cognitive impairment: linking mechanisms. J Alzheimer’s Dis. 2012;30(s2):S185–98. doi:10.3233/JAD-2012-111433
24. Hassing LB, Hofer SM, Nilsson SE, et al. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: evidence from a longitudinal study. Age Ageing. 2004;33(4):355–361. doi:10.1093/ageing/afh100
25. Alpérovitch A, Blachier M, Soumaré A, et al. Blood pressure variability and risk of dementia in an elderly cohort, the three-city study. Alzheimer’s Dement. 2014;10(5):S330–7. doi:10.1016/j.jalz.2013.05.1777
26. Pandav R, Dodge HH, DeKosky ST, Ganguli M. Blood pressure and cognitive impairment in India and the United States: a cross-national epidemiological study. Arch Neurol. 2003;60(8):1123–1128. doi:10.1001/archneur.60.8.1123
27. Ginter E, Simko V. Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol. 2012;771:42-50.
28. Akbari H, Asadikaram G, Jafari A, et al. Atorvastatin, losartan and captopril may upregulate IL-22 in hypertension and coronary artery disease; the role of gene polymorphism. Life Sci. 2018; 207:525–531. doi:10.1016/j.lfs.2018.07.005
29. Asadikaram G, Akbari H, Safi Z, et al. Downregulation of IL-22 can be considered as a risk factor for onset of type 2 diabetes. J Cell Biochem. 2018;119(March):1–7.
30. Akbari H, Asadikaram G, Vakili S, Masoumi M. Atorvastatin and losartan may upregulate renalase activity in hypertension but not coronary artery diseases: the role of gene polymorphism. J Cell Biochem. 2019;120(6):9159–9171. doi:10.1002/jcb.v120.6
31. Ryan CM, Geckle M. Why is learning and memory dysfunction in type 2 diabetes limited to older adults? Diabetes Metab Res Rev. 2000;16(5):308–315. doi:10.1002/(ISSN)1520-7560
32. Van den Berg E, Kloppenborg RP, Kessels RPC, Kappelle LJ, Biessels GJ. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: a systematic comparison of their impact on cognition. Biochim Biophys Acta (BBA)-Molecular Basis Dis. 2009;1792(5):470–481. doi:10.1016/j.bbadis.2008.09.004
33. Gottesman RF, Schneider ALC, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71(10):1218–1227. doi:10.1001/jamaneurol.2014.1646
34. Cervilla JA, Prince M, Joels S, Lovestone S, Mann A. Long-term predictors of cognitive outcome in a cohort of older people with hypertension. Br J Psychiatry. 2000;177(1):66–71. doi:10.1192/bjp.177.1.66
35. Elias PK, Elias MF, D’Agostino RB, et al. NIDDM and blood pressure as risk factors for poor cognitive performance: the Framingham Study. Diabetes Care. 1997;20(9):1388–1395. doi:10.2337/diacare.20.9.1388
36. Posner HB, Tang M-X, Luchsinger J, Lantigua R, Stern Y, Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2002;58(8):1175–1181. doi:10.1212/WNL.58.8.1175
37. Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension. 1998;31(3):780–786. doi:10.1161/01.HYP.31.3.780
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
