Back to Journals » International Journal of Nanomedicine » Volume 9 » Supplement 2
Assessment of ZnO and SiO2 nanoparticle permeability through and toxicity to the blood–brain barrier using Evans blue and TEM
Authors Shim KH, Jeong K, Bae SO, Kang MO, Maeng E, Choi C, Kim Y, Hulme J, Lee EK, Kim M, An SSA
Received 25 November 2013
Accepted for publication 24 February 2014
Published 15 December 2014 Volume 2014:9(Supplement 2) Pages 225—233
DOI https://doi.org/10.2147/IJN.S58205
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Kyu Hwan Shim,1 Kyeong-Hoon Jeong,2,3 Sun Oh Bae,1 Min O Kang,1 Eun Ho Maeng,4 Cheol Soo Choi,2,3 Yu-Ri Kim,5 John Hulme,1 Eun Kyu Lee,1 Meyoung-Kon Kim,5 Seong Soo A An1
1Department of Bionano Technology, Gachon Medical Research Institute, Gachon University, Seongnam-si, Republic of Korea; 2Korea Mouse Metabolic Phenotyping Center, Lee GilYa Cancer and Diabetes Institute, Gachon University, Incheon, Republic of Korea; 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Republic of Korea; 4Department of Analysis, Korea Testing and Research Institute (KTR), Gimpo, Republic of Korea; 5Department of Biochemistry and Molecular Biology, Korea University Medical School and College, Seoul, Republic of Korea
Abstract: As increasing variants of nanoparticles (NPs) are being used in various products, it has become apparent that size alone can no longer adequately explain the variety of generated toxic profiles. Recent studies with NPs have suggested that various sizes of NPs could determine in vitro toxicity. In an attempt to address concerns regarding neurotoxicity of zinc oxide (ZnO) and silica (SiO2) NPs, these were examined after exposing them via oral, dermal, and intravenous administrations of NPs and their toxicological effects on the brain over a prescribed period of time were assessed. After 28 days of repeated oral administrations of ZnO or SiO2 independently, possibly due to damages to the blood brain barrier (BBB), neurotoxicity, were investigated by Evans blue technique. Next, in order to assess whether ZnO NPs could compromise the BBB, ZnO NPs were intravenously injected on day 0, 7, 14, 21 and 28 no further treatment was administered for 62 days. Deposition of SiO2 in brain from repeated dermal and oral administrations for 90 days were evaluated by transmission electron microscopy coupled with scanning energy-dispersive X-ray spectroscopy. Physiochemical profiles were principally determined on particle size at the beginning of the current toxicity investigations on ZnO and SiO2 NPs. The BBB was found to be intact after independent repeated oral administrations of ZnO or SiO2 NPs for 28 days, suggesting no significant damage. Neuronal death was also not observed after the intravenous administrations of ZnO NPs. After 90 days of repeated dermal and oral administration of SiO2 NPs, no deposition of NPs was observed in hippocampus, striatum, and cerebellum regions using transmission electron microscope analyses. These observations suggest that the BBB was not compromised and was able to block penetration of ZnO and SiO2 NPs, resulting in significant neurotoxic effects. Moreover, absence of SiO2 in three regions of brain after dermal and oral administrations for 90 days suggested that brain was protected from SiO2. No behavior change was observed in all studies, suggesting that 90 days may not be long enough to assess full neurotoxicity of NPs in vivo.
Keywords: zinc oxide, silica, BBB, neurotoxicity, penetration, administration
Introduction
Potential toxicities of nanoparticles (NPs) are of growing concern in academia and for regulatory agencies, industries, and society. This, in turn, has prompted a flurry of new studies and the expansion of smaller investigations in regards to the neurotoxicity of NPs in vivo. As precise understanding of NPs toxicity remains elusive, many developed countries have recently embarked on a concerted effort to further update the in vivo toxicity profile of many different types of NPs in everyday use.
One of the oldest and most frequently used NPs is zinc oxide (ZnO). ZnO NPs are present in sunscreens and cosmetic products due to their role in preventing skin cancers by protection from ultraviolet light, and promoting pH balance, and flora regeneration. Silica (SiO2) NPs can be found in abrasives, food additives, cosmetics, and biomedical devices. Their use in the medical field in diagnosis, imaging, and drug delivery is on the rise. According to recent reports, inhaled NPs could travel to brain or systemically via lung or olfactory nerves on the upper section of the nasal cavity.1 Furthermore, direct injections of NPs to brain have been shown to cause inflammation and neurotoxicity, leading to neurological diseases.2–5
In in vitro studies, ZnO NPs caused apoptosis of neural stem cells and interfered with the ion channel current in primary hippocampal neurons from rat.6,7 Moreover, ZnO NPs in association with the onset of neurological diseases was recently reported.8 In the case of SiO2 NPs, accumulations in liver and spleen causing necrosis have been observed.9 In addition, inhalation studies with SiO2 NPs showed penetration of NPs into brain through the nasal cavity, leading to accumulations in the striatum, causing oxidative damages and inflammatory responses.10 Hence, it is possible that the accumulation of these NPs in brain may reduce dopamine generation and protein downregulation of tyrosine hydroxylase, which can lead to Parkinson’s disease.
The blood–brain barrier (BBB) is an important physical barrier for controlling homeostasis and stopping the transmission of foreign substances into the central nervous system. Based on in vivo studies, NPs in blood may cause damage to the BBB and increase overall permeability, leading to BBB disruption and penetration of NPs, causing neurological diseases.11,12 Previously, damage to the BBB was measured using Evans blue dye (Sigma-Aldrich Co., St Louis, MO, USA) after injections of 50–60 nm silver (Ag), aluminum (Al), and copper (Cu) NPs via intravenous, intraperitoneal, or intracerebral routes.13 Evans blue dye is suitable for assessing BBB damage by chemicals or NPs and has high affinity with albumin, making the dye visible in the brain or its homogenates upon BBB disruption.
After titanium dioxide NPs were repeatedly injected intranasally for 30 days, they were observed in the hippocampus, leading to obvious morphological changes with increased expressions of glial fibrillary acidic protein.14 Recent studies also reported that Fe3O4 NPs (30 nm) could move directly from the olfactory nerve to the brain after intranasal administrations, where NPs could be found in the striatum and hippocampus.15 Previously, SiO2-coated magnetic NPs were found in the brain following intraperitoneal administration.16 In order to monitor the passage of NPs after intraperitoneal administration, PEGylated fluorescein-doped magnetic SiO2 NPs was moved through astrocytes of the BBB from vascular endothelial cells to cerebral parenchyma.17 In addition, 70 nm SiO2 NPs were observed in cerebral cortex and hippocampus after dermal exposure for 28 days.18 After exposure of NPs to rats for 6 hours in a whole-body exposure chamber, the concentration of NPs was significantly increased in the cerebrum and cerebellum, but the increase was transient for only 1 additional day of the post-exposure period.19 It seems that insoluble or poorly soluble NPs could enter the brain via the olfactory neuronal pathway and through the BBB.20 Hence, the above results suggest potential neurotoxicity of NPs and the cause of neurodegenerative diseases by the accumulation of NPs in the brain.
In previous studies, bound plasma proteins or protein corona onto both ZnO and SiO2 NPs were identified. Interestingly, apolipoprotein E was found to bind to NPs, and the interaction may help NPs to pass through the BBB as part of a complex. Hence, disruptions of BBB by ZnO and SiO2 NPs was investigated and evaluated by Evans blue after oral administrations of NPs for 28 days. In addition, immunohistochemistry (IHC) analyses were performed to confirm inflammations in specific regions of the brain after 90 days of transdermal and oral administrations NPs.
Materials and methods
Preparation of NPs
Different sizes of ZnO nanomaterials were purchased from Sumitomo Osaka Cement Co, Ltd, (lot no 141319 for 20 nm; Tokyo, Japan) and American Elements (lot no 1871511079-673 for 100 nm; Los Angeles, CA, USA). Modifications of purchased ZnO NPs were progressed to change the surface charge using coating reagents, citrate (for negative charge), and L-serine (for positive charge) as reported previously.21
SiO2 nanomaterials (20 and 100 nm) were obtained from E&B Nanotech Co, Ltd, (Ansan, Republic of Korea). L-arginine was used to increase the number of available protonated silanol groups on the surface of SiO2 NPs.22 The physicochemical properties were verified, including their average sizes and morphology and zeta potentials.21,22
Evaluation of BBB damage by NPs with Evans blue
Oral administration
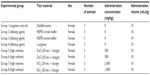
Rats (5 weeks old) were acclimatized for 1 week, and five rats of similar weight were selected for each group. Administration volume of NPs was determined by measuring the average weight of rats every week (Table 1). The dosages for each route of administration were determined from a 14-day repeated-dose study (Yu-Ri Kim, unpublished data, 2014). According to previous results, the maximum dose for causing significant systemic toxicity was selected for each group.
Evans blue procedure
After 28 days of repeated oral administrations, animals were anesthetized with isoflurane. Evans blue solution (2% solution 4 mL/kg) was injected via the tail vein and circulated for 1 hour. After the second anesthetization, animals were perfused with saline to remove the intravascular dye through the coronary artery, and paraformaldehyde (4%) was used to perfuse the brain. Brain was extracted, divided into left brain, right brain, and cerebellum, and the weight of each of these brain sections was measured. Each brain section was incubated with formamide at 55°C for 24 hours. The supernatant was separated after centrifugation at 12,000 g for 20 minutes. A spectrophotometer (VICTOR™ ×5; PerkinElmer, Waltham, MA, USA) was used to measure absorbance at 635 nm.
Evaluating inflammation responses after NPs
Intravenous administration of ZnO NPs by IHC analysis
All four types of ZnO NPs (positively/negatively charged 20 nm and 100 nm NPs) at 0.1, 10 mg/animal of 20 and 100 nm respectively, were injected intravenously into the tail of the animal for 90 days. After 90 days, animals were euthanized and whole brains were obtained immediately for IHC testing.
IHC
Left hemispheres of brain were immersed in 30% formaldehyde and fixation through tissue dehydration with ethyl alcohol and xylene. The tissues were sectioned to 6 mm thick using a microtome (Dako, RM2155 Microtome; Leica, Germany) and fixed on glass slides. First, deparaffinizing was performed. Next, endogenous peroxidase inhibitor was treated to each section and incubated for 30 minutes at room temperature. Biotin blocker was then added and incubated for 30 minutes at room temperature. The slides were immersed in Seablock (Thermo Fisher Scientific, Waltham, MA, USA), a blocking agent, for 30 minutes, and biotinylated NeuN antibody (EMD Millipore, Billerica, MA, USA) was used to treat the section for 1 hour as per the manufacturer’s recommendations (100 times dilution). The washing step was repeated two times with phosphate-buffered saline (PBS) for 5 minutes, and horseradish peroxidase-conjugated NeutrAvidin was incubated on the tissue sections at concentrations of 10 μg/mL in PBS. After following the same washing procedure, NeuN protein was visualized with diaminobenzidine plus chromogen for 5 minutes and rinsed with PBS three times prior to counterstaining. The sections were immersed in Mayer’s hematoxylin solution for 2 minutes and rinsed with tap water until the color of tissues turned blue. The sections were dehydrated in ethyl alcohol for mounting using Dako’s Mounting Medium. The tissues from the cerebellum regions were analyzed with a Nikon microscope (Eclipse TE2000-U; Nikon Corporation, Tokyo, Japan).
SiO2 NP accumulation in rat brain after repeated administration
SiO2EN100(R) and SiO2EN20(R) were orally administered to animals, and SiO2EN100(−) and SiO2EN20(−) were dermally administered to animals (Table 2). After animals were anesthetized by isoflurane, the blood in both blood vessel and the brain was removed by perfusing saline through coronary artery. Paraformaldehyde solution (1%) was injected into the coronary artery to perfuse the brain, and the extracted brain was washed in 4% paraformaldehyde. The brain was divided into hippocampus, striatum, and cerebellum using a razor blade to cut into the size of 1 mm3. Each region was stored in a vial containing 4% paraformaldehyde.
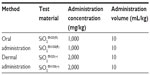  | Table 2 Administration concentrations and volumes for 90-day SiO2EN100(R) and SiO2EN20(R) oral administrations and SiO2EN100(−) and SiO2EN20(−) dermal administrations |
Small pieces of brain sample were dissected and washed three times with PBS. For postfixation, samples were placed in sodium cacodylate-buffered 1.5% osmium tetroxide for 60 minutes at 4°C before staining with the blocking agent uranyl acetate (0.5%). Brain samples were dehydrated via a series of ethanol concentrations and embedded in Epon resin (TAAB Laboratories Equipment Ltd, Aldermaston, UK). Uranyl acetate and lead citrate were used for staining ultrathin sections. An Hitachi electron microscope (H-7650; Hitachi Ltd, Tokyo, Japan) was used for the examination.
Results
Assessing damage to the BBB by NPs using Evans blue
Evans blue was dissolved in formamide, and then the solution was serially diluted to obtain a standard curve at a wavelength of 635 nm (Figure 1). The amount of Evans blue (μg) in the brain (g) was calculated from the optical density (OD) value of extracted brain using the formula from the standard curve (Figure 1).
  | Figure 1 Standard curve of Evans blue. |
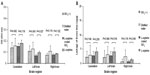
The brain extracted from rats treated with ZnO NPs was cut into right brain, left brain, and cerebellum. Then, Evans blue from each area of the brain was extracted with formamide solution, and measured OD at 635 nm wavelength. The amount of Evans blue was calculated using the stated formula according to the standard curve (Figure 1). Groups treated with NPs were measured higher OD values in comparison to the control group, but the standard deviations were high and the difference between positive and negative control samples was not statistically significant (Figure 2A). After the quantification from OD to the amount of Evans blue (μg)/g, all experimental groups showed the amount of extracted Evans blue from NPs treated rats (positive group) was higher than the negative control group. However the standard deviations were high and the difference between positive and negative control samples was not statistically significant. (Figure 2B).
The OD of extracted formamide solution from the brain of SiO2-treated animals was measured. The measured value of OD was used to calculate the amount of Evans blue (μg) in the brain (g) (Figure 3B). Significance was displayed with SiO2EN20(R)-treated group than the control group, but the value of the treated group was lower than that of the control group. Also, no significant difference was seen between negative control groups and NPs treated groups (Figure 3A). Similarly, there was no significant difference in the amount of Evans blue for all samples (Figure 3B).
IHC
The brains of animals were investigated by IHC method with NeuN-specific staining with Mayer’s hematoxylin counterstaining. In particular, cerebellums of the rats were examined for structural changes, and differences were compared to the normal control. Neuronal cells were stained specifically by using NeuN-specific antibody, and no morphological change was observed from ZnO NPs injected groups through animal’s tail vein in comparison to PBS-treated control group. No observable changes in brain slices were found in the rats treated with ZnO NPs of different sizes, surface charges, and concentrations (Figure 4).
Observation of accumulation of NPs in the brain of the repeatedly administered rats using transmission electron microscopy (TEM)
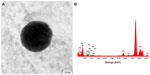
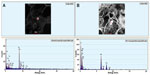
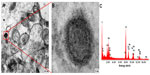
First, as a positive control test, SH-SY5Y neuroblastoma cells were incubated with SiO2 NPs (100 nm) in vitro, then NPs were observed using TEM (Figure 5A). The size of the NPs was confirmed to be 100 nm, and a silicon (Si) peak (1.7 KeV) was verified using an energy-dispersive X-ray spectroscope (EDS) (Figure 5B).
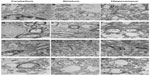
Each group treated with SiO2 NPs was monitored for the presence of NPs in cerebellum, hippocampus, and striatum using TEM after dermal administration of SiO2EN20(−) and SiO2EN100(−) and oral administration of SiO2EN20(R) and SiO2EN100(R). After 90 days of repeated administrations, the brain was extracted and cut to 1 mm2 fragments and transported to the Korea Institute of Science and Technology (Seoul, Republic of Korea) for TEM analyses. Cerebellum, hippocampus, and striatum were investigated thoroughly, since the accumulation of NPs was expected to occur in those brain areas (Figure 6). Nano-size dots were observed in each brain region, and EDS analysis was performed to confirm the presence or absence of SiO2 NPs. According to EDS analysis, the Si peak was absent and only osmium and lead (dye materials) and carbon and Cu (grid materials) peaks were present, belonging to the pretreatment chemicals (Figure 7A). In addition, a suspected iron peak was observed, which could have been from the blood vessel in the brain (Figure 7B). In SiO2EN100(−) group, suspected nano-size dot was analyzed to conform silica NPs. However no silica peak appeared in the EDS graph (Figure 8).
Discussion
Recently, the composition of protein corona on the surface of ZnO or SiO2 NPs from incubation with plasma and brain homogenates was analyzed. Interestingly, apolipoproteins were found in abundance, and other proteins involved in inflammation and complement activations were also detected. Apolipoprotein E was also present, which is known to mediate the passing of NPs across the BBB and to be involved in Alzheimer’s disease.23,24
Previous studies with rat animal models reported that Ag, Cu, and Al NPs induced BBB damages and disruptions and brain edema formation after repeated exposure by intravenous, intraperitoneal, and intracerebral administrations.13 In the current study, Evans blue analysis was performed to investigate BBB disruption from repeated oral administrations of ZnO and SiO2 NPs for 28 days. Unlike previous results with Ag, Cu, and Al NPs,13 a significant difference was not observed in the brains of rats treated with ZnO or SiO2 NPs in comparison with the normal control group. This result suggests that the repeated oral administration of ZnO or SiO2 NPs for 28 days did not induce BBB damage. In the case of oral administration, only a small percentage of NPs seemed to be absorbed through intestine.25,26 Hence, BBB damage may not have been induced because the small amount of penetrated NPs from intestine to the blood vessel could have been cleared away by liver and kidney.
Several studies have reported that insoluble or poorly soluble NPs could enter the brain via the olfactory neuronal pathway and through the BBB.20 Consequently, BBB-penetrated NPs could cause several cellular and molecular events, such as oxidative and heat stresses.10,27,28 The exposure to NP-rich diesel exhausts has been found to upregulate messenger (m)RNA levels of proinflammatory cytokines and N-methyl-D-aspartate receptor subunits in the hippocampus.29 However, the exposure of the lactoferrin-conjugated polyethylene glycol-polylactide-polyglycolide NPs showed no inflammatory reaction in the brain.30 In our study, no morphological change was found in the cerebellum after ZnO NP injections through the tail vein. However, further investigations should be performed in order to fully rule out the potential for neurotoxicity with long-term exposures.
Dermal and oral administrations of SiO2 NPs were performed for 90 days to confirm whether NPs were deposited in the brain upon penetrating the BBB, and the specific areas of the brain (hippocampus, striatum, and cerebellum), were performed by the analysis as per other reports.15,16,18 After 90 days of administrations, deposition of NPs was not found in any region of the brain. Nano-size dots were analyzed by EDS to know whether it is the aggregated dye materials. Based on other reports of intranasal administration of NPs, they were found mostly in the hippocampus, striatum, and cerebellum.1,10,15,16 After intranasal exposure, NPs can be directly transported from the nose to the brain via the olfactory nerve.20 However, NPs administered dermally or orally should penetrate skin or intestine wall, respectively, and NPs also need to penetrate the BBB to reach the brain. Hence, it was suggested that NPs in blood may not reach the brain after dermal and oral administrations. In previous studies, NPs were deposited mainly in lung, liver, spleen, and kidney after administrations.9,14,19 The majority of SiO2 NPs were excreted via feces, and 7%–8% of particles were excreted via urine (Jeong-A Lee, unpublished, 2014). Additionally, positron emission tomography analysis of SiO2 NPs revealed that they had not accumulated significantly in the brain, indicating that NPs may not have penetrated the BBB and did not deposit in the brain (Chang-Moon Lee, unpublished, 2014).
Conclusion
According to the preliminary data, oral administration of ZnO and SiO2 NPs for 90 days did not compromise or damage the BBB. Intravenous injections of ZnO NPs showed no toxic effect in the brain, and exposure to SiO2 NPs by dermal and oral administrations over the same period showed a complete absence of silica particulates in all of the samples analyzed by TEM. These results suggest that oral and dermal administrations of ZnO and SiO2 NPs do not induce significant neurotoxic effects in the brain.
Acknowledgments
This study was supported by a grant (10182MFDS991) from the Korean Ministry of Food and Drug Safety in 2012 and by a grant (NRF-2012R1A2A2A03046819) from the Korean National Research Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
Oszlánczi G, Papp A, Szabó A, et al. Nervous system effects in rats on subacute exposure by lead-containing nanoparticles via the airways. Inhal Toxicol. 2011;23(4):173–181. | |
Lee S, Pie JE, Kim YR, Lee H, Son SW, Kim MK. Effects of zinc oxide nanoparticles on gene expression profile in human keratinocytes. Molecular and Cellular Toxicology. 2012;8(2):113–118. | |
Jang YS, Lee EY, Park YH, et al. The potential for skin irritation, phototoxicity, and sensitization of ZnO nanoparticles. Molecular and Cellular Toxicology. 2012;8(2):171–177. | |
Park YH, Bae HC, Jang Y, et al. Effect of the size and surface charge of silica nanoparticles on cutaneous toxicity. Molecular and Cellular Toxicology. 2013;9(1):67–74. | |
Musa M, Kannan TP, Masudi SM, Rahman IA. Assessment of DNA damage caused by locally produced hydroxyapatite-silica nanocomposite using Comet assay on human lung fibroblast cell line. Molecular and Cellular Toxicology. 2012;8(1):53–60. | |
Deng X, Luan Q, Chen W, et al. Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology. 2009;20(11):115101. | |
Zhao J, Xu L, Zhang T, Ren G, Yang Z. Influences of nanoparticle zinc oxide on acutely isolated rat hippocampal CA3 pyramidal neurons. Neurotoxicology. 2009;30(2):220–230. | |
Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6(6):449–462. | |
Xie G, Sun J, Zhong G, Shi L, Zhang D. Biodistribution and toxicity of intravenously administered silica nanoparticles in mice. Arch Toxicol. 2010;84(3):183–190. | |
Wu J, Wang C, Sun J, Xue Y. Neurotoxicity of silica nanoparticles: brain localization and dopaminergic neurons damage pathways. ACS Nano. 2011;5(6):4476–4489. | |
Langford D, Masliah E. Crosstalk between components of the blood brain barrier and cells of the CNS in microglial activation in AIDS. Brain Pathol. 2001;11(3):306–312. | |
Beaumont A, Marmarou A, Fatouros P, Corwin F. Secondary insults worsen blood brain barrier dysfunction assessed by MRI in cerebral contusion. Acta Neurochir Suppl. 2002;81:217–219. | |
Sharma HS, Hussain S, Schlager J, Ali SF, Sharma A. Influence of nanoparticles on blood-brain barrier permeability and brain edema formation in rats. Acta Neurochir Suppl. 2010;106:359–364. | |
Wang J, Chen C, Liu Y, et al. Potential neurological lesion after nasal instillation of TiO(2) nanoparticles in the anatase and rutile crystal phases. Toxicol Lett. 2008;183(1–3):72–80. | |
Wu J, Ding T, Sun J. Neurotoxic potential of iron oxide nanoparticles in the rat brain striatum and hippocampus. Neurotoxicology. 2013;34:243–253. | |
Kim JS, Yoon TJ, Yu KN, et al. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol Sci. 2006;89(1):338–347. | |
Ku S, Yan F, Wang Y, Sun Y, Yang N, Ye L. The blood-brain barrier penetration and distribution of PEGylated fluorescein-doped magnetic silica nanoparticles in rat brain. Biochem Biophys Res Commun. 2010;394(4):871–876. | |
Nabeshi H, Yoshikawa T, Matsuyama K, et al. Systemic distribution, nuclear entry and cytotoxicity of amorphous nanosilica following topical application. Biomaterials. 2011;32(11):2713–2724. | |
Oberdörster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16(6–7):437–445. | |
Win-Shwe TT, Fujimaki H. Nanoparticles and neurotoxicity. Int J Mol Sci. 2011;12(9):6267–6280. | |
Kim KM, Kim TH, Kim HM, et al. Colloidal behaviors of ZnO nanoparticles in various aqueous media. Toxicol Environ Health Sci. 2012;4(2):121–131. | |
Kim KM, Kim HM, Choi MH, et al. Colloidal properties of surface coated colloidal silica nanoparticles in aqueous and physiological solutions. Sci Adv Mat. 2014;6(7):1573–1581. | |
Kreuter J, Shamenkov D, Petrov V, et al. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target. 2002;10(4):317–325. | |
Veronesi B, Makwana O, Pooler M, Chen LC. Effects of subchronic exposures to concentrated ambient particles. VII. Degeneration of dopaminergic neurons in Apo E-/- mice. Inhal Toxicol. 2005;17(4–5):235–241. | |
Jani P, Halbert GW, Langridge J, Florence AT. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol. 1990;42(12):821–826. | |
Schleh C, Semmler-Behnke M, Lipka J, et al. Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology. 2012;6(1):36–46. | |
Rogers EJ, Bello D, Hsieh S. Oxidative stress as a screening metric of potential toxicity by nanoparticles and ariborne [corrected] particulate matter. Inhal Toxicol. 2008;20(9):895. | |
Sharma HS, Sharma A. Nanoparticles aggravate heat stress induced cognitive deficits, blood-brain barrier disruption, edema formation and brain pathology. Prog Brain Res. 2007;162:245–273. | |
Win-Shwe TT, Yamamoto S, Fujitani Y, Hirano S, Fujimaki H. Spatial learning and memory function-related gene expression in the hippocampus of mouse exposed to nanoparticle-rich diesel exhaust. Neurotoxicology. 2008;29(6):940–947. | |
Hu K, Shi Y, Jiang W, Han J, Huang S, Jiang X. Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: preparation, characterization and efficacy in Parkinson’s disease. Int J Pharm. 2011;415(1–2):273–283. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.