Back to Journals » Infection and Drug Resistance » Volume 16
Assessment of the Risk and Symptoms of SARS-CoV-2 Infection Among Healthcare Workers During the Omicron Transmission Period: A Multicentric Study from Four Hospitals of Mainland China
Authors Chen S, Chen M, Chen Q, Zhang T, Xu B, Tung TH , Shen B , Wu X
Received 22 March 2023
Accepted for publication 17 May 2023
Published 29 May 2023 Volume 2023:16 Pages 3315—3328
DOI https://doi.org/10.2147/IDR.S412657
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Shuaishuai Chen,1,2,* Mengyuan Chen,1,2,* Qiaoming Chen,3 Tongtong Zhang,4 Bing Xu,5 Tao Hsin Tung,6 Bo Shen,1,2 Xiaomai Wu7
1Department of Clinical Laboratory, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai, People’s Republic of China; 2Key Laboratory of System Medicine and Precision Diagnosis and Treatment of Taizhou, Taizhou, People’s Republic of China; 3Department of Prevention and Health Care, Taizhou Enze Medical Center (Group) Enze Hospital, Taizhou, People’s Republic of China; 4Department of Prevention and Health Care, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai, People’s Republic of China; 5Department of Human Resources, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai, People’s Republic of China; 6Evidence-Based Medicine Center, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai, People’s Republic of China; 7Department of Respiratory and Critical Medicine, Taizhou Enze Medical Center (Group) Enze Hospital, Taizhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bo Shen, Taizhou Hospital of Zhejiang Province affiliated to Wenzhou Medical University, 150 Ximen Street of Linhai City, Linhai, 317000, People’s Republic of China, Email [email protected] Xiaomai Wu, Taizhou Enze Medical Center (Group) Enze Hospital, 1 East Tongyang Road of Luqiao, Taizhou, 318000, People’s Republic of China, Email [email protected]
Purpose: The SARS-CoV-2 omicron variant emerged and spread rapidly among the population in the early stage of China’s normalized prevention and control in December 2022. Healthcare workers (HCWs) are particularly exposed to SARS-CoV-2, it is important to evaluate the impact of the omicron pandemic on HCWs in China.
Methods: A self-administered online survey was conducted on infected HCWs from four hospitals of Taizhou. A total of 748 HCWs received the survey via DingTalk, and 328 responded to the questionnaire. The risk factors were investigated using univariate and multivariate logistic regression analysis.
Results: By December 20, 2022, 748 HCWs tested positive by PCR, and the infection rate was 11.4% (748/6581). Among 328 respondents, the most common symptoms were cough (88.4%), fever (83.5%), runny nose (77.1%), sore throat (73.2%), headache (70.1%), muscle aches (67.1%), and fatigue (53.4%). 69.8% (229/328) of the participants had five or more major onset symptoms, while no severe case was observed. The multivariate analysis indicated that the poor sleep quality (OR = 2.29, 95% CI: 1.31– 4.02, P = 0.004) was an independent risk factor for more major onset symptoms, while wore gloves ≥ 95% times in working (OR = 0.49, 95% CI: 0.28– 0.85, P = 0.011) was significantly related to fewer symptoms. In addition, 239 (72.9%) recipients reported high fever (temperature ≥ 38.5°C), less common cold (≤ 3 vs > 3 times/year, OR = 2.20, 95% CI: 1.05– 4.65, P = 0.038) was significantly associated with high fever.
Conclusion: Our findings imply rapid transmissibility of omicron and multiple-onset symptoms among HCWs. Improved autoimmunity and self-protection measures for HCWs may be helpful in controlling infection and clinical symptoms. Our results provide empirical reference values for improved countermeasures and protective measures for major public health emergencies.
Keywords: SARS-CoV-2 omicron, healthcare workers, multiple onset symptoms, poor sleep quality, China
Introduction
SARS-CoV-2 have caused global pandemics in the recent 3 years, contributing to 657.98 million infections and 6.68 million deaths by January 7, 2023.1 Devastating epidemics pose great threats to human health, especially the older adult population.2 As China has a huge population of over 1.4 billion,3 it faces much more serious situations regarding COVID-19 prevention and control than the majority of other countries and regions in the world. Since SARS-CoV-2 WA1 wave started in China in late December 2019, the Chinese government has promulgated and implemented a series of laws and regulations, scheduled a series of policies, and built shelter hospitals and urban nucleic acid bases to prevent and control epidemics. Different SARS-CoV-2 variants show different virulence, transmissibility, and immune escape abilities, leading to different prevalence outcomes.4,5 Based on the SARS-CoV-2 genotype or sub-genotype circulation and epidemic scale, COVID-19 prevention and control policies in China have been adjusted scientifically and precisely.
Vaccines are considered effective and safe for the prevention and control of SARS-CoV-2.6,7 The mass vaccination against SARS-CoV-2 was advanced from December 2020 in China. Participants in vaccination campaigns for COVID-19 in China provided informed consent and were voluntary. Currently, several types of vaccines against SARS-CoV-2 have been approved in China, including inactivated, adenovirus vector, and recombinant protein vaccines. Inactivated vaccines, including Sinovac’s CoronaVac and Sinopharm’s CoronaVac, are the most extensively used vaccines in China and included in the WHO emergency use listing.8 Until early 2023, the total coverage rates of the first-dose and full-course vaccination for the entire population of China have reached 93.0% and 90.6%, respectively.
The SARS-CoV-2 omicron variant, which has spread worldwide since December 2021, has become the main pathogen in the general population,9,10 and also became the predominant pathogen in China since January 2022. The main epidemic strains are BA.5.2 and BF.7 and their subbranches, since the adjustment of prevention and control policies in China. As omicron variants have relatively low virulence but higher transmissibility, China unveiled optimized policies one after another in late 2022 to guide the Easing Covid Policy step-by-step. In combination with the low pathogenicity, low morbidity and mortality, high vaccination coverage, and accumulated experience in prevention and control, China has adjusted COVID-19 from “Class B infectious disease and managed as Class A infectious disease” to “Class B Infectious Disease and managed as Class B Infectious Disease” in early January 202311 (Figure 1).
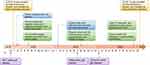 |
Figure 1 China government implemented science-based, precise, and flexible policies and laws, to prevent and control SARS-CoV-2 infections. |
Optimized Easing COVID Policies on Omicron Variants may lead to larger omicron waves. Although omicron waves have been reported in several countries and regions,12,13 the epidemiological situation and disease-associated symptoms and factors in China remain unclear. Furthermore, the majority of Chinese were not previously infected with SARS-CoV-2, the situations were totally different from any other countries and regions in the world, and studies on omicron waves in Chinese populations are urgently needed. As health-care workers (HCWs) are regularly screened for omicron infections and are exposed to high biological risks, studying the impact of omicron variants among HCWs can provide more professional and scientific data. HCWs were routinely screened for SARS-CoV-2 infection via real-time PCR analysis. HCWs underwent a twice-a-week testing schedule for SARS-CoV-2, while those operating in high-risk clinical areas such as fever clinics and designated medical institutions that centrally manage COVID-19 patients were screened daily for SARS-CoV-2. Upon entering the peak period of the omicron variant infection, medical demands will increase sharply, bringing extraordinary pressure on medical systems as well as high risks of infection for HCWs. However, the epidemiological characteristics of the SARS-CoV-2 Omicron variants among HCWs under such emergency status in China remain unclear.
In this first multicenter study of omicron infections in HCWs in mainland China under the normalization of prevention and control policy, we aimed to characterize the epidemiological and clinical features of omicron-infected HCWs and explore the risk factors associated with increased clinical symptoms and high fever from late November 2022 to December 20, 2022. Our findings contribute to provide scientific data for hospital policymakers in well-organized management and arrangement and present the impact of the omicron wave on populations from HCWs’ professional insights under emergency situations.
Materials and Methods
Study Design and Participants
This multicenter study included four hospitals in Taizhou: Taizhou Hospital, Enze Hospital, Taizhou Rehabilitation Hospital, and Enze Maternity Hospital. The inclusion criteria were HCWs who tested positive using the real-time reverse-transcription-polymerase chain reaction (RT-PCR) assay for SARS-CoV-2, consented to participate in the study and were able to complete the questionnaire online (Figure 2). The participants included clinicians (physicians, surgeons, physical/respiratory therapists, and those who had direct contact with patients), nurses, medical technicians, and administrative services, such as security staff and logistic services. The study was approved by the local ethics committee of each hospital, and all procedures were performed in accordance with the tenets of the Declaration of Helsinki.
 |
Figure 2 Flowchart of enrolled healthcare workers with omicron infection. |
The main epidemic variants of SARS-COV-2 virus were BA.5.2 and BF.7 in Taizhou, which was sampled for genomic sequencing by the Taizhou Centers for Disease Control and Prevention. In this study, the two-dose primary immunization and first booster dose of HCWs were Sinovac’s CoronaVac inactivated vaccine. Two doses of basic immunity with an interval of 2–4 weeks, and the third dose (first booster dose) was given 6 months after the second dose. A portion of HCWs received the fourth dose (second booster) of a recombinant protein vaccine or adenovirus vector vaccine against COVID-19. Occupational infections were defined as those in close contact with confirmed COVID-19 cases of colleagues or patients at work, in absence of a positive household member. Non-occupational infections were defined as those that had not contacted with confirmed patients or colleagues.
Questionnaires
A self-administered online questionnaire, referred to the WHO/2019-nCoV/HCW Surveillance Protocol/2020.1, modified according to the actual situation, was designed using the Wen-Juan-Xing platform (Changsha Ranxing Information Technology Co., Ltd., Hunan, China), and a Quick Response (QR) code was generated. A total of 748 HCWs with omicron infections in hospitals from November 18 to December 20, 2022, were invited to participate in the survey through DingTalk, one of the most commonly used chat tools in China. The respondents answered the questionnaire through scanning the Quick Response (QR) code between December 22 and 24, 2022. Age, sex, height, weight, education level, job tasks, preexisting basic diseases, physical condition, ventilation in the working environment, personal and protective equipment (PPE), vaccine dose, and symptoms were assessed. Physical exercise habits were defined as regular exercise at least three times a week for more than 30 min.
Statistical Analysis
All data were analyzed using SPSS Statistics software (version 23.0; IBM Corp., Armonk, N.Y., USA). Categorical variables were analyzed using the chi-square test and displayed as frequencies and percentages. The seven major onset symptoms include cough, fever, runny nose, sore throat, headache, muscle aches, and fatigue. The participants were separated into ≥5 major onset symptoms group and <5 major onset symptoms group, according to the symptoms, and divided into two groups based on high fever or not (≥38.5°C vs <38.5°C). The population size was calculated by Power Analysis and Sample Size Software (PASS) version 11, the minimum sample size achieved a power of 80%, and under 5% significance level, using a 2-sided hypothesis test. Potential factors associated with the multiplicity of symptom onset or high fever were assessed using univariate analyses. Variables with P < 0.2 in the univariate analysis were included in the logistic regression model and analyzed using the stepwise regression method. A P value < 0.05 was considered statistically significant.
Results
The Transmissibility of Omicron Among Healthcare Workers
The epidemiological characteristics of HCWs infected with the SARS-CoV-2 omicron variant are shown in Figure 3A. A total of 748 HCWs were infected between November 8 and December 20, 2022, in the centers. Thirteen cases were infected in November, and five cases were infected from December 1 to 8, 2022. Since December 9, the number of infections among HCWs increased daily.
Daily infection rates in the four hospitals are shown in Figure 3B. The cumulative incidence rate was 12.0% (488/4077), 10.6% (218/2064), 8.5% (31/363), and 14.3% (11/77) at Taizhou Hospital, Enze Hospital, Taizhou Rehabilitation Hospital, and Enze Maternity Hospital, respectively. In addition, we analyzed the omicron infections for HCWs in different job tasks, as shown in Figure 3C, the infection rates of nurses, clinicians, medical technicians and other job tasks were 13.9%, 11.8%, 7.6%, and 5.5%, respectively. Our results demonstrate that the risk of infection varies by job tasks; nurses and clinicians have an increased risk of infection compared with medical technicians and other position roles, as they have a higher frequency and duration of direct contact with patients.
Characteristics of the Study Population
A total of 328 of the 748 eligible HCWs participated in the survey and completed the questionnaire; the overall response rate was 43.9%. As shown in Table 1, female had a high response rate than male (45.9% vs 35.0%), <40 years aged group had high response rate than ≥40 years aged group (45.6% vs 38.7%). The proportions of clinicians, nurses, medical technicians, and HCWs with other infections were 22.2% (166/748), 48.1% (360/748), 9.7% (73/748), and 20.0% (149/748), respectively. We then compared the demographic characteristics of respondents and non-respondents among HCWs with omicron infection (Table 2). Demographic characteristics, such as age and level of education in respondents, were similar to those in non-respondents, whereas gender and job tasks between the two groups were significantly different (P < 0.05).
 |
Table 1 The Response Rate of the Omicron Infection Survey Among Healthcare Workers |
 |
Table 2 Comparisons of Demographic Characteristics Between Respondents and Non-Respondents of the Survey Among Healthcare Workers Infected with Omicron |
The baseline characteristics of participants are displayed in Table 3. The participants consisted of 278 female (84.8%) and 50 male (15.2%), their mean age was 32.9±8.7 years, and mean BMI was 22.2±3.0 kg/m2. A total of 37 (11.3%) participants had preexisting basic diseases, including hypertension, diabetes, and autoimmune diseases. A total of 97 (29.6%) participants received four vaccine doses, and all were vaccinated within 14 days. Moreover, the results showed that the percentage of HCWs immunized with either 1, 2, or 3 doses was 0.9% (3/328), 7.9% (26/328), and 60.1% (197/328), respectively. More than half of the participants (183, 55.8%) experienced fatigue before infection, and 32.3% had poor sleep. Moreover, 103 (31.4%) of participants sleep less than 7 h per day, and 14.0% keep exercise habit (≥3 times/week) in their daily life. After infection, 229 (69.8%) participants appeared to have five or more major onset symptoms. A total of 239 (72.9%) participants had temperatures higher than 38.5°C body temperature, and 244 (74.4%) of the participants experienced fever for ≥2 days. Only 139 participants (42.4%) worked in a ventilated environment. A questionnaire on personal and protective equipment (PPE) showed that 234 (71.3%) wore surgical masks, and 92 (28.0%) wore medical protective masks. A total of 294 (89.6%) participants wore masks in the work environment ≥95% of the time, whereas only 91 (27.7%) participants wore masks outside the work environment for ≥95% of the time. Moreover, 78 (23.8%) participants wore gloves, and 39 (11.9%) wore face shields in their work environment ≥95% of the time.
 |
Table 3 The Baseline Characteristics of the Participants |
Factors Associated with Major Onset Symptoms
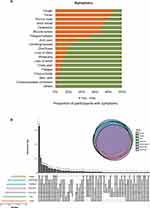
The most common symptoms were cough (290, 88.4%), fever (274, 83.5%), runny nose (253, 77.1%), sore throat (240, 73.2%), headache (230, 70.1%), muscle aches (220, 67.1%), and fatigue (175, 53.4%) (Figure 4A). Only 6 (1.8%) participants reported no symptoms. We found that 25.3% (83/328) overlapped with seven major onset symptoms and 24.7% (81/328) overlapped with six major onset symptoms (Figure 4B); however, no severe infection was observed among HCWs in this study.
In our survey, 229 participants reported five or more major onset symptoms and 99 participants reported <5 major onset symptoms; univariate analysis showed that fatigue, poor sleep quality before infection, and wore gloves ≥95% times were factors affecting multiple-onset symptoms after infection (P < 0.05). We included the index of P < 0.2 in the univariate analysis into the regression analysis and analyzed using stepwise multiple logistic regression. As shown in Table 4, poor sleep quality (yes vs no, OR = 2.29, 95% CI: 1.31–4.02, P = 0.004) was significantly related to multiple major onset symptoms of omicron infection, and wore gloves ≥95% times when working (yes vs no, OR = 0.49, 95% CI: 0.28–0.85, P = 0.011) was significantly related to less major onset symptoms. In addition, we separated the participants into two groups according to high fever (≥38.5°C) or not (<38.5°), multivariate analysis showed that those who had less common cold were more likely to have high fever (≤3 vs >3 times/year, OR = 2.20, 95% CI: 1.05–4.65, P = 0.038), as shown in Table 5.
 |
Table 4 Factors Associated with Major Symptoms by Univariate and Stepwise Multiple Logistic Regression |
 |
Table 5 Factors Associated with High Fever by Univariate and Stepwise Multiple Logistic Regression |
Occupational versus Non-Occupational Acquired by HCWs
The distribution of occupational and non-occupational infections acquired by HCWs were estimated by t-test or chi-square test and the results are shown in Table 6. The mean age of HCWs between occupational and non-occupational infections has no difference (32.8 ± 8.0 vs 32.9 ± 9.0, P = 0.896). The rate of occupational infections (107/328) was particularly lower than non-occupational infections (221/328). Considering job tasks, nurses had the highest proportion of both occupational and non-occupational SARS-CoV-2 infection. Other job task such as administrative services, those not contacted with patients, has lower rate of occupational infections than non-occupational infections.
 |
Table 6 Comparisons of SARS-CoV-2 Infections of Healthcare Workers Between Occupational and Non-Occupational Infections |
Recommendation of Healthcare Workers
We also collected suggestions from HCWs on individual protection and personal life against omicron infection, and the results are shown in Figure S1. They provide advice on individual protection and personal life habits, with most suggestions including wearing masks correctly, strengthening self-protection consciousness, maintaining good hand hygiene, and social distancing. A total of 40.3% of clinicians, 34.9% of nurses, 47.3% of medical technicians, and 21.4% of administrative staff correctly recommended wearing masks. In total, 24.2% of clinicians, 20.1% of nurses, 25.5% of medical technicians, and 21.4% of administration staff recommended strengthening self-protection consciousness. Interestingly, administration staff may provide more consideration to personal lifestyle habits than clinical workers.
Discussion
This study was a multicenter investigation of SARS-CoV-2 omicron infection among HCWs during its emergence in China. Our study aimed to describe the symptoms and epidemic characteristics of the acute outbreak of omicron among HCWs in Taizhou, to provide a reference for the public and HCWs, and also provide an evidence-based basis for the prevention and control of epidemics. The main epidemic variants of the SARS-COV-2 virus were BA.5.2 and BF.7 in this study, which were sampled for genomic sequencing by the Taizhou Centers for Disease Control and Prevention. In this study, we observed the rapid transmissibility of omicrons and multiple onsets of symptoms after infection among HCWs. Clinical frontline HCWs had an increased risk of infection compared with those in other job tasks. Poor sleep quality and wearing gloves <95% times in working were associated with multiple major onset symptoms of omicron infection. Fever is a common symptom of SARS-COV-2 infection, and those with ≤3 times of common cold episodes per year are more likely to have high fever after omicron infection.
The SARS-CoV-2 omicron variant has spread rapidly worldwide and become the dominant variant globally.14 Omicron in Europe had a negligible impact on human health in terms of morbidity and mortality in general,9,15 and so in China. In China, the proportion of asymptomatic infections and mild cases of infected individuals exceeds 90%, with extremely low severity and mortality rates, may be due to the low pathogenicity, high vaccination coverage, and accumulated experience in prevention and control. The high transmissibility of the omicron variants is associated with several factors. The genome of the omicron variant showed more than 30 mutations in the spike protein compared with the original sequence, which may increase transmissibility by affecting binding affinity and evading the immune response.16 The present study showed that the transmissibility of omicron among HCWs was rapid, and the number of infections increased daily, which was consistent with previous findings.
Since the beginning of the pandemic, HCWs begun to pay attention to personal protective measures. Our results showed that the rate of occupational infections was particularly lower than non-occupational infections, consistent with a recent study on HCWs in Italy, during the omicron transmission period, the proportion of occupational infections was relatively low as compared to infections acquired by same HCWs outside hospital.10 Several previous studies have shown that the infection risk of clinical tasks involving direct or close contact with COVID-19 patients is higher than administrative services.9,10,15 Our results demonstrated that frontline HCWs are at a high risk of omicron infection, nurses and clinicians have an increased risk of infection compared with medical technicians and other job tasks, as they have a higher frequency and duration of direct contact with patients, consistent with previous research. The proportion of infections among HCWs reported in this survey was consistent with previous reports.17
In addition to the respiratory tract, SARS-CoV-2 affects various organs, causing myocardial dysfunction, gastrointestinal symptoms, dermatological complications, and liver and kidney impairment.18 Hui et al reported that omicron replicates faster in the bronchus but has a lower replication efficiency in the lung parenchyma than other SARS-CoV-2 variants.19 Our results showed that the most common symptoms of omicron infection were cough, fever, runny nose, sore throat, headache, muscle aches, and fatigue, mainly expressed as upper respiratory tract infection. No severe pulmonary infection was observed among HCWs at the deadline of our study, mainly because of the high vaccination rate of HCWs. More than two-thirds of the participants had an overlap of five or more onset symptoms. A study of UK participants with positive symptomatic PCR or antigen test results for SARS-CoV-2 showed that the most frequently reported symptoms among omicron infections were runny nose, headache, sore throat, sneezing, and persistent cough, which are similar to our results.20
Interestingly, poor sleep quality led to increased symptoms after infection. Several studies have shown that sleep is important in maintaining the stability of the immune system and health of the population.21 Sleep deprivation has been shown to alter the immune system and impair the immune response, possibly causing immune dysregulation, inflammation, and facilitating viral infection.22,23 Previous studies have shown that sleep deprivation, poor sleep quality, and high fatigue are possible risk factors for SARS-CoV-2 infection and are related to negative outcomes.24,25 Sleep disorders are common among clinical frontline staff because of their extended and/or night shift work, which may facilitate SARS-CoV-2 infection and aggravate symptoms during infection.26,27 In this study, approximately one-third of the participants slept for less than 7 h per day or had poor sleep quality, and long-term sleep deficiency may lead to immune dysregulation. Alleviating long-term fatigue and sleep deficiency is important through adjustment of the shift arrangements and working states to maintain optimal immune function and prevent repeated infections and severe symptoms.
Moreover, our results have an interesting finding that those who had common cold ≤3 times per year were more likely to have a high fever after omicron infection. Although the common cold is a relatively mild, self-limiting illness, immunocompromised individuals are more susceptible to infections caused by common cold viruses.28 As fever is the predominant thermoregulatory response to infection and inflammatory diseases, high body temperatures enhance the effectiveness of systemic immune responses during infection via stimulating both innate and adaptive immunity.29
Interestingly, the vaccination status was unrelated to symptom diversity. The neutralizing antibody titer of the vaccine immunization gradually decreased after 6 months. Several studies have demonstrated that the omicron variant escapes most monoclonal antibodies and is neutralized by antibodies generated by a stronger vaccine dose.4,30 As three doses of vaccine are enough to be protected against symptomatic disease, and the fourth-dose vaccination of the participants was within 14 days in this study, which may be the reason the vaccination dose was not related to multiple onset symptoms.
However, this study had several limitations. First, the sample included a larger proportion of female and less of male, which may have resulted in sampling bias. Second, the response rate was low, and the structure of gender and positions was inconsistent, which may have resulted in a bias in sample representativeness. Finally, as we included infected HCWs at the initial stage of the epidemic, the sample size was small, and the short duration of the survey may have resulted in a bias in some onset symptoms. However, these results require further large-scale external verification.
Conclusion
The SARS-CoV-2 epidemic is ongoing globally and remains a challenge for the public. This study analyzed the infections and symptoms in HCWs during the first wave of the epidemic after the normalization of prevention and control measures in China. The present findings imply rapid transmissibility of omicron and multiple-onset symptoms after omicron infection among HCWs in mainland China. Clinical frontline workers have an increased risk of infection compared with other job tasks such as administrative services. Sleep disorders are common among HCWs, poor sleep quality is a risk factor associated with more onset symptoms, and the common cold ≤3 times/year is a risk factor for high fever after infection. Protective measures, such as ensuring an adequate supply of personal protective measures, adequate rest and sleep time, improving sleep quality, and alleviating fatigue, are of great significance in protecting HCWs from infection and alleviating symptoms, thus helping maintain the hospital’s treatment capacity. Improved autoimmunity and self-protection measures for HCWs at high biological risk may be helpful in controlling COVID-19 infection and clinical symptoms. This study also provided empirical reference values for countermeasures and improved protective measures for the public during major public health emergencies.
Ethics Approval
The study was approved by the ethics committee of Taizhou Hospital of Zhejiang province.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. WHO coronavirus (COVID-19) dashboard; 2023. Available from: https://covid19.who.int/.
2. O’Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590(7844):140–145. doi:10.1038/s41586-020-2918-0
3. China National Bureau of Statistics. Interpretation for the 7th census of China; 2021. Available from: http://www.stats.gov.cn/xxgk/jd/sjjd2020/202105/t20210512_1817342.html.
4. Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602(7898):657–663. doi:10.1038/s41586-021-04385-3
5. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. doi:10.1016/S0140-6736(22)00462-7
6. Su S, Du L, Jiang S. Learning from the past: development of safe and effective COVID-19 vaccines. Nat Rev Microbiol. 2021;19(3):211–219. doi:10.1038/s41579-020-00462-y
7. Zeng G, Wu Q, Pan H, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled Phase 2 clinical trials. Lancet Infect Dis. 2022;22(4):483–495. doi:10.1016/S1473-3099(21)00681-2
8. Huang Z, Xu S, Liu J, et al. Effectiveness of inactivated COVID-19 vaccines among older adults in Shanghai: retrospective cohort study. Nat Commun. 2023;14(1). doi:10.1038/s41467-023-37673-9
9. Cegolon L, Negro C, Mastrangelo G, Filon FL; and group O.w. Primary SARS-CoV-2 infections, re-infections and vaccine effectiveness during the omicron transmission period in healthcare workers of Trieste and Gorizia (Northeast Italy). Viruses. 2021;14(12). doi:10.3390/v14122688
10. Cegolon L, Ronchese F, Ricci F, Negro C, Larese-Filon F. SARS-CoV-2 infection in health care workers of Trieste (North-Eastern Italy), 2022: occupational risk and the impact of the Omicron Variant. Viruses. 2022;14(8):1663. doi:10.3390/v14081663
11. National health commission of the People’s Republic of China, policies on COVID-19 prevention and control. Available from: http://www.nhc.gov.cn/xcs/zhengcwj/list_gzbd.shtml.
12. Tegally H, Moir M, Everatt J, et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat Med. 2022;28(9):1785–1790. doi:10.1038/s41591-022-01911-2
13. Wang Q, Iketani S, Li Z, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186(2):279–286 e278. doi:10.1016/j.cell.2022.12.018
14. Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in Southern Africa. Nature. 2022;603(7902):679–686. doi:10.1038/s41586-022-04411-y
15. Basso P, Negro C, Cegolon L, Larese Filon F. Risk of vaccine breakthrough SARS-CoV-2 infection and associated factors in healthcare workers of Trieste teaching hospitals (North-Eastern Italy). Viruses. 2022;14(2). doi:10.3390/v14020336
16. Tsui WNT, Hamill V, Noll L, et al. Molecular detection of SARS-CoV-2 and differentiation of Omicron and Delta variant strains. Transbound Emerg Dis. 2022;69(5):e1618–e1631. doi:10.1111/tbed.14497
17. Firew T, Sano ED, Lee JW, et al. Protecting the front line: a cross-sectional survey analysis of the occupational factors contributing to healthcare workers’ infection and psychological distress during the COVID-19 pandemic in the USA. BMJ Open. 2020;10(10):e042752. doi:10.1136/bmjopen-2020-042752
18. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
19. Hui KPY, J.c.w. H, Cheung MC, et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603(7902):715–720. doi:10.1038/s41586-022-04479-6
20. Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618–1624. doi:10.1016/S0140-6736(22)00327-0
21. Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. doi:10.1152/physrev.00010.2018
22. Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012;16(2):137–149. doi:10.1016/j.smrv.2011.05.001
23. Ragnoli B, Pochetti P, Pignatti P, et al. Sleep deprivation, immune suppression and SARS-CoV-2 infection. Int J Environ Res Public Health. 2022;19(2):904. doi:10.3390/ijerph19020904
24. Mello MT, Silva A, Guerreiro RC, et al. Sleep and COVID-19: considerations about immunity, pathophysiology, and treatment. Sleep Sci. 2020;13(3):199–209. doi:10.5935/1984-0063.20200062
25. Kim H, Hegde S, LaFiura C, et al. COVID-19 illness in relation to sleep and burnout. BMJ Nutr Prev Health. 2021;4(1):132–139. doi:10.1136/bmjnph-2021-000228
26. Parry DA, Oeppen RS, Amin MSA, Brennan PA. Sleep: its importance and the effects of deprivation on surgeons and other healthcare professionals. Br J Oral Maxillofac Surg. 2018;56(8):663–666. doi:10.1016/j.bjoms.2018.08.001
27. Ferini-Strambi L, Zucconi M, Casoni F, Salsone M. COVID-19 and sleep in medical staff: reflections, clinical evidences, and perspectives. Curr Treat Options Neurol. 2020;22(10):29. doi:10.1007/s11940-020-00642-4
28. Passioti M, Maggina P, Megremis S, Papadopoulos NG. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413. doi:10.1007/s11882-013-0413-5
29. Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15(6):335–349. doi:10.1038/nri3843
30. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671–675. doi:10.1038/s41586-021-04389-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.