Back to Journals » Journal of Blood Medicine » Volume 10
Assessment of soluble cytotoxic T lymphocyte-associated antigen-4, transforming growth factor β1, and platelet-derived microparticles during dasatinib therapy for patients with chronic myelogenous leukemia
Authors Nomura S , Ito T , Satake A , Ishii K
Received 10 September 2018
Accepted for publication 20 November 2018
Published 19 December 2018 Volume 2019:10 Pages 1—8
DOI https://doi.org/10.2147/JBM.S187005
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin Bluth
Shosaku Nomura, Tomoki Ito, Atsushi Satake, Kazuyoshi Ishii
First Department of Internal Medicine, Kansai Medical University, Hirakata, Osaka, Japan
Background: The outcome for chronic myelogenous leukemia (CML) patients presented in the chronic phase has changed dramatically since the introduction of tyrosine kinase inhibitor (TKI) therapy. Notably, an increased incidence of large granular lymphocytes (LGLs), which is related to immunological conditions, appears to be predictive of a favorable outcome for dasatinib therapy. We therefore examined the immunological characteristics of CML patients during dasatinib therapy by determining the plasma concentrations of five different biomarkers.
Methods: The plasma levels of biomarkers, specifically interleukin-6, platelet-derived microparticles (PDMPs), soluble vascular cell adhesion molecule 1 (sVCAM-1), transforming growth factor (TGF) β1, and soluble cytotoxic T lymphocyte-associated antigen-4 (sCTLA-4), were measured by ELISA at baseline and after 2 and 6 months of TKI treatment. The incidence of LGLs was estimated by microscopic examination.
Results: The levels of PDMPs, sVCAM-1, and TGFβ1 were significantly elevated in patients with CML. Dasatinib treatment was associated with a significant reduction in the levels of these markers and with an increased incidence of LGLs compared with imatinib or nilotinib treatment. In addition, an increased incidence of LGLs was significantly correlated with a decreased sCTLA-4 level during dasatinib therapy.
Conclusion: The assessment of the levels of specific biomarkers may be beneficial to understand the immunological conditions of patients with CML during dasatinib treatment.
Keywords: CML, TKI, LGL, PDMP, TGFβ1, sCTLA-4
Introduction
Chronic myelogenous leukemia (CML) is a myeloproliferative disorder in which leukemic cells display the Philadelphia chromosome generated from a reciprocal t(9:22) (q34:q11) translocation.1 The chromosome 9 and chromosome 22 transposal of t (9:22) and (q34:q11) causes the cancer gene C-ABL at 9q34 to link with the BCR gene at 22q11, forming the BCR–ABL gene on chromosome 22.2 Before the era of tyrosine kinase inhibitor (TKI) therapy, the main therapeutic option for patients with CML was an allogeneic stem cell transplantation with the aim of achieving complete remission. Unfortunately, the outcomes of this strategy remain suboptimal.3 However, the prognosis of CML patients presenting in the chronic phase has changed dramatically since the introduction of TKI therapy,4 in which treatment objectives are now focused on achieving a deep molecular response (DMR).5,6 Patients with a sustained DMR have no significant risk of CML progression or CML-related death, so the likelihood of a new increase in the number of leukemic cells is quite low; thus, this treatment option is considered to be relatively safe.7
Among the various types of TKIs, dasatinib has unique characteristics in that it is a multi-kinase inhibitor that acts on SRC-family kinases, as well as on BCR–ABL, c-kit, EPHA2, and PDGF receptor.8,9 Studies have shown that dasatinib increases the number of lymphocytes in circulation, particularly natural killer cells and/or cytotoxic T lymphocytes which present as large granular lymphocytes (LGLs) with a differentiated immune phenotype.10,11 In addition, the incidence of LGLs has been shown to be closely associated with a favorable treatment response.12 Taken together, these studies indicate that dasatinib potentially modulates the host immune response.
CD4+ regulatory T cells (Tregs) play a critical role in the maintenance of peripheral tolerance by suppressing the activation and proliferation of immune cells.13,14 Tregs express cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and the transcription factor Foxp3.13 Soluble CTLA-4 (sCTLA-4) can modulate and terminate immune responses.15 Several studies have shown that sCTLA-4 levels are altered in patients with certain autoimmune disorders.15,16 However, to our knowledge, sCTLA-4 levels have not previously been investigated in patients with CML receiving treatment with TKIs.
In the present study, we assessed the levels of various biomarkers, specifically interleukin (IL)-6, platelet-derived microparticles (PDMPs),17–20 soluble vascular cell adhesion molecule 1 (sVCAM-1), transforming growth factor (TGF) β1, and sCTLA-4, in TKI-treated patients with CML. The purpose of this study was to measure the fluctuations in the levels of sCTLA-4, TGFβ1, and PDMPs, and to clarify the clinical significance of these biomarkers during dasatinib therapy in patients with CML.
Patients and methods
Patients
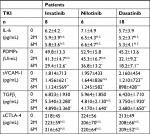
The study cohort included 67 patients with CML, who received TKI treatment (imatinib, 25; nilotinib, 18; and dasatinib, 24), selected among those admitted to our hospital between September 2011 and November 2017. The standard diagnosis of CML was made with reference to the European Leukemia Net.21 All patients were untreated upon their first examination. A control group comprising 10 healthy volunteers was also included. This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of Kansai Medical University, and written informed consent was obtained from each patient. The three groups of patients and the healthy volunteers were compared in terms of age, sex, and serum biochemistry data. Moreover, the levels of biomarkers were compared between the groups. Baseline data were obtained prior to TKI treatment.
Measurement of PDMPs
PDMPs were measured using an ELISA kit (JIMRO Co., Ltd., Takasaki, Japan) that included monoclonal antibodies against glycoproteins CD42b and CD42a.22 For the assay, 1.0 U/mL of PDMPs was defined as the amount of PDMPs obtained from 24,000 solubilized platelets/mL. Blood samples (2 mL) were collected from the peripheral veins of control and CML patients into vacutainers containing EDTA–acid citrate dextrose (NIPRO Co., Ltd., Osaka, Japan) using 21-gauge needles to minimize platelet activation. The samples were gently mixed by inversion and maintained at room temperature for a maximum of 2–3 hours. Immediately after centrifugation at 8,000× g for 5 minutes, 200 µL of supernatant was carefully collected from the upper layer of each sample to avoid platelet contamination and then stored at –40°C until analysis. PDMP levels were measured twice per sample, and the mean values were recorded.
Measurement of cytokines and soluble molecules
Blood samples from fasted CML patients and controls were collected into tubes with sodium citrate and allowed to clot at room temperature for a minimum of 1 hour. Citrated plasma was isolated by centrifugation at 1,000× g for 20 minutes at 4°C and stored at –30°C until analysis. Plasma concentrations of IL-6, TGFβ1, and sVCAM-1 were measured using monoclonal antibody-based ELISA kits (Thermo Fisher Scientific, Waltham, MA, USA), and sCTLA-4 was measured using an ELISA kit from BioLegend, Inc. (San Diego, CA, USA). The recombinant products and standard solutions provided with the commercial kits were used as positive controls in each assay. All kits were used according to the manufacturer’s instructions.
Biomarker cutoff values
The most suitable cutoff level of each biomarker was set based on a receiver operating characteristic (ROC) curve. Each ROC analysis was performed using the false-positive rate as the vertical axis and the sensitivity as the cross axis. The optimal cutoff value for 28-day mortality was calculated using biomarkers selected in multivariate logistic regression.
Assessment of LGLs
Complete blood cell counts were routinely performed before the initiation of imatinib, nilotinib, or dasatinib treatment and every month thereafter. The morphology of peripheral blood leukocytes was analyzed by microscopic examination of May–Grünwald–Giemsa-stained smears. LGLs were defined as described previously.11,23
Treatment and safety assessment
All TKIs were administered as monotherapy, and dose selection was performed according to the patient’s condition. For example, imatinib was typically administered at a dose of 400 mg daily with dose elevations up to 600 mg, nilotinib was typically administered at a dose of 400 mg daily, and dasatinib was typically administered at a dose of 100 mg daily with dose elevations up to 140 mg. Adverse events were assessed via laboratory examinations, including blood cell counts, assessment of liver and renal function, and physical examination. Chest radiographs were routinely examined after the initiation of dasatinib.
Statistics
Data are expressed as the mean ± SD. Between-group comparisons were analyzed using Scheffe’s test, and P-values of less than 0.05 were considered statistically significant. All analyses were performed using the StatFlex program (ver. 6).
Results
The demographic and clinical characteristics of patients are shown in Table 1. Age and sex were similar between the three groups of patients receiving different TKIs. Furthermore, there were no significant differences in the baseline laboratory data such as red blood cells, hemoglobin, white blood cells (WBCs), platelet count (Plt), aspartate aminotransferase, alanine aminotransferase, total bilirubin, lactate dehydrogenase (LD), and C-reactive protein between the TKI groups. After TKI treatment, the WBCs, Plt, and LD were significantly decreased at 2 months after therapy, whereas the other laboratory data did not exhibit significant changes (data not shown).
The plasma levels of IL-6, PDMPs, sVCAM-1, TGFβ1, and sCTLA-4 were compared between patients with CML and healthy volunteers (Figure 1). The levels of PDMPs, sVCAM-1, and TGFβ1 were found to be significantly elevated in patients with CML compared with controls (Figure 1B,C,D). However, there were no significant differences in the levels of IL-6 and sCTLA-4 observed between the two groups (Figure 1 A–E).
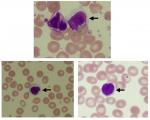
The appearance of LGLs was examined at 6 months after TKI treatment (Figure 2). No differences in lymphocytosis or the incidence of LGLs was observed between the imatinib and nilotinib groups (Figure 3). However, significantly high lymphocytosis and a higher incidence of LGLs were observed in patients receiving dasatinib compared with those receiving either imatinib or nilotinib (Figure 3).
The administration of each TKI significantly reduced the plasma concentrations of PDMPs, sVCAM-1, and TGFβ1 relative to baseline after both 2 and 6 months (Table 2). In particular, these changes in dasatinib-treated patients were remarkable compared with those in patients receiving imatinib or nilotinib (Table 2). In contrast, no significant differences in the levels of IL-6 or sCTLA-4 were observed in any of the patient groups (Table 2).
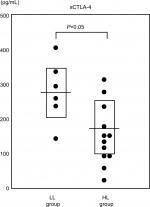
We divided the dasatinib patients into two subgroups according to the level of lymphocytosis observed at 6 months after dasatinib treatment: a low lymphocyte (LL) group and a high lymphocyte (HL) group. The HL group showed a significant reduction in the plasma concentration of sCTLA-4 compared with the LL group (P<0.05; Figure 4).
Discussion
Although the introduction of TKIs has greatly improved the outcomes in patients with CML, some do still progress from the chronic phase, and the prognosis of these patients remains poor.24 Various types of TKIs are currently available, each with specific characteristics.25 In the present study, we investigated the specific influence of dasatinib therapy on the immune system of patients with CML.
We measured the serum levels of various biomarkers related to immune system or vascular function in patients with CML. Many patients exhibited elevated levels of PDMPs, sVCAM-1, and TGFβ1, and the levels of these markers were significantly reduced following dasatinib treatment.
PDMPs play an important role in the clotting process, so an increase in PDMPs is likely to cause hypercoagulability.18,26 Because PDMPs promote the expression of adhesion molecules by monocytes and endothelial cells,19,26 it is possible that they may participate in the development or progression of vascular dysfunction.19 Villmow et al27 previously reported the elevation of platelet activation and vascular dysfunction-related biomarkers such as sVCAM-1 in patients with CML. In the present study, dasatinib therapy significantly decreased the levels of PDMPs and sVCAM-1. Dasatinib has also been reported to induce platelet dysfunction in patients with CML28 and to inhibit platelet formation.29 These findings are in agreement with the results presented in the current study.
TGFβ1 has been described as a platelet-related biomarker. Previous studies have shown that thrombocytopenic patients with improved Plt after treatment with immunosuppressive drugs, high-dose dexamethasone, intravenous immunoglobulin, or splenectomy have increased TGFβ1 levels.30,31 Therefore, dasatinib might decrease TGFβ1 levels via the inhibition of platelet generation.
An interesting phenomenon observed during dasatinib therapy is the increased incidence of LGLs.10,11 In addition, Kreutzman et al32 reported that dasatinib-associated LGLs contribute to the link between elevated levels of cytokines/chemokines and favorable outcome in dasatinib-treated CML patients. LGLs represent 10%–15% of the total peripheral blood mononuclear cells in normal adults,33 and play an essential role in acquired immunity against viral infections and neoplasm development.34 In the present study, CML patients receiving dasatinib had elevated levels of LGLs compared with those receiving imatinib. There are several potential explanations for this observation, including an inhibition of Treg function.10,11 In the present study, we determined the serum levels of sCTLA-4 and TGFβ1 as Treg-related biomarkers. Although dasatinib treatment caused a reduction in TGFβ1, the sCTLA-4 levels did not change significantly after dasatinib treatment. However, a significant reduction in the plasma concentration of sCTLA-4 was observed in the HL group compared with the LL group, indicating that the increase in lymphocytes is closely related to the reduction in sCTLA-4 level. While we could not directly clarify the relationship between the level of sCTLA-4 and LGLs, our data suggest that the LGL level is very likely to be related to the sCTLA-4 level, because the LGLs showed the same level change as the lymphocyte (Figure 3). Although sCTLA-4 specifically inhibits early T-cell activation by blocking the interaction of CD80/CD86 with costimulatory receptor CD28, the biological significance of increased sCTLA-4 in serum remains unclear.35 However, the loss of sCTLA-4 appears to impair the function of Treg cells.36 Our results suggest that the functional decline of Tregs reflected by a reduction in sCTLA-4 levels leads to the elevation of LGLs observed during dasatinib therapy.
TGFβ1 is an important inhibitor of B-cell proliferation and autoantibody production.37 In addition, it suppresses certain Th2- and Th2 cell-mediated autoimmune diseases,38 potentially via its ability to modulate Tregs. TGFβ1 has been shown to induce the conversion of Foxp3-negative T cells to Foxp3-positive Tregs and is essential for the maintenance of Treg function.39 In addition, PDMPs can derive Treg proliferation in the presence of TGFβ1.40 Therefore, our data indicate that the functional decline of Treg cells observed during dasatinib therapy may be modulated via PDMPs and TGFβ1.
This study has two potential strengths. First, we showed that the levels of PDMPs, sVCAM-1, and TGFβ1 were significantly elevated in patients with CML, and that these elevated levels were reduced following TKI treatment, particularly with dasatinib. Second, we showed that the elevation of LGLs was significantly higher during dasatinib therapy compared with during imatinib or nilotinib therapy, and that this effect may be related to a reduction in the sCTLA-4 level. However, this study also has several limitations. First, the number of included patients was small. Second, the detection of Tregs using specific surface markers and flow cytometry was not performed. Third, we were unable to assess the relationship between the evaluated biomarkers and the therapeutic effects of dasatinib. Dasatinib frequently causes pleural effusion, suggesting that pleural effusion may be associated with the increase in LGLs or with the therapeutic effects of dasatinib. Fourth, we could not study the correlation between the levels of plasma cytokines and the leukemic cell burden, because the BCR–ABL levels were not measured at each sample point. Larger and more specific studies would therefore be useful to validate our findings. Finally, we were unable to evaluate the therapeutic effects of LGLs in vitro. In vitro experiments using cell lines and LGLs, such as natural killer cells, would likely be beneficial.
Conclusion
Patients with CML were found to have significantly elevated levels of PDMPs, sVCAM-1, and TGFβ1. Dasatinib caused a significant reduction in the levels of these markers and increased the incidence of LGLs compared with imatinib and nilotinib. In addition, the increase in LGLs was likely associated with a decrease in the sCTLA-4 level during dasatinib therapy. The assessment of these biomarkers in patients with CML may be beneficial during the administration of dasatinib therapy. However, larger clinical trials are required to verify these findings.
Acknowledgments
This study was partly supported by a grant from the Japan Foundation of Neuropsychiatry and Hematology Research. The authors thank Clare Cox, PhD, and Katie Oakley, PhD, from Edanz Group for editing a draft of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243(5405):290–293. | ||
Kurzrock R, Kantarjian HM, Druker BJ, Talpaz M. Philadelphia chromosome-positive leukemias: from basic mechanisms to molecular therapeutics. Ann Intern Med. 2003;138(10):819–830. | ||
Bortin MM, Horowitz MM, Rowlings PA, et al. 1993 progress report from the International Bone Marrow Transplant Registry. Advisory Committee of the International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1993;12(2):97–104. | ||
Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109(1):58–60. | ||
Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2014;123(4):494–500. | ||
Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014;123(9):1353–1360. | ||
Laneuville P. When to Stop Tyrosine Kinase Inhibitors for the Treatment of Chronic Myeloid Leukemia. Curr Treat Options Oncol. 2018;19(3):15. | ||
Rix U, Hantschel O, Dürnberger G, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110(12):4055–4063. | ||
Lindauer M, Hochhaus A. Dasatinib. Recent Results Cancer Res. 2014;201:27–65. | ||
Mustjoki S, Ekblom M, Arstila TP, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23(8):1398–1405. | ||
Kim DH, Kamel-Reid S, Chang H, et al. Natural killer or natural killer/T cell lineage large granular lymphocytosis associated with dasatinib therapy for Philadelphia chromosome positive leukemia. Haematologica. 2009;94(1):135–139. | ||
Schiffer CA, Cortes JE, Hochhaus A, et al. Lymphocytosis after treatment with dasatinib in chronic myeloid leukemia: Effects on response and toxicity. Cancer. 2016;122(9):1398–1407. | ||
Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. | ||
von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6(4):338–344. | ||
Yu J, Heck S, Patel V, et al. Defective circulating CD25 regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood. 2008;112(4):1325–1328. | ||
Liu MF, Wang CR, Chen PC, Fung LL. Increased expression of soluble cytotoxic T-lymphocyte-associated antigen-4 molecule in patients with systemic lupus erythematosus. Scand J Immunol. 2003;57(6):568–572. | ||
Nomura S, Ozaki Y, Ikeda Y. Function and role of microparticles in various clinical settings. Thromb Res. 2008;123(1):8–23. | ||
Nomura S, Shimizu M. Clinical significance of procoagulant microparticles. J Intens Care. 2015;3(1):2. | ||
Nomura S. Microparticle and Atherothrombotic Diseases. J Atheroscler Thromb. 2016;23(1):1–9. | ||
Miyazaki Y, Nomura S, Miyake T, et al. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88(9):3456–3464. | ||
Baccarani M, Cortes J, Pane F, et al; European LeukemiaNet. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–6051. | ||
Osumi K, Ozeki Y, Saito S, et al. Development and assessment of enzyme immunoassay for platelet-derived microparticles. Thromb Haemost. 2001;85(2):326–330. | ||
Chang H, Kamel-Reid S, Hussain N, Lipton J, Messner HA. T-cell large granular lymphocytic leukemia of donor origin occurring after allogeneic bone marrow transplantation for B-cell lymphoproliferative disorders. Am J Clin Pathol. 2005;123(2):196–199. | ||
Shah NP. Advanced CML: therapeutic options for patients in accelerated and blast phases. J Natl Compr Canc Netw. 2008;6(Suppl 2):S31–SS6. | ||
Soverini S, Mancini M, Bavaro L, Cavo M, Martinelli G. Chronic myeloid leukemia: the paradigm of targeting oncogenic tyrosine kinase signaling and counteracting resistance for successful cancer therapy. Mol Cancer. 2018;17(1):49. | ||
Shimazu T, Inami N, Satoh D, et al. Effect of acarbose on platelet-derived microparticles, soluble selectins, and adiponectin in diabetic patients. J Thromb Thrombolysis. 2009;28(4):429–435. | ||
Villmow T, Kemkes-Matthes B, Matzdorff AC. Markers of platelet activation and platelet-leukocyte interaction in patients with myeloproliferative syndromes. Thromb Res. 2002;108(2-3):139–145. | ||
Quintás-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009;114(2):261–263. | ||
Mazharian A, Ghevaert C, Zhang L, Massberg S, Watson SP. Dasatinib enhances megakaryocyte differentiation but inhibits platelet formation. Blood. 2011;117(19):5198–5206. | ||
Mouzaki A, Theodoropoulou M, Gianakopoulos I, Vlaha V, Kyrtsonis MC, Maniatis A. Expression patterns of Th1 and Th2 cytokine genes in childhood idiopathic thrombocytopenic purpura (ITP) at presentation and their modulation by intravenous immunoglobulin G (IVIg) treatment: their role in prognosis. Blood. 2002;100(5):1774–1779. | ||
Guo C, Chu X, Shi Y, et al. Correction of Th1-dominant cytokine profiles by high-dose dexamethasone in patients with chronic idiopathic thrombocytopenic purpura. J Clin Immunol. 2007;27(6):557–562. | ||
Kreutzman A, Ladell K, Koechel C, et al. Expansion of highly differentiated CD8+ T-cells or NK-cells in patients treated with dasatinib is associated with cytomegalovirus reactivation. Leukemia. 2011;25(10):1587–1597. | ||
Lamy T, Loughran TP. Large Granular Lymphocyte Leukemia. Cancer Control. 1998;5(1):25–33. | ||
Qiu ZY, Xu W, Li JY. Large granular lymphocytosis during dasatinib therapy. Cancer Biol Ther. 2014;15(3):247–255. | ||
Simone R, Pesce G, Antola P, et al. The soluble form of CTLA-4 from serum of patients with autoimmune diseases regulates T-cell responses. Biomed Res Int. 2014;2014:215763. | ||
Gerold KD, Zheng P, Rainbow DB, Zernecke A, Wicker LS, Kissler S. The soluble CTLA-4 splice variant protects from type 1 diabetes and potentiates regulatory T-cell function. Diabetes. 2011;60(7):1955–1963. | ||
Cross D, Cambier JC. Transforming growth factor beta 1 has differential effects on B cell proliferation and activation antigen expression. J Immunol. 1990;144(2):432–439. | ||
Bridoux F, Badou A, Saoudi A, et al. Transforming growth factor beta (TGF-beta)-dependent inhibition of T helper cell 2 (Th2)-induced autoimmunity by self-major histocompatibility complex (MHC) class II-specific, regulatory CD4(+) T cell lines. J Exp Med. 1997;185(10):1769–1775. | ||
Wan YY, Flavell RA. ‘Yin-Yang’ functions of transforming growth factor-beta and T regulatory cells in immune regulation. Immunol Rev. 2007;220:199–213. | ||
Vajen T, Mause SF, Koenen RR. Microvesicles from platelets: novel drivers of vascular inflammation. Thromb Haemost. 2015;114(2):228–236. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.