Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 12
Assessment of Serum Lipid Profiles and High-sensitivity C-reactive Protein Among Patients Suffering from Rheumatoid Arthritis at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia: A Cross-Sectional Study
Authors Dessie G , Tadesse Y, Genet S
Received 29 May 2020
Accepted for publication 7 August 2020
Published 23 September 2020 Volume 2020:12 Pages 223—232
DOI https://doi.org/10.2147/OARRR.S264466
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Chuan-Ju Liu
Gashaw Dessie,1 Yewondwossen Tadesse,2 Birhanu Demelash,2 Solomon Genet3
1Department of Biochemistry, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Internal Medicine, School of Medicine, Addis Ababa University, Addis Ababa, Ethiopia; 3Department of Biochemistry, School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Gashaw Dessie
Department of Biochemistry, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Tel +251 975152796
Email [email protected]
Background: Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by severe joint pain, swelling, damage, and disability which leads to joint destruction and loss of function. The complication of RA is associated with cardiovascular diseases, particularly due to systemic inflammation and dyslipidemia. The purpose of this study was to assess the development of atherosclerosis, which acts as a major risk factor for cardiovascular complications in RA patients.
Methods: A hospital-based cross-sectional study was conducted at the Rheumatology Clinic of Tikur Anbessa Specialized Hospital. The study made a comparison of risk factors (dyslipidemia and inflammatory status) between individuals having RA as a case group and apparently healthy individuals as a control group. Simple descriptive statistics, one-way ANOVA, independent sample t-test and multivariate analysis were utilized for statistical analysis. p-value of < 0.05 at the 95% confidence level was considered as statistically significant.
Results: The result of this study demonstrated that there was a significant elevation of mean ±SD of TC, TC/HDL, LDL/HDL, and lowered value of HDL-C was seen among RA patients than controls (P-value < 0.05). The mean ±SD of inflammatory marker, high-sensitivity C-reactive protein (hsCRP), was significantly higher among RA patients compared to controls (P< 0.05). HDL-C had a significant negative correlation with a hsCRP whereas TC/HDL-C and LDL/HDL-C had a significant positive correlation with hsCRP (P< 0.05).
Conclusion: In this study, RA patients had lipid abnormalities and elevated systemic inflammation than controls. An increase in hsCRP and dyslipidemia status among RA patients indicates the possible development of an atherosclerotic event. Therefore, assessment of lipid profiles and hsCRP in early RA patients may be helpful to assess the possible development of cardiovascular complications.
Keywords: rheumatoid arthritis, lipid profiles, high-sensitivity C-reactive protein
Introduction
Rheumatoid arthritis (RA) is a heterogeneous and progressive autoimmune disease which affects all ethnic groups throughout the world.1 The World Health Organization (WHO) considered it as a disease with the greatest impact on society. According to the global burden of 2010 study, clinical and epidemiological research, the prevalence of RA was 0.24% and continued without change from 1990 to 2010.1 Rheumatoid arthritis is a chronic systemic inflammatory disease characterized by chronic inflammation of the joints with severe pain, swelling, joint damage and disability, which leads to joint destruction and loss of function. It is characterized by a high systemic inflammation with increased mortality and reduced life expectancy.2 It also causes a significant morbidity effect due to synovial inflammation, joint destruction and associated disability. Epidemiological studies have shown that mortality is increased in patients with RA compared to the general population.3
RA is caused by genetic and environmental factors, which results in immune dysregulation and inflammation.4 It is an inflammatory disorder characterized by chronic inflammation of synovial joints associated with proliferation of synovial cells and infiltration of activated immune inflammatory cells, which leads to progressive destruction of cartilage and bone.5 Complication of RA is mainly associated with the risk of cardiovascular disease. The morbidity and mortality of cardiovascular diseases increase in RA patients due to factors including, hypercholesterolemia and elevated systemic inflammation.6 Several studies show that an increase in the serum cholesterol level increases the risk of cardiovascular complication. Systemic inflammation in RA contributes to an abnormality in serum lipid profiles. This is because of infectious agents, autoimmune dysregulation and oxidative stress that causes endothelial dysfunction. Injury to vascular endothelium is also a critical event in acute inflammatory disease processes. Endothelial cells play a major role in the development of atherosclerosis.7 The oxidation of lipoprotein activate the endothelial cells for subsequent expression of selectins and adhesion molecules.8,9 Macrophages express scavenger receptors for oxidized LDL to internalized lipoprotein particles. After a short period of hyperlipidemic condition, cholesterols are produced and deposited on endothelial space. The triglycerides, cholesteryl ester or free cholesterol contributed to much cholesterol formation in endothelial cells.10 This leads to atherosclerotic plaque formation and increases in lipid profiles, including cholesterol levels. Untreated RA leads to elevated levels of the TC, LDC-C and decreased concentration of the HDL-C.11 However, early diagnosed RA patients taking disease-modifying antirheumatic drugs, including hydroxychloroquine, methotrexate, and nonsteroidal anti-inflammatory drugs show elevation of HDL-C and lower disease activity.12
The American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) state the common criteria to measure disease activity, including acute phase response, physical disability and swollen joint counts.13 The intention of disease activity score (DAS) is to manage inflammatory status of RA patients. With regard to measurement of acute phase response, CRP is preferred to assess pathophysiological features of synovial inflammation in RA.14 Reducing disease activity is one of the targets of RA treatment by lowering inflammation.15 An increase in C-reactive protein (CRP) levels and its duration are closely related to the severity and activity of inflammatory diseases, hence CRP determination is useful for the diagnosis and treatment of inflammation.16 CRP has been shown to have a significant value as an inflammatory marker in the pathogenesis of RA and has been suggested to mediate a part of the complement activation in RA.17 It is a sensitive marker of the systemic inflammation and elevates in untreated RA patients. Consequently, studies states that disease-modifying antirheumatic drugs, including methotrexate (MTX) have better treatment option to reduce inflammation in RA.6 The purpose of conducting this study was to investigate the concentration of the serum lipid profiles, and hsCRP in patients with RA. Although the risk factors for cardiovascular diseases are assessed by different studies, the study related to biochemical analysis of RA has not yet been investigated in Ethiopia. Since there is no adequate research related to this topic in Ethiopia, it will serve as a baseline for future research. It helps policy-makers to give much attention to serum lipid profiles, and hsCRP in RA patients to control dyslipidemia and inflammation. The management of dyslipidemia and inflammatory status are important to reduce the risk of development of cardiovascular disease at early stages in RA patients.
Materials and Methods
Objective of Study
The purpose of conducting this study was to assess the level of serum lipid profiles, an inflammatory marker (hsCRP) among RA patients and control groups. The other objective of the study was to assess the risk factors for RA.
Study Design
A hospital based cross-sectional comparative study was conducted. It was conducted at the Rheumatology Clinic of Tikur Anbessa Specialized Hospital, the largest referral teaching hospital under the administration of Addis Ababa University, located in Addis Ababa, Ethiopia. The study made a comparison of risk factors (dyslipidemia and inflammatory status) between individuals having RA as a case group and apparently healthy individuals as a control group.
Study Population
Patients who fulfill the American College of Radiology (ACR) and European League Against Rheumatism (EULAR) of RA criteria were utilized for the selection of patients during the study period.18 The ACR/EULAR criteria used by the physicians were the number of joints and types of joint (small or large) affected by the disease. In addition to joint involvement, both serological and acute phase responses were examined. Consequently, patients who fulfilled the inclusion criteria were selected for data collection during the study period.
Eligibility Criteria for Patients
Study participants who were volunteer to participate, 18 years old and above, and those who were at a different progression of the disease were included in this study. Thus, both early and active RA patients with less than or greater than one year of disease duration were incorporated in the study. In contrast, participants with mental health problems, hearing impairment or any other serious health problems, and those patients who were not able to provide the appropriate information were excluded. In addition, RA patients who were diabetic, pregnant women, and patients taking antituberculosis (TB) drugs were excluded. Patients with other chronic diseases like cancer were also excluded.
Eligibility Criteria for Control Group
Inclusion and exclusion of healthy control groups were done carefully by professional nurses. Study participants were included as a control group, if he or she volunteered to participate and was above 18 years old. Apparently healthy individuals who take care of RA patients and other patients in an outpatient departments other than the Rheumatology Clinic were also involved in the study as controls. Some staff members of the hospital (n=5) were also selected as a control group, and included in the study. Participants with mental health problems, hearing impairment or any other serious health problems and those patients who were not able to provide the appropriate information were excluded. In addition, Individuals with chronic disease like RA, diabetes mellitus, hypertension, cancer, tuberculosis, and pregnant women were excluded from the study.
Blood Sample Collection Procedure and Process
Blood was collected from both RA patients and control after overnight fasting state immediately before the procedure. After overnight fasting, 5 mL venous blood sample was collected using a BD Vacutainer containing EDTA by the nurses under aseptic conditions. Serum was isolated by centrifugation at 3000 rpm for five minutes. After separation, serum was stored at −20°C. Then, serum lipid profiles and hsCRP were measured by calibrated, fully automated mind array, Cobas Integra 400 Plus (Roche Diagnostics GmbH, Mannheim, Germany) clinical chemistry analyzer according to manufacturer’s instruction in the central laboratory of Zewditu referral hospital, and Ethiopian public health institute (EPHI).
Statistical Analysis
The data were checked, cleaned and entered into EpiData software and imported to SPSS version-20 software for analysis. Simple descriptive statistics were used to analyze sociodemographic and clinical characteristics of the study subjects. One-way ANOVA was used to compare the three levels of hsCRP based on other continuous variables. Independent sample t-test was used to compare case and control groups based on clinical and demographic data. The adjusted odds ratio was calculated for the multivariate analysis to assess risk factors for RA. P-value of <0.05 at the 95% confidence level was considered as statistically significant.
Results
Sociodemographic Characteristics
In this study, 73 rheumatoid arthritis (RA) patients who fulfilled the appropriate clinical definition for RA were included. Of the total cases 64 (87%) were females and nine (13%) were males and among 40 control subjects, 13 (32.5%) were males and 27 (67.5%) were females respectively. In this study, 25 (34.4%), 38 (52%) and 10 (13.6%) of the total case group fall under the age groups of 20–39, 40–59, and 60–79 years respectively. In the control group, 15 (37.5%), 20 (50%) and 5 (12.5%) were in the age groups 20–39, 40–59, and 60–79 years respectively. The levels of hsCRP were categorized as low, moderate, and high risk to evaluate the inflammatory status of study participants. Among 73 RA patients, 14 (19.1%), 16 (22%), and 43 (58.9%) fall in <1 mg/L, 1–3 mg/L, and >3 mg/L levels of hsCRP respectively. In the control group, 19 (47.5%), 14 (35%), and 7 (17.5%) showed hsCRP levels of <1 mg/L, 1–3 mg/L, and > 3 mg/L respectively (Figure 1).
 |
Figure 1 Comparision of catagorical value of high sensitivity c-reactive protein for case and control groups. |
Estimated Levels of Biochemical Parameters
Serum Levels of Lipid Profiles and hsCRP in RA Patients and Control Groups
The mean ±SD of ages among patients and controls were 44.41±11.53 and 41.93±12.84 respectively, and the difference was not statistically significant. Out of 73 total RA patients, 52 were positive for IgG rheumatoid factor test. The sensitivity was 71%. The body mass index of both cases and controls were measured and the difference was not statistically significant. High-sensitivity C-reactive protein (hsCRP) and triglyceride (TG) were statistically higher among patients than controls, whereas the levels of high-density lipoprotein (HDL-C) were statistically lower compared to controls. However, the value of low-density lipoprotein (LDL-C) was not statistically significant (Table 1).
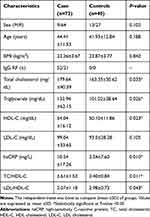 |
Table 1 Comparison of Serum Lipid Profiles and hsCRP Among Case and Control Group |
Categorical Values of Serum Lipid Profiles and hsCRP in Rheumatoid Arthritis Patients and Control Groups
Out of the total 73 RA patients, 19 (26.1%), 18 (24.6%), 24 (32.8%), 11 (15%), and 42 (57.5%) were above baseline values of the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guideline for TC, HDL-C, TG, LDL-C, and hsCRP respectively. These patients were found to have serious dyslipidemia. On the contrary, out of 40 controls six (15%), nine (22.5%), four (10%), four (10%), and 7 (17.5%) were above the baseline values of the NCEP ATP III guideline for TC, HDL, TG, LDL, and hsCRP respectively. We compared the level of dyslipidemia between case and control groups in reference to baseline values. The patients had 22.8%, 11%, and 5%, for TG, TC, and LDL-C higher values than controls respectively. The RA patients showed 2% lower HDL-C value compared to control group (Table 2).
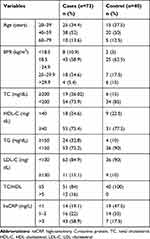 |
Table 2 Categorical Values of Clinical and Demographic Data for Case and Control Groups |
Atherogenic Indices Among Case and Control Groups
The atherogenic indices are important to investigate atherosclerosis. The logarithmic value of TG/HDL-C was not statistically significant; hence the result showed that no significant variation existed between the groups. The mean values of both TC/HDL-C and LDL/HDL-C for RA patients were 3.64 and 2.07, respectively. The mean values of both TC/HDL-C and LDL-C/HDL-C for controls were 3.40 and 1.98, respectively. Calculated atherogenic ratios for TC/HDL-C and LDL-C/HDL-C were statistically significant and higher in cases compared to controls. Of the total enrolled 73 RA patients, TC/HDL-C value of 12 patients was beyond the baseline value (>5). But, controls had values lower than the baseline values.
Comparison of Serum Lipid Profiles Among Different Levels of hsCRP in RA Patients
Serum lipid profiles and demographic data of RA patients were compared according to the levels of hsCRP. The hsCRP, LDL-C, and TG levels elevated with an increase in age group. The three levels of hsCRP indicate the severity status of the disease. A patient having <1 mg/L, 1–3 mg/L, and >3 mg/L was categorized as low, moderate, and high inflammatory risk respectively. The level of HDL-C was negatively or inversely correlated with hsCRP value of RA patients (r=−0.328). The value of TC/HDL-C and LDL/HDL-C significantly varied across the three levels of hsCRP (Table 3). The level of hsCRP showed a positive correlation with TC/HDL-C (r=0.318). The association between the two variables was statistically significant. The level of hsCRP positively correlated with LDL/HDL-C value (r=0.327). The association between variables was statistically significant. The risk of myocardial infarction increases considerably as the ratio of the TC/HDL-C increases. See results in Table 3 below.
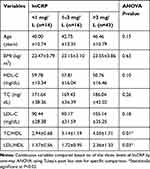 |
Table 3 Characteristics of Serum Lipid Profiles and Demographic Data of the RA Patient According to Three Levels of hsCRP |
The adjusted odd ratio was calculated for multivariate analysis to assess risk factors for RA. P-value of <0.05 at the 95% confidence level was considered statistically significant (Table 4).
 |
Table 4 Multivariate Analysis of Demographic and Clinical Risk Factors for RA |
Discussion
In this study, 73 rheumatoid arthritis (RA) patients and 40 age and sex-matched apparently healthy controls were included as case and control groups respectively. The RA patients both in early and active stages of RA have been included in the study. Recent clinical trial studies revealed that the clinical outcome of RA patients is similar in various diseases duration period.19 From the total enrolled RA patients, 87.6% and 13.4% were females and males respectively, whose age ranged from 26 to 73 years old. The assessment of this study shows that females were more affected by RA than males. Being female was significantly associated as a risk factor for the development of the disease (P<0.05, Table 4). Women whose age ranged from 40–60 showed a three-fold increased risk of developing RA than men. This finding agreed with another study.20 RA was also seen among middle-aged women.21 The review by McGill et al discussed the effect of residential area in the development of RA. According to this review, women who are living with fewer facilities, in rural areas are more prone to develop this chronic inflammatory disease. With regard to residential area, we collected the data of study participants through well-structured questionnaires. We found that there were patients who live in rural areas, especially farmers, who were suffering from the disease.
The risk of developing RA is associated with the socioeconomic status of the individual. According to an extended report done in Sweden,22 socioeconomic status is associated with education, and occupation, and this agrees with our result, which showed that people with low economic and educational status are more vulnerable to the disease (Table 4). Patients who are illiterate and completed primary school were more exposed for RA than others who completed college or university. This is due to the fact that low educational status leads to low accessibility to medical care service, and lifestyle modification. Therefore, RA more frequently occurs in an individual with lower educational status.23 The 1987 ACR criteria states that the diagnosis of RA is characterized by the laboratory investigation of rheumatoid factor (RF). In addition to RF, the recent 2010 ACR/EULAR criteria incorporates anticyclic citrullinated peptide antibody (ACPA) for the diagnosis of RA.24 Concerning sensitivity and specificity, RF has lower specificity and sensitivity than ACPA. ACPA has 95–98% specificity and serves as a prognostic and predicative marker for the diagnosis of RA.25 From the total of 73 RA patients, 52 patients were positive for IgG rheumatoid factor test whereas the other 21 patients were negative for the test (Table 1). The result of our study shows that the sensitivity of IgG rheumatoid factor test is 71%. The result of this study agrees with the previous research.26 The study mentioned sensitivity of IgG rheumatoid factor and ACPA within the range of 60–80%, 70–90% respectively.
This study showed that there were statistically significant elevations of TC, hsCRP, and TG among RA patients than controls. LDL-C did not show statistically significant variation among patients compared to controls, whereas HDL-C was lower in patients compared to controls (Table 1). The inflammatory marker, hsCRP, was significantly (P<0.05) higher among RA patients compared to controls (Table 1). Patients had 22.8%, 11%. and 5%, for TG, TC. and LDL-C higher values than controls, respectively in reference to baseline values, NCEP ATP III. RA patients showed 2% lowered HDL-C value compared to control group (Table 2). The significant elevation of hsCRP level among cases indicates the presence of the systemic inflammation. The result of our study agreed with previous findings,27 which indicated the association of elevated level of hsCRP with cardiovascular risk among RA and osteoarthritis patients. The higher level of the hsCRP among RA patients shows that elevation of systemic inflammation is the underlying cause of future cardiovascular risk.28 Choy et al also explained that hsCRP serves as an independent predictor of cardiovascular risk, especially for MI. Three levels of hsCRP concentration were used to evaluate the inflammatory status. The levels of hsCRP used in our study are <1 mg/L, 1–3 mg/L, and >3 mg/L (Figure 1). Many other researchers used these baseline values for comparison.29–31 The level beyond 3 mg/L indicates higher inflammatory status, whereas hsCRP with 1–3 mg/L and <1 mg/L levels indicate moderate and lower inflammatory risk respectively. Therefore, we evaluated the inflammatory status of patients using these three levels of hsCRP. The level of HDL-C was negatively or inversely correlated with hsCRP value of RA patients; however, there were no statistically significant variations (Table 3). This result was in line with a previous work.32 The other laboratory test evaluated in this study was the measurement of triglycerides (TG), where 32.8% of total cases showed higher TG values compared to baseline values of the NCEP ATP III guideline. The TG value of the RA patients was 22.8% higher than controls. Statistically significant elevation of TG was observed among cases (Table 2). In addition, our study was in line with another research report.33
According to this study, the concentration of both hsCRP and TC showed a statistically significant elevation among cases compared to controls. However, the concentration of HDL-C was significantly lower among RA patients than age and sex-matched control subjects. Elevated serum hsCRP level serves as an independent predictor of CVD.34 This was also supported by another group of researchers.32 In line with these works, our study also correlated to future cardiovascular risk complication as the levels of hsCRP increased. The elevation of hsCRP among RA patients shown in this study is due to diminished anti-inflammatory activity of HDL-C. HDL-C serves as an independent predictor of future cardiovascular risk complication.35 Therefore, statistically significant lowering of HDL-C level among RA patients showed the possible development of future cardiovascular risk complication. The possible mechanism for the development of the cardiovascular risk associated with a lower HDL-C level has been stated by different researchers. The presence of higher inflammation among active RA patients leads to structural modification of HDL-C.36 Paraoxonase-1 is an enzymatic component of HDL-C which has an anti-oxidant effect.36 Therefore, oxidation of LDL-C will be continued and causes progression of the atherosclerosis. According to the present study, higher inflammatory status among RA patients may cause loss of the antioxidant activity of HDL-C. This leads to the pathogenesis of the atherosclerosis, which is the underlying cause of the cardiovascular risk.
The ratio of TC:HDL-C and LDL:HDL-C was statistically elevated among patients in contrast to the controls. Mean ±SD ratio of TC:HDL-C among cases and controls was 3.64±1.52 and 3.40±0.84 respectively (Table 5). The mean value of TC/HDL-C was 3.64 and 3.40 for patients and controls respectively (Table 5). This study showed that there was a significant (P<0.05) elevation of the atherogenic indices as the level of hsCRP increases among RA patients (Table 3). From the total enrolled 73 RA patients, 12 patients had TC/HDL-C value beyond the baseline value (>5). However, controls had a lower value than the baseline values. This agreed with the report of the group of researcher.3 The risk of MI increases considerably if the ratio of the TC:HDL-C is greater than five.3 The significant elevation of these atherogenic indices among cases agreed with one previous research work.12
 |
Table 5 Comparison of Atherogenic Indices Between Case and Control Groups |
Conclusion
The results of this study showed significant elevation of TC, TC/HDL, LDL/HDL, and lowered value of HDL-C was seen among RA patients than controls. Therefore, it is possible to conclude that there was dyslipidemia among RA patients than controls. The inflammatory marker, hsCRP, was significantly higher among patients compared to controls. The higher hsCRP shows high grade of systemic inflammation. Elevated value of hsCRP and atherogenic indices indicate the elevation of atherosclerotic events in RA patients. Dyslipidemia and increased systemic inflammation status were observed among RA patients than controls. Both indices indicate the presence of an atherosclerotic event in RA patients. Therefore, it is possible to conclude that RA patients may become more prone to developing future cardiovascular coronary artery diseases compared to normal subjects. Measurement of lipid profiles and hsCRP in early RA patients may help to prevent coronary artery diseases.
Abbreviations
CVD, cardiovascular diseases; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive proteins; LDL-C, low-density lipoprotein cholesterol; RA, rheumatoid arthritis; TC, total cholesterol.
Data Sharing Statement
Data are available from the corresponding author on a reasonable request.
Ethics Approval
Ethical approval was obtained from the Research and Ethics Committee of the Department of Biochemistry, School of Medicine, Addis Ababa University, Ethiopia. Department of Ethics and Research Committee decided and approved the protocol with reference number, SOM/BCHM/137/2010 on 11/06/2018.
Consent
Consent forms are available from the corresponding author on a reasonable request. All participants provided informed consent, and this study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We sincerely acknowledge University of Gondar, Addis Ababa University, Tikur Anbessa Specialized Hospital for funding, and support during the research work. We thank all the participants who were enrolled in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–1322. doi:10.1136/annrheumdis-2013-204627
2. Ungurianu A, Margină D, Grădinaru D, et al. Lipoprotein redox status evaluation as a marker of cardiovascular disease risk in patients with inflammatory disease. Mol Med Rep. 2017;15(1):256–262. doi:10.3892/mmr.2016.5972
3. Georgiadis AN, Papavasiliou EC, Lourida ES, et al. Atherogenic lipid profile is a feature characteristic of patients with early rheumatoid arthritis: effect of early treatment–a prospective, controlled study. Arthritis Res Ther. 2006;8(3):R82. doi:10.1186/ar1952
4. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Eng J Med. 2011;365(23):2205–2219. doi:10.1056/NEJMra1004965
5. Filippin LI, Vercelino R, Marroni N, Xavier RM. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin Exp Immunol. 2008;152(3):415–422. doi:10.1111/j.1365-2249.2008.03634.x
6. van Halm VP, Nurmohamed MT, Twisk JW, Dijkmans BA, Voskuyl AE. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther. 2006;8(5):R151. doi:10.1186/ar2045
7. Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54(1):24–38. doi:10.1373/clinchem.2007.097360
8. Klingenberg R, Lüscher TF. Rheumatoid arthritis and coronary atherosclerosis: two cousins engaging in a dangerous liaison. Eur Heart J. 2015;36(48):3423. doi:10.1093/eurheartj/ehv489
9. Nanchen D, Gencer B, Auer R, et al. Prevalence and management of familial hypercholesterolaemia in patients with acute coronary syndromes. Eur Heart J. 2015;36(36):2438–2445. doi:10.1093/eurheartj/ehv289
10. Baumer Y, McCurdy S, Weatherby TM, et al. Hyperlipidemia-induced cholesterol crystal production by endothelial cells promotes atherogenesis. Nat Commun. 2017;8(1):1129. doi:10.1038/s41467-017-01186-z
11. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70(3):482–487. doi:10.1136/ard.2010.135871
12. Parveen S, Jacob R, Rajasekhar L, Srinivasa C, Mohan IK. Serum lipid alterations in early rheumatoid arthritis patients on disease modifying anti rheumatoid therapy. Indian J Clin Biochem. 2017;32(1):26–32. doi:10.1007/s12291-016-0566-9
13. Smolen J, Breedveld F, Schiff M, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology. 2003;42(2):244–257. doi:10.1093/rheumatology/keg072
14. Hensor EM, McKeigue P, Ling SF, et al. Validity of a two-component imaging-derived disease activity score for improved assessment of synovitis in early rheumatoid arthritis. Rheumatology. 2019;58(8):1400–1409. doi:10.1093/rheumatology/kez049
15. Fautrel B, Kirkham B, Pope JE, et al. Effect of baricitinib and adalimumab in reducing pain and improving function in patients with rheumatoid arthritis in low disease activity: exploratory analyses from RA-BEAM. J Clin Med. 2019;8(9):1394. doi:10.3390/jcm8091394
16. Peltola H, Laipio M, Siimes M. Quantitative C‐reactive protein (CRP) determined by an immunoturbidimetric method in rapid differential diagnosis of acute bacterial and viral diseases of children. Acta Pædiatrica. 1984;73(2):273–274. doi:10.1111/j.1651-2227.1984.tb09944.x
17. Molenaar ET, Voskuyl AE, Familian A, van Mierlo GJ, Dijkmans BA, Hack CE. Complement activation in patients with rheumatoid arthritis mediated in part by C‐reactive protein. Arthritis Rheumatism. 2001;44(5):997–1002. doi:10.1002/1529-0131(200105)44:5<997::AID-ANR178>3.0.CO;2-C
18. Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheumatism. 1992;35(5):498–502. doi:10.1002/art.1780350502
19. Aletaha D, Maa J-F, Chen S, et al. Effect of disease duration and prior disease-modifying antirheumatic drug use on treatment outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. 2019;78(12):1609–1615. doi:10.1136/annrheumdis-2018-214918
20. Liu Y, Aryee MJ, Padyukov L, et al. Epigenome-wide association data implicate DNA methylation as an intermediary of genetic risk in rheumatoid arthritis. Nat Biotechnol. 2013;31(2):142. doi:10.1038/nbt.2487
21. McGill P. Rheumatoid arthritis in sub-Saharan Africa. Ann Rheum Dis. 1991;50(12):965. doi:10.1136/ard.50.12.965
22. Bengtsson C, Nordmark B, Klareskog L, Lundberg I, Alfredsson L. Socioeconomic status and the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis. 2005;64(11):1588–1594. doi:10.1136/ard.2004.031666
23. Pincus T. Aggressive treatment of early rheumatoid arthritis to prevent joint damage. Bull Rheum Dis. 1998;47(8):2.
24. Chang PY, Yang CT, Cheng CH, Yu KH. Diagnostic performance of anti‐cyclic citrullinated peptide and rheumatoid factor in patients with rheumatoid arthritis. Int J Rheum Dis. 2016;19(9):880–886. doi:10.1111/1756-185X.12552
25. Hashemzadeh K, Saghafi M, Khajedaluee M, Sahebari M. Synovial Anti Cyclic Citrullinated Peptide Antibodies in Comparison with Serum Antibodies in Early and Late Rheumatoid Arthritis. Fort J Rheumatol. 2020;2(02):067–073. doi:10.26502/fjr.26880019
26. Song Y, Kang E. Autoantibodies in rheumatoid arthritis: rheumatoid factors and anticitrullinated protein antibodies. QJM. 2009;103(3):139–146. doi:10.1093/qjmed/hcp165
27. Pearle A, Scanzello C, George S, et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15(5):516–523. doi:10.1016/j.joca.2006.10.010
28. Choy E, Ganeshalingam K, Semb AG, Szekanecz Z, Nurmohamed M. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology. 2014;53(12):2143–2154. doi:10.1093/rheumatology/keu224
29. Koenig W, Khuseyinova N, Baumert J, Meisinger C. Prospective study of high-sensitivity C-reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg Cohort Study, 1984–1998. Clin Chem. 2008;54(2):335–342. doi:10.1373/clinchem.2007.100271
30. Rifai N, Ridker PM. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem. 2001;47(3):403–411. doi:10.1093/clinchem/47.3.403
31. Graf J, Scherzer R, Grunfeld C, Imboden J. Levels of C-reactive protein associated with high and very high cardiovascular risk are prevalent in patients with rheumatoid arthritis. PLoS One. 2009;4(7):e6242. doi:10.1371/journal.pone.0006242
32. Gotto AM, Moon JE. Management of cardiovascular risk: the importance of meeting lipid targets. Am J Cardiol. 2012;110(1):3A–14A. doi:10.1016/j.amjcard.2012.04.002
33. Ansari SK, Jaiswal G. Raised Lipid Profile in Rheumatoid Arthritis-A Risk for CVD.
34. Galarraga B, Khan F, Kumar P, Pullar T, Belch J. C-reactive protein: the underlying cause of microvascular dysfunction in rheumatoid arthritis. Rheumatology. 2008;47(12):1780–1784. doi:10.1093/rheumatology/ken386
35. Navarro-Millán I, Yang S, DuVall SL, et al. Association of hyperlipidaemia, inflammation and serological status and coronary heart disease among patients with rheumatoid arthritis: data from the National Veterans Health Administration. Ann Rheum Dis. 2016;75(2):341–347. doi:10.1136/annrheumdis-2013-204987
36. Zhou C, Cao J, Shang L, et al. Reduced paraoxonase 1 activity as a marker for severe coronary artery disease. Dis Markers. 2013;1:35.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
