Back to Journals » Drug Design, Development and Therapy » Volume 17
Assessment of Response to Different Induction Chemotherapy Regimens in Locally Advanced Nasopharyngeal Carcinoma
Authors Lian CL, Zhou R, Zhou Y, Zhou P, Wu SG
Received 2 December 2022
Accepted for publication 9 February 2023
Published 22 February 2023 Volume 2023:17 Pages 551—562
DOI https://doi.org/10.2147/DDDT.S399937
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Chen-Lu Lian,1– 3,* Rui Zhou,2,* Yuan Zhou,2,* Ping Zhou,2 San-Gang Wu1,2
1The School of Clinical Medicine, Fujian Medical University, Fuzhou, People’s Republic of China; 2Department of Radiation Oncology, Xiamen Cancer Center, Xiamen Key Laboratory of Radiation Oncology, The First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, People’s Republic of China; 3Department of Radiation Oncology, Fudan University Shanghai Cancer Center (Xiamen Branch), Xiamen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: San-Gang Wu, Department of Radiation Oncology, Xiamen Cancer Center, Xiamen Key Laboratory of Radiation Oncology, The First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, 361003, People’s Republic of China, Email [email protected]
Purpose: To compare the short-term treatment response and survival of the three induction chemotherapy (IC) regimens, including gemcitabine and cisplatin (GP), docetaxel and cisplatin (TP), and docetaxel, cisplatin, and fluoropyrimidines (TPF) in locally advanced nasopharyngeal carcinoma (LANPC).
Methods: We included stage III–IVA NPC patients who received ≥ 3 cycles of IC in this study. The chi-square test, multivariate logistic regression analysis, and Kaplan–Meier method were used for statistical analysis.
Results: A total of 227 patients were included. The overall response rate (ORR) of the primary nasopharyngeal tumors after IC with GP, TP, and TPF was 91.9%, 83.8%, and 91.7%, respectively (P=0.729), and the ORR of the cervical lymph nodes was 94.6%, 72.3%, and 85.0%, respectively (P< 0.001). For the primary nasopharyngeal tumor, there was no significant difference in the ORR among the three IC regimens. For cervical lymph nodes, patients treated with GP had significantly higher ORR compared to those treated with the TP regimen (P=0.014), and comparable ORR was found between TPF and GP regimens (P=0.161). Similar progression-free survival (PFS) (P=0.501) and overall survival (OS) (P=0.504) were found among three IC regimens. There were comparable PFS (P=0.123) and OS (P=0.478) among those with complete response (CR), partial response (PR), and stable disease (SD)/progressive disease (PD) in the primary nasopharyngeal tumors. However, patients who had CR in the primary nasopharyngeal tumor (P=0.014) and the cervical lymph nodes (P=0.022) had better PFS compared to those who had PR or SD/PD.
Conclusion: GP and TPF regimens are equivalent to the TP regimen in the response to primary nasopharyngeal tumors after IC, but with better ORR in the cervical lymph nodes than the TP regimen. The response to IC may be a powerful indicator for predicting prognosis and developing individualized follow-up and treatment strategies for LANPC patients.
Keywords: nasopharyngeal carcinoma, induction chemotherapy, short-term response, lymph node, survival
Introduction
Nasopharyngeal carcinoma (NPC) is a unique head and neck cancer with a distinct geographic distribution, which is endemic in Southern China and Southeast Asia.1 The morbidity and mortality rate of NPC in China accounts for 46.8% and 43.5% of the world, respectively.2 Due to the insidiousness of symptoms, approximately 70% of NPC patients have locally advanced disease at diagnosis.3,4 The results of the Intergroup trial 0099 demonstrated that the addition of chemotherapy to radiotherapy significantly improved the survival outcomes of locally advanced NPC (stage III–IVA disease) (LANPC).5 Several prospective studies have also confirmed the clinical value of chemotherapy in NPC.6–9 Induction chemotherapy (IC) followed by concurrent chemoradiotherapy (CCRT) is the preferred treatment strategy in the current National Comprehensive Cancer Network (NCCN) guidelines for stage II–IVA NPC.10 A similar recommendation was found in the recent Chinese Society of Clinical Oncology (CSCO) NPC guidelines regarding stage III–IVA (except for T3N0) patients.11
Gemcitabine and cisplatin (GP) or docetaxel, cisplatin, and 5-fluorouracil (TPF) regimens are the preferred IC regimens for NPC in the current NCCN guidelines [10]. In addition to GP and TPF regimens, the docetaxel and cisplatin (TP) regimen has also been recommended as an IC regimen in the CSCO guidelines.11 However, the optimal IC regimen for LANPC remains unclear due to differences in overall response rate (ORR) among the different IC regimens.12–15 Since the response to IC was associated with long-term survival of NPC,16–21 it is of great value to explore the efficacy of different IC regimens in this population. In light of this, this study aimed to compare the short-term treatment response and survival outcomes of the three IC regimens, including GP, TP as well as TPF in patients with LANPC.
Methods and Materials
Patients
We retrospectively included patients who were diagnosed with NPC between April 2013 and July 2021 in the NPC database of our institution. Patients who met the following criteria were included: 1) histologically diagnosed with NPC; 2) stage III–IVA disease with positive lymph nodes in the neck; 3) receiving ≥3 cycles of IC with GP, TPF (F including 5-fluorouracil [5-FU], and tegafur, gimeracil and oteracil potassium capsules), or TP regimens; 4) available contrast-enhanced magnetic resonance images (MRI) of the nasopharynx and whole neck before and after IC. Patients with the following criteria were excluded: 1) underwent resection of the primary nasopharyngeal tumor and/or the metastatic cervical lymph nodes; 2) had other malignancy before NPC diagnosis or was simultaneously diagnosed with NPC and other second primary cancers. All patients were staged according to the 8th edition of the UICC/AJCC staging system.22 This study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of the First Affiliated Hospital of Xiamen University. Informed consent was obtained from all the patients before treatment.
Treatment
All the eligible patients were administered ≥3 cycles of cisplatin-based IC every 3 weeks. The IC regimens included the TPF (docetaxel 75 mg/m2 on day 1, cisplatin 25 mg/m2 on days 1–3, and 5-FU 600–750 mg/m2 per day as a continuous 120 hours infusion or tegafur, gimeracil and oteracil potassium capsules 60 mg bid on day 1-14), TP (docetaxel 75 mg/m2 on day 1, cisplatin 25 mg/m2 on days 1–3), or GP regimens (gemcitabine 1000 mg/m2 on days 1 and 8, cisplatin 25 mg/m2 on days 1–3). The selection of IC regimens was mainly based on individual patient characteristics, previous clinical trial results, treatment guidelines from NCCN and CSCO, and willing of patients. Radiotherapy was administered to the nasopharynx and neck using intensity-modulated radiation therapy (IMRT) or image-guided radiation therapy (IGRT) after 3 weeks of the last cycle of IC. CCRT was delivered during radiotherapy and consisted of cisplatin (80–100 mg/m2 every 3 weeks), nedaplatin (30–40 mg/m2 every 3 weeks), or lobaplatin (30 mg/m2 every 3 weeks). The administration of concurrent chemotherapy was based on the physical tolerance of patients. Acute toxicities during IC were graded according to the Common Terminology Criteria for Adverse Events version 4.0.
Evaluation of Treatment Response
The assessment of tumor response was performed after the completion of IC, which was based on the MRI and nasopharyngeal fiberscope findings according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The overall response rate (ORR) was defined as the proportion of patients with a partial response (PR) or complete response (CR) in the primary nasopharyngeal tumors or cervical lymph nodes.
Clinical Endpoints
The primary survival endpoints of this study were progression-free survival (PFS) and overall survival (OS). PFS was defined as the time from the initial diagnosis of NPC to the first detection of locoregional recurrence and/or distant metastasis. OS was calculated from the date of NPC diagnosis to the date of death from all causes.
Statistical Analysis
The chi-square test or Fisher’s exact test was used to compare patients’ characteristics among the three IC regimens. Binary logistic regression analysis was used to determine the predictive factors associated with the treatment response of IC. The PFS and OS were calculated using the Kaplan–Meier method, and differences were compared by Log rank tests. All statistical analyses were conducted with the IBM SPSS 26.0 software package (IBM Corp., Armonk, NY). P values <0.05 were considered statistically significant.
Results
Patient Characteristics
A total of 227 patients were included in this study. Of these patients, 96 (42.3%) and 131 (57.7%) were stage IVA and IVB diseases, respectively. The baseline patient characteristics have listed in Table 1. There were 37 (16.3%), 130 (57.3%), and 60 (26.4%) patients receiving GP, TP, and TPF regimens, respectively. In the TPF group, 29 patients (48.3%) received 5-FU and 31 patients (51.7%) received tegafur, gimeracil, and oteracil potassium capsule. Female patients (P=0.002) were more likely to receive a GP regimen (P=0.002). Similar distributions were found regarding age, histology, tumor (T) stage, nodal (N) stage, and clinical stage among the three IC regimens.
 |
Table 1 Baseline Characteristics of Patients |
A total of 212 (93.4%) patients received CCRT. Among these patients, 192 (90.6%), 11 (5.2%), and 9 (4.2%) received cisplatin-, lobaplatin-, and nedaplatin-based CCRT. There were 34 (16.0%) and 178 (84.0%) patients who received one and two cycles of concurrent chemotherapy during radiotherapy, respectively.
Treatment Response After Induction Chemotherapy
The details of treatment responses after IC in the primary nasopharyngeal tumor and cervical lymph nodes have listed in Table 2 and Figures 1–2.
 |
Table 2 Treatment Responses After Induction Chemotherapy by Different Induction Chemotherapy Regimens |
 |
Figure 1 Treatment responses of the primary nasopharyngeal tumors after induction chemotherapy with different induction chemotherapy regimens. |
 |
Figure 2 Treatment responses of the cervical lymph nodes after induction chemotherapy with different induction chemotherapy regimens. |
In those receiving GP regimens, 6 patients (16.2%) had CR, 28 (75.7%) had PR, 3 (8.1%) had stable disease (SD), and no patient had progressive disease (PD) in the primary nasopharyngeal tumor. Regarding the TP regimen, 24 (18.5%), 85 (65.4%), 20 (15.4%), and 1 (0.8%) patients had CR, PR, SD, and PD, respectively. In the TPF regimen group, 13 (21.7%), 42 (70.0%), 5 (8.3%), and 0 patients had CR, PR, SD, and PD, respectively. However, there were no statistically significant differences in ORRs of the primary nasopharyngeal tumors among the three IC regimens (P=0.229) (Table 2) (Figure 1).
In those receiving the GP regimen (n=37), CR, PR, and SD rates were 32.4% (n=12), 62.2% (n=23), and 5.4% (n=2) for the cervical lymph nodes after IC. No patients had PD in the cervical lymph nodes in the GP group. Regarding TP regimen (n=130), CR, PR, SD, and PD rates were 11.5% (n=15), 60.8% (n=79), 26.9% (n=35), and 0.8% (n=1) in the cervical lymph nodes after IC, respectively. In the TPF group (n=60), CR, PR, and SD rates were 18.3% (n=11), 66.7% (n=40), and 15.0% (n=9) in the cervical lymph nodes after IC, respectively. Moreover, no patients had PD in the cervical lymph nodes in the TPF group. The ORR of the GP group was significantly higher than that of the TP and TPF groups (94.6% vs 72.3% vs 85.0%, P<0.001) (Table 2) (Figure 2).
Predictive Factors for Overall Response Rate After Induction Chemotherapy
The binary logistic regression analysis was performed to determine the predictive factors for ORR after IC. Age, gender, histology, T stage, N stage, IC regimen, and pretreatment EBV DNA level were considered as potential predictive factors and then included in the binary logistic regression analysis for determining the predictive factors associated with the ORR of the primary nasopharyngeal tumor and cervical lymph node after IC.
For the primary nasopharyngeal tumor, the results showed that the IC regimen was not a predictive factor of ORR (TP vs GP, odds ratio [OR] 0.505, P=0.322; TPF vs GP, OR 0.996, P=0.996) (Table 3). However, those with detective EBV-DNA had a significantly higher ORR rate in the primary nasopharyngeal tumor compared to those with undetected EBV-DNA (OR 3.225, P=0.030). For cervical lymph nodes, the results demonstrated that the IC regimen was an independent predictive factor for ORR. Patients treated with GP had significantly higher ORR compared to those treated with the TP regimen (TP vs GP, OR 0.155, P=0.014). However, comparable ORR was found between those treated with TPF and GP regimens (TPF vs GP, OR 0.318, P=0.161) (Table 4).
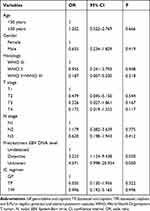 |
Table 3 Multivariate Logistic Regression Analysis of Predictive Factors for an Overall Response Rate of the Primary Nasopharyngeal Tumors |
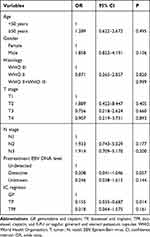 |
Table 4 Multivariate Logistic Regression Analysis of Predictive Factors for an Overall Response Rate of the Cervical Lymph Nodes |
Survival Outcomes According to Different Induction Chemotherapy Regimens
In this study, the TPF regimen was performed between April 2013 and September 2021, the TP regimen was applied between June 2014 and March 2021, and the GP regimen was applied between April 2020 and July 2021. The median follow-up time was 31.2 months (range, 6.9–110.3 months) in the entire cohort, and was 26.1 (range, 6.9–110.3 months), 36.8 (range, 9.0–96.8 months), and 19.3 (range, 11.0–26.1 months) in those receiving TPF, TP, and GP regimens, respectively. During the follow-up, 20 (8.8%) patients suffered from locoregional recurrence, and 34 (15.0%) patients developed distant metastasis, of which 6 patients had both locoregional recurrence and distant metastasis. A total of 25 patients died during the follow-up and 23 (92.0%) of them died from NPC. The 3-year PFS and OS were 75.8% and 88.2%, respectively. There were no statistically significant differences in the PFS (P=0.501) and OS rates (P=0.504) among the three IC regimens (Figures 3A and B).
 |
Figure 3 Progression-free survival (A) and overall survival (B) curves for patients receiving different induction chemotherapy regimens. |
Survival Outcomes According to Treatment Response After Induction Chemotherapy
For primary nasopharyngeal tumors, there were 43 (18.9%) patients who showed CR, and 155 (68.3%) patients had PR after IC. There were 28 (12.3%) patients who showed SD and one (0.4%) patient who showed PD in the primary nasopharyngeal tumors. Patients who had CR in the primary nasopharyngeal tumors had better PFS compared to those who had PR or SD/PD in the primary nasopharyngeal tumors (P=0.014), the 3-year PFS was 81.8%, 77.3%, and 61.1%, respectively (Figure 4A). However, a similar OS (P=0.141) (Figure 4B) was found among those with CR, PR, and SD/PD in the primary nasopharyngeal tumors.
 |
Figure 4 Progression-free survival (A) and overall survival (B) curves according to treatment response in the primary nasopharyngeal tumors after induction chemotherapy. |
For cervical lymph nodes, there were 38 (16.7%) patients who showed CR, and 142 (62.6%) patients who had PR after IC. There were 46 (20.3%) patients who showed SD and 1 (0.4%) patient who showed PD in the cervical lymph nodes. Patients who had CR in the cervical lymph nodes had better PFS compared to those who had PR or SD/PD in the cervical lymph nodes (P=0.022), the 3-year PFS was 90.4%, 77.2%, and 64.5%, respectively (Figure 5A). Moreover, patients who had CR in the cervical lymph nodes had better OS compared to those who had PR or SD/PD in the cervical lymph nodes (P=0.017), the 3-year OS was 93.8%, 91.5%, and 77.5%, respectively (Figure 5B).
 |
Figure 5 Progression-free survival (A) and overall survival (B) curves according to treatment response in the cervical lymph nodes after induction chemotherapy. |
Toxicity and Side Effects During Induction Chemotherapy
Table 5 lists the grade 3/4 acute toxicities among the three IC regimens. During the whole IC, the most common events were leukopenia (27.3%, n=62) and neutropenia (25.1%, n=57). Patients who received the TPF regimen had slightly higher grade 3 or 4 leukopenia (35.0% vs 23.8–27.0%, P=0.287), neutropenia (31.7% vs 20.0–24.3%, P=0.219), and mucositis (25.0% vs 10.8–16.2%, P=0.174) than those treated with GP and TP regimens, but without significant statistical differences. Patients who received GP regimen had slightly higher grade 3 or 4 Anemia (8.1% vs 3.1–5.0%, P=0.362) and thrombocytopenia (10.8% vs 3.3–3.8%, P=0.189) compared to those treated with TP and TPF regimens, but without significant statistical differences. Only 1.8% (n=4) and 1.3% (n=3) had grade 3 or 4 hepatotoxic and nephrotoxic events, respectively.
 |
Table 5 Grade 3/4 Acute Toxicities During Induction Chemotherapy Among the Three Chemotherapy Regimens |
Discussion
In this study, we investigated the short-term treatment response and survival outcomes among the GP, TP, and TPF IC regimens in NPC patients. Our results indicated that there was no significant correlation between the IC regimen and ORR in the primary nasopharyngeal tumor. However, GP and TPF regimens showed better ORR regarding the cervical lymph node compared to the TP regimen. Patients who had CR in the primary nasopharyngeal tumor and the cervical lymph nodes had significantly better PFS compared to those with PR or SD/PD.
In 2009, a randomized Phase II trial firstly showed that two cycles of TP-based IC followed by CCRT significantly improved the OS compared to those treated with CCRT alone in LANPC.6 Several Phase III randomized trials also confirmed that three cycles of GP- or TPF-based IC followed by CCRT could significantly improve the survival outcomes for this population.8,23 In the current NCCN guidelines, GP or TPF regimens are the preferred IC regimens for stage II–IVA NPC.10 However, GP, TPF as well TP regimens are the preferred IC regimens for NPC in the current CSCO guidelines.11 In our study, there were 16.3%, 57.3%, and 26.4% of patients received GP, TP, and TPF regimens, respectively. The selection of an IC regimen for individual patients was normally based on the clinical characteristics of the patient, previous clinical trial results, NCCN and CSCO treatment guidelines, and willing of patients. The results of the GP-based IC regimen in LANPC were published in September 2019.8 In our institution, the GP-based IC regimen began to use in LANPC patients in 2020.
In terms of the primary nasopharyngeal tumor, our findings were consistent with the results of Wang et al, which showed similar ORR in the primary nasopharyngeal tumor among the three IC regimens.14 However, the majority of patients receiving two cycles of IC, and ORR in the TP regimen was 96%, which was significantly higher than our study and the previous prospective studies.6,24 A previous study from Zang et al showed the opposite result that GP was better than TP in the regression of primary nasopharyngeal tumors.13 However, it should be noted that it was a study conducted in a non-endemic area of NPC in China, and the WHO III subtype accounted for only 72.2–74.6% of their enrolling patients (88.6% of patients with the WHO III subtype in our study). Several studies including ours have found that NPC patients with the WHO II subtype have a lower response to cisplatin and a higher risk of recurrence compared to those with WHO III NPC.25–27 Therefore, different chemotherapy cycles and different pathological subtypes may have an impact on the ORR of NPC patients treated with IC.
Regarding the cervical lymph nodes, our study found that the TP-based IC regimen had an inferior ORR compared with GP and TPF regimens. Several studies have found that the TPF regimen had a better ORR in the cervical lymph nodes than TP (80.0% vs 68.4%) and the GP regimen had a similar response to the TPF regimen.15,28,29 However, the results from Wang et al showed an ORR of 97.3%, 98.2%, and 97.4% in those treated with GP, TPF, and TP, respectively (P=0.977).14
Several studies have demonstrated that the addition of 5-FU or S1 could significantly inhibit lymph node metastasis in cancer cells.30,31 The results from NPC-9901 and NPC-9902 also showed that the addition of fluorouracil during the adjuvant phase was associated with significantly improved distant metastasis-free survival in this population.32 In our study, we found that the addition of fluorouracil to TP in the induction phase contributes to better ORR in the cervical lymph nodes.
After a short follow-up, we found similar PFS and OS rates among the three IC regimens. However, patients who had CR in the primary nasopharyngeal tumor and the cervical lymph nodes had better PFS compared to those who had PR or SD/PD in the cervical lymph nodes. Although our study did not explore the effect of different IC regimens on the long-term survival of these patients, several previous studies have demonstrated that the response to IC could predict long-term survival outcomes in NPC patients.33–38 A previous prospective study enrolling 185 NPC patients with stage III–IVB diseases found that an unsatisfactory tumor response (SD or PD) after IC was associated with poor progression-free survival and locoregional relapse-free survival.38 The results from Dwijayanti et al also showed that the 5-year OS was higher in the CR group than in the PR and PD groups (71.0% vs 30.4% vs.10.6%).35 Therefore, timely assessment after IC can assist clinicians to evaluate the prognosis of patients more accurately and then make a reasonable intensive treatment decision for those with a high risk of disease recurrence.
Several limitations should be acknowledged in this study. First, this is a retrospective study that has inherent selection bias. Second, a small sample size of patients and a short follow-up time of the patients limited our study to the general NPC population. Third, the proportions of patients receiving different IC regimens were quite an imbalance and the time range of patients receiving various IC regimens was quite different. However, patients who received radiotherapy were treated with IMRT or IGRT-based radiotherapy techniques in our study, which could reduce the potential impact of radiotherapy techniques on survival outcomes. Finally, a small proportion of patients might experience serious toxicities during IC and could not tolerate a such standard dose of IC.
In conclusion, our study suggests that GP and TPF regimens are equivalent to the TP regimen in the response to the primary nasopharyngeal tumors after IC, but with better ORR in the cervical lymph nodes than the TP regimen. The response to IC may be a powerful indicator for predicting prognosis. Patients with CR in the primary nasopharyngeal tumor and the cervical lymph nodes have significantly better PFS compared to those with PR or SD/PD. The findings of our study could offer additional information to develop individualized follow-up and treatment strategies for NPC patients.
Data Sharing Statement
Data will be made available on request.
Acknowledgments
Thanks to all the patients who participated in this study.
Author Contributions
CLL, RZ, and YZ drafted the manuscript. CLL acquired the datasets. SGW conceived the study. CLL conducted the statistical analyses. CLL, RZ, PZ, and YZ participated in the study design. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was partly supported by the Natural Science Foundation of Fujian Province (No. 2020J011220) and the Key Medical and Health Projects in Xiamen (No. 3502Z20209002).
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi:10.1016/S0140-6736(19)30956-0
2. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
3. Pan JJ, Ng WT, Zong JF, et al. Prognostic nomogram for refining the prognostication of the proposed 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122:3307–3315. doi:10.1002/cncr.30198
4. Yi JL, Gao L, Huang XD, et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: ten-year experience of a single institution. Int J Radiat Oncol Biol Phys. 2006;65:161–168. doi:10.1016/j.ijrobp.2005.12.003
5. Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi:10.1200/JCO.1998.16.4.1310
6. Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27:242–249. doi:10.1200/JCO.2008.18.1545
7. Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a Phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509–1520. doi:10.1016/S1470-2045(16)30410-7
8. Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med. 2019;381:1124–1135. doi:10.1056/NEJMoa1905287
9. Petit C, Lee AWM, Carmel A, et al. Network-meta-analysis of chemotherapy in nasopharyngeal carcinoma (MAC-NPC): an update on 8221 patients. J Clin Oncol. 2020;38:6523. doi:10.1200/JCO.2020.38.15_suppl.6523
10. Caudell JJ, Gillison ML, Maghami E, et al. NCCN Guidelines® Insights: head and Neck Cancers, Version 1.2022. J Natl Compr Canc Netw. 2022;20:224–234. doi:10.6004/jnccn.2022.0016
11. Tang LL, Chen YP, Chen CB, et al. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun. 2021;41:1195–1227. doi:10.1002/cac2.12218
12. Liu SL, Sun XS, Xie HJ, et al. Comparing three induction chemotherapy regimens for patients with locoregionally advanced nasopharyngeal carcinoma based on TNM stage and plasma Epstein-Barr virus DNA level. BMC Cancer. 2020;20:89. doi:10.1186/s12885-020-6555-7
13. Zang J, Xu M, Li C, et al. Gemcitabine and cisplatin versus docetaxel and cisplatin as induction chemotherapy followed by concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma from non-endemic area of China. J Cancer Res Clin Oncol. 2020;146:2369–2378. doi:10.1007/s00432-020-03229-3
14. Wang F, Chuner J, Lei W, et al. Optimal induction chemotherapeutic regimen followed by concurrent chemotherapy plus intensity-modulated radiotherapy as first-line therapy for locoregionally advanced nasopharyngeal carcinoma. Medicine. 2020;99:e22283. doi:10.1097/MD.0000000000022283
15. Lokesh KN, Chaudhuri T, Lakshmaiah KC, et al. Induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma in adults: results from a nonendemic region. Indian J Cancer. 2018;55:257–260. doi:10.4103/ijc.IJC_115_18
16. Peng H, Tang LL, Chen BB, et al. Optimizing the induction chemotherapy regimen for patients with locoregionally advanced nasopharyngeal Carcinoma: a big-data intelligence platform-based analysis. Oral Oncol. 2018;79:40–46. doi:10.1016/j.oraloncology.2018.02.011
17. Zeng Z, Yan RN, Tu L, et al. Assessment of Concurrent Chemoradiotherapy plus Induction Chemotherapy in Advanced Nasopharyngeal Carcinoma: cisplatin, Fluorouracil, and Docetaxel versus Gemcitabine and Cisplatin. Sci Rep. 2018;8:15581. doi:10.1038/s41598-018-33614-5
18. Zhu J, Duan B, Shi H, et al. Comparison of GP and TPF induction chemotherapy for locally advanced nasopharyngeal carcinoma. Oral Oncol. 2019;97:37–43. doi:10.1016/j.oraloncology.2019.08.001
19. Bongiovanni A, Vagheggini A, Fausti V, et al. Induction chemotherapy plus concomitant chemoradiotherapy in nasopharyngeal carcinoma: an updated network meta-analysis. Crit Rev Oncol Hematol. 2021;160:103244. doi:10.1016/j.critrevonc.2021.103244
20. Peng H, Chen B, He S, et al. Efficacy and Toxicity of Three Induction Chemotherapy Regimens in Locoregionally Advanced Nasopharyngeal Carcinoma: outcomes of 10-Year Follow-Up. Front Oncol. 2021;11:765378. doi:10.3389/fonc.2021.765378
21. He Y, Guo T, Wang J, et al. Which induction chemotherapy regimen followed by cisplatin-based concurrent chemoradiotherapy is the best choice among PF, TP and TPF for locoregionally advanced nasopharyngeal carcinoma? Ann Transl Med. 2019;7:104. doi:10.21037/atm.2019.02.15
22. Tang LL, Chen YP, Mao YP, et al. Validation of the 8th Edition of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma From Endemic Areas in the Intensity-Modulated Radiotherapy Era. J Natl Compr Canc Netw. 2017;15:913–919. doi:10.6004/jnccn.2017.0121
23. Li WF, Chen NY, Zhang N, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J Cancer. 2019;145:295–305. doi:10.1002/ijc.32099
24. Zhong YH, Dai J, Wang XY, et al. Phase II trial of neoadjuvant docetaxel and cisplatin followed by intensity-modulated radiotherapy with concurrent cisplatin in locally advanced nasopharyngeal carcinoma. Cancer Chemother Pharmacol. 2013;71:1577–1583. doi:10.1007/s00280-013-2157-2
25. Guo R, Wu H, Wang J, et al. Lymph Node Status and Outcomes for Nasopharyngeal Carcinoma According to Histological Subtypes: a SEER Population-Based Retrospective Analysis. Adv Ther. 2019;36:3123–3133. doi:10.1007/s12325-019-01100-7
26. Lee HW, Hwang YH, Han JH, et al. High expression of excision repair cross-complementation group 1 protein predicts poor outcome in patients with nasopharyngeal cancer. Oral Oncol. 2010;46:209–213. doi:10.1016/j.oraloncology.2009.12.007
27. Cheung F, Chan O, Ng WT, et al. The prognostic value of histological typing in nasopharyngeal carcinoma. Oral Oncol. 2012;48(5):429–433. doi:10.1016/j.oraloncology.2011.11.017
28. Jin Y, Shi YX, Cai XY, et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2012;138:1717–1725. doi:10.1007/s00432-012-1219-x
29. Chen J, Qi J, Yu B, et al. A Retrospective Study to Compare Five Induction Chemotherapy Regimens Prior to Radiotherapy in the Reduction of Regional Lymph Node Size in Patients with Nasopharyngeal Carcinoma. Med Sci Monit. 2018;24:2562–2568. doi:10.12659/MSM.906625
30. Tsuruo T, Naganuma K, Iida H, et al. Lymph node metastasis and effects of 1-beta-D-arabinofuranosylcytosine, 5-fluorouracil, and their lipophilic derivatives in an experimental model system using P388 leukemia. Cancer Res. 1980;40:4758–4763.
31. Sato H, Hatori M, Ando Y, et al. S-1 mediates the inhibition of lymph node metastasis in oral cancer cells. Oncol Rep. 2009;22:719–724. doi:10.3892/or_00000492
32. Lee AW, Tung SY, Ngan RK, et al. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer. 2011;47:656–666. doi:10.1016/j.ejca.2010.10.026
33. Liu LT, Tang LQ, Chen QY, et al. The Prognostic Value of Plasma Epstein-Barr Viral DNA and Tumor Response to Neoadjuvant Chemotherapy in Advanced-Stage Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys. 2015;93:862–869. doi:10.1016/j.ijrobp.2015.08.003
34. Peng H, Chen L, Li WF, et al. Tumor response to neoadjuvant chemotherapy predicts long-term survival outcomes in patients with locoregionally advanced nasopharyngeal carcinoma: a secondary analysis of a randomized phase 3 clinical trial. Cancer. 2017;123:1643–1652. doi:10.1002/cncr.30520
35. Dwijayanti F, Prabawa A, Besral F, et al. The Five-Year Survival Rate of Patients with Nasopharyngeal Carcinoma Based on Tumor Response after Receiving Neoadjuvant Chemotherapy, Followed by Chemoradiation, in Indonesia: a Retrospective Study. Oncology. 2020;98:154–160. doi:10.1159/000504449
36. Wang W, Peng S, Wu H, et al. Association of tumor downstaging after neoadjuvant chemotherapy with survival in patients with locally advanced nasopharyngeal carcinoma: a retrospective cohort study. J Cancer Res Clin Oncol. 2021;147:2913–2922. doi:10.1007/s00432-021-03690-8
37. Jiang YT, Chen KH, Liang ZG, et al. A nomogram based on tumor response to induction chemotherapy may predict survival in locoregionally advanced nasopharyngeal carcinoma. Head Neck. 2022;44(6):1301–1312. doi:10.1002/hed.27020
38. Xu Y, Wu Z, Ye W, et al. Prognostic value of serum uric acid and tumor response to induction chemotherapy in locally advanced nasopharyngeal carcinoma. BMC Cancer. 2021;21:519. doi:10.1186/s12885-021-08285-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
