Back to Journals » Drug, Healthcare and Patient Safety » Volume 13
Assessment of Potential Drug–Drug Interactions and Their Predictors in Chronic Outpatient Department of Dessie Referral Hospital, Dessie, Northeast Ethiopia
Authors Gobezie MY , Bitew HB, Tuha A , Hailu HG
Received 8 October 2020
Accepted for publication 28 January 2021
Published 11 February 2021 Volume 2021:13 Pages 29—35
DOI https://doi.org/10.2147/DHPS.S279371
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hemalkumar B Mehta
Mengistie Yirsaw Gobezie,1 Hailu Birhanu Bitew,1 Abdu Tuha,1 Haftom Gebregergs Hailu2
1Department of Pharmacy, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia; 2Department of Pharmacology and Toxicology, School of Pharmacy, College of Health Sciences, Mekelle University, Mekelle, Ethiopia
Correspondence: Haftom Gebregergs Hailu Tel +251912085606
Fax +251344416681
Email [email protected]
Objective: To assess the prevalence and predictors of Potential drug–drug interactions (DDIs) at the chronic outpatient department of Dessie Referral Hospital, Dessie, Northeast Ethiopia.
Patients and Methods: A cross-sectional study was carried out on the medical records of patients treated in the chronic ambulatory department of Dessie Referral Hospital (DRH), from March 1/2019 to May 30/2019. Ethical clearance was granted from the department of pharmacy, college of medicine, and health sciences, Wollo University. Lexi-comp computer program database was used to detect pDDIs. SPSS version 22 was used to produce a descriptive analysis of the background data and logistic regression to identify predictors of pDDIs.
Results: In this study, the medical record of 300 patients has been reviewed and 489 pDDIs have been identified. The prevalence of pDDIs per patient was 1.63. Of all the identified pDDIs, the moderate severity interactions were the majority, 88.55% (n=433) followed by 8.38% (n=41) of minor, 2.66% (n=13) of major, and 0.41% (n=2) of contraindicated drug interactions. Taking three or more drugs at a time has been found as a statistically significant predictor of the occurrence of pDDIs.
Conclusion: A high rate of moderate severity pDDIs have been recorded. A system of checks and balances should be developed and executed for all those who are involved in prescribing, dispensing, and administration of medications for effective identification and prevention of pDDIs.
Keywords: patient safety, polypharmacy, prevalence, drug selection
Introduction
Because of their greater impact on medical and societal issues, adverse drug events (ADEs) in recent decades have become a major area of concern in the health care system.1,2 ADEs carries a significant burden of inpatient hospital care, raise total health care cost, and leads to increased loss of life. A retrospective analysis of a large inpatient database estimated that ADE is responsible for the hospitalization of 6.28% of all patients. The costs due to preventable adverse drug reactions (ADRs) in the out-patient setting might stretch to € 8515 and 9.2 days of hospital stay.3,4
A significant percentage of ADEs are preventable if adequate emphasis is given and detected early.3,5 Potential Drug–Drug Interactions (pDDIs) constitute one of the often preventable Causes of ADEs.6,7 Stockley’s drug interaction definition declares that interaction is said to occur when the pharmacological effects of one drug are changed by the presence of another drug, herbal medicine, food, drink, or by some environmental chemical agent(s).8
Drug–drug interactions (DDIs) are classified based on their mechanism of interaction as, pharmacokinetics and pharmacodynamics, but there are few unknown mechanisms of interactions.8–10 Based on the severity and significance of interactions, pDDIs can be rated as, severe, moderate, and minor.9,11–13 The occurrence of pDDIs ranges from 19.3% to 91.6% across different health settings and patients.11,14 The rate of hospital admissions in the general population due to pDDIs stretches to 1.1%.15 The prevalence of clinically important potential pDDIs was found to be 47.4% and the incidence of DDI-related ADRs in chronic geriatric patients reaches about 6.5% and most of the events presented important clinical consequences.16
The prevalence of pDDIs relates to the severity of the illness, age of the patient, the health care setting, number of medications, and length of hospital stay, presence of multiple comorbidities, and gender of the patient.7,11,17,18
Although pDDIs has been reported in different populations, there are no known data from Ethiopia and relatively little from under-developed nations. Therefore, the study aimed to measure the prevalence, clinical significance, and associated factors of pDDIs in the chronic outpatient department of DRH, Dessie, Northeast Ethiopia.
Patients and Methods
A cross-sectional study was carried out at the outpatient department for 3 months (March–May 2019) in DRH, Dessie, Northeast Ethiopia. DRH is the biggest hospital in the Northeast region of the country. The study was approved by the ethical committee of the department of pharmacy, college of medicine and health sciences, Wollo University. It was approved through a letter written on January 28, 2019, with a reference number of WU phar/205/19. The ethical committee waived the need for patient consent provided that data collectors ensured privacy and confidentiality during the review of patients’ records. As a result, the name and addresses of patients were not recorded in the data collection forms.
The chronic outpatient department has three rooms, which can serve 100 patients per day. Patients who visited the chronic outpatient department during the study period were included. Those with incomplete medical records were excluded from the study.
Information picked from the medical records included demographic characteristics, diagnosed main disorder, and other comorbidities, and number and type of prescribed drugs. All drugs were checked for pDDIs. The Ethiopian standard treatment guideline was used for grouping of patient diagnosis; accordingly, patients were categorized into six groups: Cardiovascular, Endocrine, Neurologic, Cardiovascular and endocrine, Cardiovascular and neurologic, and the others.
In the hospital, there is no any pDDIs screening and detection mechanism. Pharmacy professionals simply dispense prescribed medication without reconciliation of interactions. In this study, pDDIs were identified using Lexi-comp’s Drug–Drug Interactions Checker computer application.19 The detected DDIs were classified as level: X–interaction, D-interaction C-interaction, and B-interaction. Detected pDDIs were manually checked for the presence of sound scientific literature. Based on the severity of the pDDIs, interactions’ were grouped according to Lexicomp® classification as:
X-interaction (avoid combination) – the risks associated with concomitant use of these agents usually outweigh the benefits. These agents are generally considered contraindicated.
D-interaction (consider therapy modification) – a patient-specific assessment must be conducted to determine whether the benefits of concomitant therapy outweigh the risks. Specific actions must be taken to realize the benefits and/or minimize the toxicity resulting from the concomitant use of the agents and considered as major interactions.
C-interaction (monitor therapy) – the benefits of concomitant use of these two medications usually outweigh the risks. An appropriate monitoring plan should be implemented to identify potential negative effects and considered them as moderate interactions.
B-interactions (no action needed) – data demonstrate that the specified agents may interact with each other, but there is little to no evidence of clinical concern resulting from their concomitant use and generally classified as minor interactions.
Data analysis was done using the computer program SPSS version 22. The frequencies and percentages of the demographic characteristics, main diagnosis, number of drugs per patient, and the prevalence of the pDDIs are summarized in tables. Initially, a univariate analysis was performed for each independent variable, and variables having a p-value less than 0.25 were fitted into a multivariate logistic regression to determine the presence of an association between variables. The number of drugs per patient, diagnosis, and the age of the subjects are summarized with an odds ratio of 95% confidence interval. P-value was set at 0.05 or less.
Operational Definition
Chronic outpatient department: it is the part of a hospital designed for the treatment of outpatients with chronic health problems who visit the hospital for diagnosis or treatment, but do not require a bed at the time or to be admitted for overnight care.
Polypharmacy: regular use of at least four medications.
Results
During the study period, 315 chronic outpatients came to DRH. Of those, the medical records of 15 patients were discarded because of incompleteness.
Sociodemographic and Clinical Data
From the total 300 patients, 176 were females and their ages ranged from 18 to 84 years, and the mean age was 51.54 ± 15.568 years. The majority of the study participants (54.7%) came with cardiovascular diseases and nearly half (49.3%) were diagnosed with two disease conditions. The number of drugs per patient ranged from 2 to 7 and the mean number of it was 3.24 ± 1.153. Polypharmacy was practiced in 46 (15.3%) participants (Table 1).
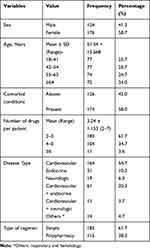 |
Table 1 Sociodemographic and Clinical Data of the Study Subjects |
Prevalence and Severity of Potential DDIs
A total of 973 drugs were prescribed for 300 chronic ambulatory patients and 489 pDDIs were identified. The prevalence of pDDI per patient was 1.63 (489/300). Regardless of the severity of interactions, 228 (76%) patients had at least one pDDIs. Based on the severity of pDDIs, 433 (88.55%) and 41 (8.38%) interactions are classified as moderate and minor interactions, respectively (Table 2).
 |
Table 2 Prevalence and Severity of pDDIs |
Common Interacting Drug Combinations
Among 31 drug combinations which were responsible for 489 potential DDIs, 280 (57.3%) were contributed by: enalapril + hydrochlorothiazide (46), aspirin + enalapril (34), spironolactone + enalapril (57), enalapril + furosemide (69), metformin + enalapril (32) and metformin + glibenclamide (42). The most serious drug interactions were found among salbutamol with propranolol and carbamazepine with nifedipine (Table 3).
 |
Table 3 Common Interacting Drug Combinations |
Factors Associated with Potential DDIs
Multivariate analyses showed that patients taking more than three concomitant drugs (polypharmacy) are at higher risk of pDDIs (adjusted odds ratio (AOR) (95% confidence interval (CI) =5.794 (2.719–12.348)) and P<0.001), but other variables did not show significant association with pDDIs (Table 4).
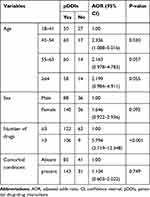 |
Table 4 Factors Associated with pDDIs |
Discussion
This study aimed at assessing the prevalence and severity of pDDIs in outpatient settings. Drug interaction is a very important issue in drug therapy. In this study, 973 drugs were prescribed for 300 chronic outpatients visiting DRH and 489 pDDIs were identified. This study showed that the overall prevalence of at least one pDDI in 76% (228/300) of study participants would have resulted from the combinations of the prescribed drugs. This is lower than reports in Saudi Arabia (90.64%),20 however, comparable with a result of studies conducted at the internal medicine ward of Gondar University hospital (78%) and Black Lion tertiary hospital (78.02%).10,21 This might indicate the need for equal attention of pDDIs during the prescription of medicines for chronic patients visiting the outpatient department and admitted patients. But, lower figures were reported by other studies in Brazil (26.5%),22 in Thailand (27.9%)18 and pediatrics ward of Gondar University hospital (45.8%),12 this might be partially explained by the difference in availability of drugs, use of less number of drugs per patient in younger age groups.13
The result of this study revealed that the prevalence of pDDIs per patient was 1.63. This figure agrees with a finding in Pakistan 1.7423 and India 1.68.24 However, the present result is lower than results reported in adult medical wards of Gondar University and Black Lion tertiary hospitals which were 4.2 and 2.9, respectively,10,21 even though, higher than a finding in Brazil (0.22),22 and pediatric wards of Gondar University hospital (1.02).12 This may be due to the differences in the study setting, type, and the number of drugs used by study participants.
All pDDIs were not equally detrimental, though, recognition of the severity of each pDDI was central to assess the clinical importance and appropriate management. The list of interacting drug combinations, particularly prevailing major and moderate interactions, is useful for health care professionals to monitor patient profiles for pDDIs. Of the total 489, pDDIs identified, major interactions covered only 2.66% of the interactions which is comparable with a finding in Saudi Arabia (2.6%),20 but much lower when compared with findings in other studies from Gondar University hospital (10–13%) and Black Lion hospital (13.1%). This wide difference may be due to the differences in the study population, two of the studies were conducted in internal medicine wards and the other one was conducted in the pediatrics ward.10,12,21 Around half of the major severity pDDIs were observed with Amlodipine + phenytoin (6 combinations) which can potentially result in toxicity of phenytoin in chronic patients. Most interacting drug combinations were moderate severity, which covers 88.55% of the identified pDDIs. This figure was higher in comparison to other studies in Ethiopia.10,12,21 Enalapril was responsible for the occurrence of 238 moderate severity level pDDIs which may result in negative outcomes for the intended treatments. Two contraindicated pDDIs were identified (propranolol + salbutamol and carbamazepine + nifedipine) which may end up in antagonized bronchoconstriction and poor control of hypertension, respectively.19
The other result of this study showed that polypharmacy (the use of three or more drugs for a patient) has a statistically significant association with pDDIs in chronic outpatients [AOR = 5.794; 95% CI (2.719–12.348); P =000]. The risk of pDDIs has increased when the number of drugs per individual was more than three which is in line with other studies.12,16,21,25,26 In this study, other variables like gender, age, and comorbid conditions did not show a statistically significant association with the incidence of pDDIs which did not correspond with findings in Saudi Arabia20 and Brazil.16,22
The limitations of the study should not be overlooked. The drug–drug interactions found were only potential (it is not clear whether they had resulted in any harm to the patients). The results of this study might be slightly underestimated since only prescribed medications were included. The software used for the analysis of pDDIs unable to distinguish between the two different doses scheme. Therefore, dose-dependent pDDIs were not assessed.
This study successfully identified the prevalence, pattern, and factors associated with pDDIs in the outpatient department of DRH. The overall prevalence of pDDIs was found to be 76% and the majority of the pDDIs were moderate in severity. Enalapril was responsible for around half of all the pDDIs identified. Moreover, a statistically significant association was found between the number of drugs prescribed per patient (≥3 drugs per patient) and the occurrence of pDDIs.
Finally, drug–drug interactions are avoidable and the role of pharmacists in its prevention is irreplaceable. However, effective identification and prevention of pDDIs need a systematic check and balances by all those who are involved in prescribing, dispensing, and administration of medications.
Data Sharing Statement
The data used to generate this result are available from the corresponding author upon reasonable request.
Ethical Review
This study was approved by the ethical committee of the department of pharmacy, college of medicine and health sciences, Wollo University. The ethical committee waived the need for patient consent provided that data collectors ensured privacy and confidentiality during the review of patients’ records. As a result, the name and addresses of patients were not recorded in the data collection forms. This study was done with the consideration and compliance with the declaration of Helsinki.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
No funding has been received to conduct this study.
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Harris Y, Hu DJ, Lee C, Mistry M, York A, Johnson TK. Advancing medication safety: establishing a national action plan for adverse drug event prevention. Jt Comm J Qual Patient Saf. 2015;41(8):351–360. doi:10.1016/S1553-7250(15)41046-3
2. Kohn LT, Corrigan JM, Molla S. To Err is Human: Building a Safer Health System. Vol. 6. Washington DC: National academy press. 2000. doi:10.1016/j.yrtph.2007.09.017
3. Poudel DR, Acharya P, Ghimire S, Dhital R, Bharati R. Burden of hospitalizations related to adverse drug events in the USA: a retrospective analysis from large inpatient database. Pharmacoepidemiol Drug Saf. 2017;26(6):635–641. doi:10.1002/pds.4184
4. Formica D, Sultana J, Cutroneo PM, et al. The Economic Burden of Preventable Adverse Drug Reactions: A Systematic Review of Observational Studies. Vol. 17. Taylor & Francis; 2018. doi:10.1080/14740338.2018.1491547
5. Kuklik N, Stausberg J, Amiri M, Jöckel KH. Improving drug safety in hospitals: a retrospective study on the potential of adverse drug events coded in routine data. BMC Health Serv Res. 2019;19(1):1–7. doi:10.1186/s12913-019-4381-x
6. Mirosevic Skvrce N, Macolic Sarinic V, Mucalo I, Krnic D, Bozina N, Tomic S. Adverse drug reactions caused by drug-drug interactions reported to croatian agency for medicinal products and medical devices: a retrospective observational study. Croat Med J. 2011;52(5):604–614. doi:10.3325/cmj.2011.52.604
7. Bucşa C, Farcaş A, Cazacu I, et al. How many potential drug-drug interactions cause adverse drug reactions in hospitalized patients? Eur J Intern Med. 2013;24(1):27–33. doi:10.1016/j.ejim.2012.09.011
8. Baxter K. Stockley’s Drug Interactions.
9. Jimmy OD, ShobhaRani RH, Indira R, Ramjan S. Study of drug-drug interactions in the medication charts in medicine wards at a tertiary care hospital, Bangalore. Indian J Pharmacy Practice. 2012;5:4.
10. Tesfaye ZT, Nedi T. Potential drug–drug interactions in inpatients treated at the internal medicine ward of Tikur Anbessa Specialized Hospital. Drug Healthc Patient Saf. 2017;9:71–76. doi:10.2147/DHPS.S126336
11. Murtaza G, Khan MYG, Azhar S, Khan SA, Khan TM. Assessment of potential drug-drug interactions and its associated factors in the hospitalized cardiac patients. Saudi Pharm J. 2016;24(2):220–225. doi:10.1016/j.jsps.2015.03.009
12. Getachew H, Assen M, Dula F, Bhagavathula AS. Potential drug-drug interactions in pediatric wards of Gondar University Hospital, Ethiopia: a cross sectional study. Asian Pac J Trop Biomed. 2016;6(6):534–538. doi:10.1016/j.apjtb.2016.04.002
13. Feinstein J, Dai D, Zhong W, Freedman J, Feudtner C. Potential drug-drug interactions in infant, child, and adolescent patients in Children’s Hospitals. Pediatrics. 2015;135(1):e99–e108. doi:10.1542/peds.2014-2015
14. Reimche L, Forster AJ, Van Walraven C. Incidence and contributors to potential drug-drug interactions in hospitalized patients. J Clin Pharmacol. 2011;51(7):1043–1050. doi:10.1177/0091270010378858
15. Supinya D, Sirada M, Bodin B, Chuenjid K. Hospital admissions/visits associated with drug–drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2014. doi:10.1002/pds.3592
16. Neto PRO, Nobili A, Marusic S, et al. Prevalence and predictors of potential drug-drug interactions in the elderly: a cross-sectional study in the Brazilian primary public health system. J Pharm Pharm Sci. 2012;15(2):344–354. doi:10.18433/j37k5w
17. Reis AMM, Cassiani SHDB. Prevalence of potential drug interactions in patients in an intensive care unit of a university hospital in Brazil. Clinics. 2011;66(1):9–15. doi:10.1590/S1807-59322011000100003
18. Janchawee B, Wongpoowarak W, Owatranporn T, Chongsuvivatwong V. Pharmacoepidemiologic study of potential drug interactions in outpatients of a university hospital in Thailand. J Clin Pharm Ther. 2005;30(1):13–20. doi:10.1111/j.1365-2710.2004.00598.x
19. Lexicomp (Computer Software). Riverwoods, IL:W olters Kluwer clinical drug information. Wolters Kluwer Health, Inc; May 2019.
20. Aljadani R, Aseeri M. Prevalence of drug–drug interactions in geriatric patients at an ambulatory care pharmacy in a tertiary care teaching hospital. BMC Res Notes. 2018;11(1):1–7. doi:10.1186/s13104-018-3342-5
21. Bhagavathula AS, Berhanie A, Tigistu H, et al. Prevalence of potential drug-drug interactions among internal medicine ward in University of Gondar Teaching Hospital, Ethiopia. Asian Pac J Trop Biomed. 2014;4(Suppl 1):S204–S208. doi:10.12980/APJTB.4.2014C1172
22. Secoli S-R, Figueras A, Lebrão ML, de Lima FD, Santos JLF. Risk of potential drug-drug interactions among Brazilian elderly. Drugs Aging. 2010;27(9):759–770. doi:10.2165/11538460-000000000-00000
23. Farooqui R, Hoor T, Karim N, Muneer M. Potential drug-drug interactions among patient’s prescriptions collected from medicine out-patient setting. Pakistan J Med Sci. 2018;34(1):144–148. doi:10.12669/pjms.341.13986
24. Chavda NB, Solanky PP, Baria H, Naik R, Bharti K. Study of potential drug–drug interaction between prescribed drugs in patients attending outpatient department of medicine at tertiary-care hospital in south Gujarat region. Natl J Physiol Pharm Pharmacol. 2015;5(3):236–242. doi:10.5455/njppp.2015.5.0508201428
25. Obreli-Neto PR, Nobili A, De Oliveira Baldoni A, et al. Incidence and predictors of adverse drug reactions caused by drug-drug interactions in elderly outpatients: a prospective cohort study. J Pharm Pharm Sci. 2012;12(2):332–343. doi:10.1007/s00228-012-1309-3
26. Doubova SV, Reyes-Morales H, Torres-Arreola LDP, Suárez-Ortega M. Potential drug-drug and drug-disease interactions in prescriptions for ambulatory patients over 50 years of age in family medicine clinics in Mexico City. BMC Health Serv Res. 2007;7(147):1–8. doi:10.1186/1472-6963-7-147
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
