Back to Journals » Patient Preference and Adherence » Volume 12
Assessment of medication adherence and responsible use of isotretinoin and contraception through Belgian community pharmacies by using pharmacy refill data
Authors Biset N, Lelubre M, Senterre C, Amighi K, Bugnon O, Schneider MP , De Vriese C
Received 17 August 2017
Accepted for publication 20 October 2017
Published 19 January 2018 Volume 2018:12 Pages 153—161
DOI https://doi.org/10.2147/PPA.S149355
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Natacha Biset,1,* Mélanie Lelubre,1–3,* Christelle Senterre,4 Karim Amighi,1 Olivier Bugnon,2,3 Marie P Schneider,2,3 Carine De Vriese1
1Department of Pharmacotherapy and Pharmaceutics, Faculté de Pharmacie, Université libre de Bruxelles (ULB), Brussels, Belgium; 2School of Pharmaceutical Sciences, University of Geneva, University of Lausanne, Geneva, Switzerland; 3Community Pharmacy, Department of Ambulatory Care & Community Medicine, University of Lausanne, Lausanne, Switzerland; 4Research Center of Epidemiology, Biostatistics and Clinical Research, School of Public Health, Université libre de Bruxelles (ULB), Brussels, Belgium
*These authors contributed equally to this work
Purpose: The aims of the study were to evaluate medication adherence and the influencing factors for isotretinoin and contraception (oral, patches, and rings) and to evaluate the concomitant use of contraception and isotretinoin.
Methods: Reimbursed prescription data from January 2012 to August 2015 of all patients in Belgium were received from Pharmanet–National Institute for Health and Disability Insurance. Medication adherence was measured according to the medication possession ratio. The influence of gender and age was analyzed using the Mann–Whitney test and the Spearman coefficient correlation. The independence between adherence to contraception and adherence to isotretinoin was analyzed using the Pearson chi-square test of independence. Persistence was defined as the number of days between initiation and presumed end of treatment. The Kaplan–Meier method was used to plot the medication persistence curves, and the log-rank test was used to compare the curves. The concomitant use of contraception and isotretinoin was analyzed using descriptive statistics.
Results: The medication possession ratio was ≥0.8 for 46.1% of patients receiving isotretinoin and for 74.0% of women using contraception. For isotretinoin, this percentage decreased as the number of attempts increased (29.8% for the second attempt and 19.8% for more than two attempts). Men seemed more adherent than women, and a weak negative correlation between adherence and age was observed. The adherence data of isotretinoin and contraception were independent. The median persistence for isotretinoin treatment was 139 days (interquartile range 71–209) and was higher for men. Among women between 12 and 21 years old taking isotretinoin, 63.8% received at least one contraceptive prescription. However, 15.7% of women taking isotretinoin adhered to the use of contraception 1 month before, during, and 1 month after treatment.
Conclusion: Medication adherence to isotretinoin and contraception and compliance with the isotretinoin safety recommendation could be improved. Health service interventions, using pharmacy refill data, should be delivered to ensure patient safety and strict adherence to contraception when under isotretinoin treatment.
Keywords: medication possession ratio, persistence, patient safety, pregnancy prevention program
Introduction
Acne is described as a common chronic inflammatory dermatosis that significantly impacts patients’ quality of life, especially on an emotional, social and psychological level.1,2 The first-line treatment, according to the Up-to-Date guidelines, is a combination of a topical retinoid, such as adapalene or tretinoin, and an oral antibiotic. Oral isotretinoin, a second-line treatment, is generally recommended for patients with severe acne or in the case of resistance to other treatments.3
Acne requires prolonged treatment, and medication adherence to anti-acne treatment is ∼50%.2 The consequences of low medication adherence to anti-acne treatment result in additional unnecessary treatments, frustration, patient dissatisfaction and increased medical expenses.4 Medication adherence is a longitudinal behavior defined as the process by which patients take their medications as prescribed.5 It can be measured by direct methods such as electronic monitoring, which registers the opening of the pillbox every day, or by indirect methods such as pharmacy refill data or self-report questionnaires that are easy to collect.6
Isotretinoin is a vitamin A derivative usually given over a 20-week course (16–24 weeks), with a dosage of 0.5–1 mg/kg/day, depending on clinical response and side effects. The treatment seems to be more effective when a cumulative dose of 120 mg/kg is taken. Most of the time, a single attempt is sufficient to cure patients. If necessary, a second attempt can be considered at least 8 weeks after treatment discontinuation as the improvement of acne can continue until 8 weeks after discontinuation. Frequent side effects are mucous membrane and skin dryness, hepatotoxicity, lipid disorders, psychiatric disorders and teratogenicity.3,7,8 Because of the high teratogenic risk of the treatment, a pregnancy prevention program (PPP) for women of childbearing age was developed by Roche in 1988 and implemented in Europe for all oral isotretinoin generic drugs in 2003.9 One of the recommendations of this PPP is the use of an effective contraception method 1 month before, during and 1 month after treatment.10 Despite the implementation of this program, exposed pregnancies still occur in Europe and compliance with recommendations to prevent a birth defect risk seems to be limited.11 Beyond this, unintended pregnancies may occur if medication adherence to contraception is not sufficient.12,13 However, medication adherence is not considered in the isotretinoin PPP and has never been assessed to a national level in Belgium.
The two aims of this study were, 1) to evaluate medication adherence and influencing factors of isotretinoin for men and women and for contraception (oral, patches and rings) for women, using the Belgian community pharmacy refill data, and 2) to evaluate the concomitant use of contraception 1 month before, during and 1 month after isotretinoin treatment for women in Belgium.
Methods
Data source
Community pharmacy refill data were extracted for all Belgian patients from Pharmanet. Pharmanet is a national health care claims database of the National Institute for Health and Disability Insurance (INAMI), which contains all administrative information on reimbursed medicines delivered to all patients in Belgium.14 Pharmanet coded data from January 2012 to August 2015 and sent to us two Excel files with the data of, 1) isotretinoin delivered to men and women, and 2) contraception delivered to women who received at least one prescription of isotretinoin.
Study design and study population
This was a retrospective study using pharmacy refill data.
For evaluating medication adherence and influencing factors of isotretinoin and contraception, patients eligible for measuring medication adherence and persistence were those who received at least two prescriptions on two different days during the study period. More specifically for women’s contraception, we considered only oral contraceptives, patches and rings. Injections, implants, intrauterine devices and emergency contraception were excluded as we needed more than one prescription to apply the medication possession ratio (MPR) calculation.
For evaluating the concomitant use of contraception and isotretinoin, the inclusion criterion for evaluating the concomitant use of contraception and isotretinoin was women between 12 and 21 years old who received at least one prescription of isotretinoin. In Belgium, contraception methods are reimbursed for women aged ≤21 years but not systematically for older women. Therefore, data for women aged >21 years who did not receive a reimbursement were not accessible. To avoid underestimating compliance with this recommendation, we only considered women aged between 12 and 21 years. To evaluate the concomitant use of contraception and isotretinoin, all reimbursed contraception methods were considered for this analysis (oral contraceptives, patches, rings, injections, implants, intrauterine devices and emergency contraception).
Measurement
Medication adherence and medication persistence
To measure medication adherence, we used the MPR. For each patient, we divided the total days of medication supplied within the refill interval by the number of days in the refill interval (with this method).15 The total days of medication supplied were measured by multiplying the number of packages delivered by the average daily dose (number of defined daily doses [DDDs] per package). The refill interval was defined as the interval of days between initiation (date of the first prescription delivered) and the date of the last prescription delivered. For isotretinoin, data were divided according to the different attempts. A new attempt was considered to be made if a patient had a gap of >3 months between the presumed end of treatment (considering the medication supplied in the last prescription) and the next prescription. The date of the next prescription was then considered as the initiation of a new attempt. The interval of 3 months was chosen because a minimum of 8 weeks of treatment discontinuation is recommended between two attempts of isotretinoin, to which we added 1 month because a patient might have made a stockpile of medications. For contraception, we measured medication adherence regardless of the method used (oral contraceptives, rings or patches) as a patient could have changed to another contraception method. Medication adherences for oral contraceptives, rings and patches were also measured separately for information.
As dosage was not accessible to calculate the number of days of medication supplied and because dosage of isotretinoin varies according to the patient, and the dosage of contraception varies according to the method used (oral contraceptive, patch and ring dosage), we used the defined daily dose (DDD). The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults and is fixed by the WHO Collaborating Centre for Drug Statistics Methodology.16 For example, the isotretinoin DDD is fixed at 30 mg per day.17
Patients were considered as adherent if the MPR was ≥0.8, which is the most common cutoff level used in the literature across chronic diseases.6 However, this cutoff level may vary depending on treatments and pathologies, such as HIV. A higher cutoff could be determined for contraception as only one missed dose could lead to a higher risk of pregnancy, depending on the timing of the missed dose. To the best of our knowledge, there is no defined cutoff level for isotretinoin or contraceptives in the literature. In order to compare results between isotretinoin and contraception and to compare our results with the findings from the literature, we chose 0.8 as the cutoff for isotretinoin and contraception.
Medication persistence was defined as the time between the initiation (date of the first prescription) and the presumed end of treatment (date taking into account the medication supplied in the last prescription). Patients who had a presumed end of treatment during or after the first 3 months and the last 3 months of the available database were removed from the persistence analysis as discontinuation was impossible to determine.
Concomitant use of isotretinoin and contraception
To measure the concomitant use of isotretinoin and contraception for women, we merged the isotretinoin and the contraceptive databases, using a unique ID code for each patient. As the age of sexual consent is defined as 16 years in Belgium, we also separated results into two age groups in the analysis: 12–15 years and 16–21 years.
Ethical approval
The protocol and the request to obtain insurance data were sent to Pharmanet-INAMI in December 2015, who submitted our demand to the Evaluation Committee for Drugs. This committee, which sits at INAMI, considers, in particular, the possible risks in respect of privacy. The approbation was received in February 2016. All received data were already processed and anonymized by Pharmanet. The use of anonymous data for retrospective research in Belgium does not require approval from an ethics committee.
Statistical analysis
We used descriptive statistics – median and interquartile range (P25–P75) for continuous variables and percentages for categorical variables – to describe medication adherence and concomitant use of isotretinoin and contraception, using STATA IC13®. The influence of gender and age on adherence was analyzed using the Mann–Whitney test and the Spearman coefficient correlation, respectively. The independence between medication adherence for contraception and isotretinoin was analyzed using the Pearson chi-square test of independence. The Kaplan–Meier method was used to plot the medication persistence curves. This analysis provided a visualization of medication persistence for isotretinoin treatment based on gender. The log-rank test was used to compare the medication persistence curves. We only considered medication adherence for isotretinoin in the first attempts to evaluate the influence of gender and age, to evaluate the independence between medication adherence for contraception and isotretinoin, and to plot the medication persistence curves.
Results
Study population
We received a database of 86,754 patients to whom at least one prescription of isotretinoin had been delivered. The final study population for each part is described in Figure 1.
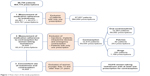  | Figure 1 Flow chart of the study population. |
Medication adherence and medication persistence
Men represented 52.5% of the isotretinoin database, and isotretinoin was mostly prescribed by dermatologists (79.4%). Contraception were mostly prescribed by general practitioners (52.8%). For isotretinoin, 46.1% of patients had an MPR ≥0.8 in the first attempt, 29.8% in the second attempt and 19.8% for more than two attempts. The median (interquartile range [IQR]) persistence was 139 days (71–209) in the first attempt, 40 days (20–104) in the second attempt and 21 days (20–70) for more than two attempts. For contraception, an MPR ≥0.8 was observed for 74.0% of patients, and the median persistence was 826 days (491–1,185). When contraception data were separated based on the contraception method, an MPR ≥0.8 was observed for 73.4% of patients with oral contraceptives, 72.0% of patients with rings and 77.4% of patients with patches (Table 1).
Isotretinoin – influence of gender
For the first attempt, men had a median MPR of 0.82 (0.53–1.16) and 52.0% had an MPR ≥0.8. For women, the median MPR was 0.66 (0.42–1.03) and 39.4% had an MPR ≥0.8. According to the Mann–Whitney test, men were more adherent than women (p-value <0.001).
Isotretinoin and contraception – association with age
A weak but significant negative correlation between medication adherence and age was observed for isotretinoin (rs=−0.0461, p-value <0.001) and contraception (rs=−0.1096, p-value <0.001).
Isotretinoin and contraception – test of independence
According to the Pearson chi-square test, medication adherences for isotretinoin and contraception were independent (p-value =0.842).
Isotretinoin – medication persistence curves
As shown in Figure 2, medication persistence seems higher for men than for women. The log-rank test, which compares the curves based on gender, is statistically significant (p<0.001). The medication persistence median (95% CI) is higher for men, at 145 (144–147) days, than for women, at 131 (129–133) days.
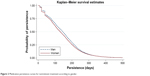  | Figure 2 Medication persistence curves for isotretinoin treatment according to gender. |
Concomitant use of isotretinoin and contraception
In the database, 17,112 women who received isotretinoin were aged between 12 and 21 years (n=3,568 for women aged between 12 and 15 years and n=13,544 for women aged between 16 and 21 years) and 63.8% (n=10,919) received at least one prescription of a contraception method (44.7% for 12–15 years and 68.8% for 16–21 years).
Among women who were prescribed at least one prescription of a contraception method, 24.5% (n=2,679/10,919) followed the recommendation to have an effective contraception method 1 month before, during and 1 month after treatment (14.1% for 12–15 years and 26.3% for 16–21 years). In other words, among the 17,112 women between 12 and 21 years old taking isotretinoin, 15.7% (2,679/17,112) followed the recommendation. More women used a contraception method during and 1 month after isotretinoin treatment (74.1% and 72.1%, respectively) than 1 month before treatment and at initiation (35.7% and 46.5%, respectively). Results of the concomitant use before, at initiation, during and after treatment by age groups are presented in Figure 3.
Discussion
Medication adherence
Isotretinoin
In our study, 46.1% of patients taking isotretinoin had an MPR ≥0.8 during the first attempt. This percentage decreased as the number of attempts increased (29.8% for the second attempt and 19.8% for more than two attempts). A retrospective cohort study by Tan et al18 (USA), using the same method, found that 57.3% of patients taking isotretinoin were adherent during the 90 days following the first prescription. They also showed that medication adherence was higher with isotretinoin than with other anti-acne treatments, such as topical treatments or antibiotics. In a cross-sectional study by Dreno et al,2 using questionnaires across America, Europe and Asia, it was found that 54% of patients were adherent to isotretinoin. Finally, in another study by Zaghloul et al19 (England), using questionnaires and counting, it was found that mean adherence was 64.7%±24% and that patients taking isotretinoin are more adherent when they are taking the treatment for the first time rather than for an additional attempt (87.5% vs 60.5% mean adherence), which we also showed in our results. The authors put forward the hypothesis that patients lost confidence in the treatment following the first attempt failure.19 Our result (46.1%) is lower than the literature findings but the study population, the method and the defined period for measuring medication adherence were not the same. For example, the period used to measure medication adherence in this study depended on the patient, whereas in the studies by Tan et al and Zaghloul et al, medication adherence for 3 months following the first prescription was examined.
The median persistence for isotretinoin treatment is 139 days (19.8 weeks), which corresponds to the usual treatment duration for a dosage of 0.5–1 mg/kg/day (16–24 weeks).7 The median persistence, like the MPR, decreased as the number of attempts increased (40 days for the second attempt and 21 days for more than two attempts). Isotretinoin treatment seems to be more effective when taking a cumulative dose of 120 mg/kg. To our knowledge, there is no information about persistence for isotretinoin treatment in the literature.
Contraception
Tan et al18 showed that 49.0% of patients taking an oral contraceptive for an anti-acne effect had an MPR ≥0.8. With the same method (MPR) but with a higher cutoff level of 0.9, Kazerooni et al20 showed that 39.4% of women taking or not taking isotretinoin were adherent to oral contraceptives. Our study showed higher results for contraception (74.0%), but our results only considered women who received a reimbursement for their contraception method and who received at least one prescription of isotretinoin. It is possible that women could be more motivated to use a contraception method due to a high teratogenic risk, and if they received a reimbursement.
However, 26.6%, 28.0% and 22.6% of the population were not adherent to oral contraceptives, rings and patches, respectively. For contraception, one missed dose can lead to pregnancy, depending on the stage of the menstrual cycle. Considering this, patient education is important not only to avoid a missed dose but also to teach patients how to react in the case of a missed dose, according to their menstrual cycle. Therapeutic education is even more important for women taking a teratogenic medication. Stuart et al,21 who studied discontinuation for oral contraceptives, rings and patches, showed that 47%, 49% and 58% of women, respectively, discontinued their treatment during the first year of treatment. In our study, 50% of women stopped their contraception before 2.3 years (829 days), which is longer than for the results of Stuart et al. However, again, our results only considered women who received at least one prescription of isotretinoin. A review of strategies to improve medication adherence to contraception methods concluded that a combination of intensive counseling, multiple contacts and reminders is needed.12
Influencing factors
Many factors influence medication adherence for chronic treatment, such as young age, which is often associated with a lower medication adherence.2,4,13,18,20,21 Zaghloul et al19 found a significant negative correlation between medication adherence for anti-acne treatment and age, with a Spearman coefficient of −0.30. Our results showed a significant negative correlation for isotretinoin and contraception. However, the correlation coefficient was weak, which means that age was poorly associated with medication adherence in our study. This weak correlation may be explained by the tight distribution of age in our population.
Concerning gender, the literature has shown mixed results. Tan et al18 showed that men seem to be more adherent, whereas Zaghloul et al19 found the opposite. In our study, men were more adherent to isotretinoin than women (52.0% vs 39.4%, respectively). One hypothesis is that men are heavier than women overall and therefore received higher doses of isotretinoin. As the MPR was calculated according to the DDD, a higher dose for men would result in a higher MPR. According to the test of independence, medication adherences for isotretinoin and contraception were not associated. Other factors can influence medication adherence to isotretinoin and contraception, such as side effects, lack of satisfaction or lack of knowledge and education,2,4,13 but these factors were not evaluated in this study. Concerning persistence, the median persistence was 2 weeks longer for men (Figure 2). A hypothesis is that severe nodular acne and acne fulminans occur mostly in men, which could lead to longer treatment.22
Concomitant use of isotretinoin and contraception
Among women 12–21 years old taking isotretinoin, 44.7% of women between 12 and 15 years received at least one prescription of contraception compared to 68.8% of women from 16 to 21 years. For both age groups, a low proportion of these women followed the main safety recommendation linked to isotretinoin; 14.1% and 26.3%, respectively. The difference between groups could be explained by an inactive sexual life at a younger age, as the age of sexual consent is fixed at 16 years in Belgium. However, Figure 3 shows that the proportion of women using a contraception method during and after isotretinoin treatment was higher for women between 12 and 15 years old, in contrast to the lower proportion of these women using a contraception method 1 month before the treatment (19.7%).
An American study analyzed the compliance with this recommendation before and after the implementation in 2006 of a stricter PPP (IPledge), among 113,578 and 77,072 women, respectively.23 This study showed that after 2006, 32% of women between 13 and 45 years old used isotretinoin and contraception concomitantly. This proportion was lower for younger women (13–17 years), 27% compared to 34% for older women (18–45 years). A Dutch study found that 53.9% of women (15–45 years) use a contraception method at the initiation of isotretinoin.24
Our results are low, particularly 1 month before treatment and at initiation, suggesting that health care professionals prefer to initiate isotretinoin and contraception together instead of following the recommendation to postpone isotretinoin treatment. The importance of initiating the use of contraception before isotretinoin treatment should be clarified in the PPP in order to increase the adoption of the recommendation. Moreover, different reasons can lead practitioners not to prescribe contraception methods, such as sexual inactivity, tubal ligation or hysterectomy. In Belgium, 8% of women aged between 15 and 54 years use sterilization as a contraception method.25 The development of future interventions to ensure patient safety should take these results into account, and recommendations for women taking a teratogenic medicine should be flexible depending on the woman’s condition.
Strengths and weaknesses
First, all reimbursed prescriptions pass through the same institute in Belgium (Pharmanet-INAMI). Therefore, patients taking isotretinoin and women aged between 12 and 21 years taking a contraceptive at the same time are all represented in the analysis. However, we have a more restricted view of women aged >21 years because reimbursement of contraception takes place only in some situations and depends on the woman’s health insurance. Because of this restriction, the second aim of this study was not fully met as it did not represent all women of childbearing age.
Second, considering the easy access to Belgian prescription data, we used the MPR to measure medication adherence. MPR is a method that is non-invasive for patients and is a rapid and economic method of analyzing a large retrospective database. However, medication adherence is longitudinal and can vary over time.5 MPR cannot monitor medication ingestion and does not reflect all aspects of medication adherence such as initiation, implementation and persistence.6 To complete our analysis, we measured medication persistence and visualized it using the Kaplan–Meier method. Moreover, data on dosage and changes in treatment were not available, which can under- or overestimate medication adherence.
Third, we used the DDD to calculate the number of days of medication supplied, which reflects the normal dosage for contraception and isotretinoin. The DDD for isotretinoin is 30 mg per day but the pharmaceutical specialties available on the market only have doses of 10, 20 and 40 mg. Dosage of isotretinoin depends on the patient weight and varies according to the clinical response and the occurrence of side effects, which can lead to a higher dosage at the beginning of treatment and a lower dosage at the end.8 Because we cannot predict the dosage evolution over time, we decided to use the DDD to decrease the dosage variation effect.
Fourth, the medication adherence results for contraception may have been underestimated as only three methods were taken into account due to the chosen measurement method. This limitation means that the first aim of this study, to evaluate medication adherence for contraception, was not fully met.
Finally, it is well known that a large enough sample size always leads to statistically significant results despite small or even trivial size effects.26 However, our data represented the entire population who received a prescription of isotretinoin in Belgium, which is rare in the literature, and it allowed us to discuss our results in a more clinical sense.
Conclusion
Medication adherence and compliance with the PPP are important for treatment effectiveness and patient safety. Health care professionals should pay more attention to women of childbearing age taking a teratogenic medication, particularly 1 month before treatment and at initiation. Interventions should be developed to improve compliance with isotretinoin safety recommendations, particularly 1 month before and at initiation of the isotretinoin treatment, but these recommendations should remain flexible and depend on the woman’s sexual condition. Pharmacy refill data are easily usable for estimating medication use in community pharmacies. Tools integrated into pharmacy software based on these data could be developed and implemented in practice to help pharmacists to detect low-adherent patients, for example using the MPR calculation, and to check the concomitant use of a contraceptive method.
Acknowledgments
We would like to thank Pharmanet-INAMI for giving us access to the pharmacy refill data and Arnaud Künzi who helped us with the data management. This paper was published with the support of the Belgian University Foundation (Fondation Universitaire de Belgique).
Disclosure
The authors report no conflicts of interest in this work.
References
Strauss JS, Krowchuk DP, Leyden JJ, et al. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56(4):651–663. | ||
Dreno B, Thiboutot D, Gollnick H, et al. Large-scale worldwide observational study of adherence with acne therapy. Int J Dermatol. 2010;49(4):448–456. | ||
Graber E. Treatment of Acne Vulgaris. UpToDate; 2015. Available from: http://www.uptodate.com/contents/treatment-of-acne-vulgaris. Accessed June 12, 2015. | ||
Snyder S, Crandell I, Davis SA, Feldman SR. Medical adherence to acne therapy: a systematic review. Am J Clin Dermatol. 2014;15(2):87–94. | ||
Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. | ||
Lehmann A, Aslani P, Ahmed R, et al. Assessing medication adherence: options to consider. Int J Clin Pharm. 2014;36(1):55–69. | ||
Information patient du Compendium Suisse des Médicaments®. Isotretinoin-Mepha Solucaps® [Patient information from the Switzerland Compendium of medicine®. Isotretinoin-subway Solucaps®]. Available from: http://compendium.ch/mpub/pnr/116903/html/fr. Accessed January 22, 2015. French. | ||
CBIP BCFI. Répertoire Commenté des Médicaments: Acné; Isotrétinoïne [Commented directory of medication: Acne; Isotretinoin]. Available from: http://www.cbip.be/fr/chapters/16?frag=14522. Accessed March 18, 2016. French. | ||
EMEA. Summary of Information on a Referral Opinion Following an Arbitration Pursant to Article 29 of Directive 2001/83/EC, for Isotretinoin/Lurantal/Trivane/Rexidal/Scheritonin; 2013. Available from: www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Isotretinoin_29/WC500010882.pdf. Accessed August 18, 2016. | ||
Fagg-afmps. Plan de gestion des risques – Activités additionnelles de minimisation des risques (additional RMA) approuvés – Roaccutane (Roche) [Risk management plan – minimization of risks (additional RMA) approved additional activities – Roaccutane (Roche)]; 2005. Available from: https://www.afmps.be/fr/humain/medicaments/medicaments/bon_usage/programme_de_gestion_de_risques/rma/r/roaccutane. Accessed January 6, 2018. French. | ||
Crijns I, Straus S, Luteijn M, Gispen-de Wied C, Raine J, de Jong-van den Berg L. Implementation of the harmonized EU isotretinoin Pregnancy Prevention Programme: a questionnaire survey among European regulatory agencies. Drug Saf. 2012;35(1):27–32. | ||
Halpern V, Lopez LM, Grimes DA, Stockton L, Gallo MF. Strategies to improve adherence and acceptability of hormonal methods of contraception. Cochrane Database Syst Rev. 2013;(10):CD004317. | ||
Steinkellner A, Chen W, Denison SE. Adherence to oral contraception in women on Category X medications. Am J Med. 2010;123(10):929.e1–934.e1. | ||
INAMI. Statistiques sur les médicaments délivrés en pharmacies publiques (Pharmanet) [Statistics on medicines delivered in community pharmacies (Pharmanet)]. Available from: http://www.inami.fgov.be/fr/statistiques/medicament/Pages/statistiques-medicaments-pharmacies-pharmanet.aspx#.WEmenVzpUjI. Accessed December 9, 2016. French. | ||
Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–1288. | ||
WHO. Introduction to Drug Utilization Research; 2003. Available from: http://apps.who.int/medicinedocs/fr/d/Js4876e/7.html. Accessed September 21, 2016. | ||
WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index. Available from: https://www.whocc.no/atc_ddd_index/. Accessed September 21, 2016. | ||
Tan X, Al-Dabagh A, Davis SA, et al. Medication adherence, healthcare costs and utilization associated with acne drugs in Medicaid enrollees with acne vulgaris. Am J Clin Dermatol. 2013;14(3):243–251. | ||
Zaghloul SS, Cunliffe WJ, Goodfield MJD. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152(5):1015–1021. | ||
Kazerooni R, Takizawa A, Vu K. Predictors of adherence to hormonal contraceptives in a female veteran population. Contraception. 2014;89(4):292–298. | ||
Stuart JE, Secura GM, Zhao Q, Pittman ME, Peipert JF. Factors associated with 12-month discontinuation among contraceptive pill, patch, and ring users. Obstet Gynecol. 2013;121:330–336. | ||
Afssaps. Recommendations de bonne pratique – Traitement de l’acné par voie locale et générale [Good practice recommendations: local and oral acne treatments]; 2007. Available from: http://ansm.sante.fr/Dossiers/Antibiotiques/Dermatologie/(offset)/2. Accessed July 6, 2017. French. | ||
Pinheiro S, Kang E, Kim C, Governale L, Zhou E, Hammad T. Concomitant use of isotretinoin and contraceptives before and after iPledge in the United States. Pharmacoepidemiol Drug Saf. 2013;22(12):1251–1257. | ||
Teichert M, Visser LE, Dufour M, et al. Isotretinoin use and compliance with the Dutch Pregnancy Prevention Programme. Drug Saf. 2010;33(4):315–326. | ||
Institut scientifique de santé publique [Scientific Institute for Public Health]. Enquête de santé 2013 – Rapport 2: comportements de santé et style de vie [Health survey 2013 – Report 2: health behaviour and lifestyle]; 2013. Available from: https://his.wiv-isp.be/fr/Documents partages/RH_FR_2013.pdf. Accessed August 16, 2016. French. | ||
Kaplan R, Chambers D, Glasgow R. Big data and large sample size: a cautionary note on the potential for bias. Clin Transl Sci. 2014;7(4):342–346. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


