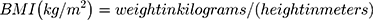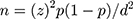Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Assessment of Cardiovascular Risk Factors in Patients with Type 2 Diabetes in Upper Egypt Villages
Authors Hussein A , Mahmoud SED , Awad MS, Mahmoud HEM
Received 20 September 2020
Accepted for publication 3 November 2020
Published 3 December 2020 Volume 2020:13 Pages 4737—4746
DOI https://doi.org/10.2147/DMSO.S282888
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Antonio Brunetti
Ahmed Hussein,1 Sharaf ED Mahmoud,1 Mohammad Shafiq Awad,2 Hossam Eldin M Mahmoud3
1Department of Internal Medicine, Faculty of Medicine, Sohag University, Nasser City, Sohag 82524, Egypt; 2Department of Cardiology, Faculty of Medicine, Beni Suef University, Beni Suef City 62511, Egypt; 3Department of Internal Medicine, Faculty of Medicine, South Valley University, Qena City 83511, Egypt
Correspondence: Ahmed Hussein
Department of Internal Medicine, Faculty of Medicine, Sohag University, Nasser City, Sohag 82524, Egypt
Tel +20 1011145537
Fax +20 934600349
Email [email protected]
Background: A large percentage of diabetic patients also have other components of metabolic syndrome, which is a group of cardiovascular (CV) hazard factors related to both diabetes mellitus (DM) and cardiovascular diseases (CVD). We do not know about the prevalence of CV risk factors in diabetic patients in Upper Egypt. We aimed to assess the CV risk factors in type 2 diabetic patients in Upper Egypt villages.
Methods: We conducted a cross-sectional study that included 800 patients with type 2 DM. We classified the participants into three groups according to the hemoglobin A1c (HbA1c) levels. We assessed the prevalence of other cardiovascular risk factors and their association with HbA1c levels through a detailed history, full clinical examination, and laboratory tests.
Results: We found that 75% of the participants were males, 25.5% elderly, 60.25% had hypertension, 60.75% had dyslipidemia, 33.25% were overweight or obese, 19.75% had a family history of coronary artery disease (CAD), 55.75% had established CVD, 42.5% were smokers, and only 12.25% were physically inactive. We found that 84% of the participants had ≥ two cardiovascular risk factors other than DM. HbA1c level was ≥ 7% in 77% of patients. After multivariate regression analysis, we found a significant association of higher systolic blood pressure (BP), more elevated diastolic BP, higher body mass index (BMI), increased waist circumference, old age, long duration of DM, and an increase in the number of clustered CV risk factors with a higher HbA1c level. At the same time, insulin therapy was significantly associated with a lower HbA1c level.
Conclusion: All type 2 diabetic patients in Upper Egypt villages have other associated CV risk factors. The clustering of cardiovascular risk factors showed a significant association with higher HbA1c levels. These findings require the thought of associated CV risk factors in choosing medical treatments to optimize glycemic control and multifactorial intervention to improve CV risk.
Keywords: DM, HbA1c, CV risk factors, CVD
Introduction
Many diabetic patients also have metabolic syndrome,1 a group of CV hazard factors related to both DM and CVD.2 The cardiovascular risk factors are considered as clinical markers of macro and microvascular DM complications.3
Patients with type 2 DM have two-to triple more danger of CV events compared to subjects without DM, and CVD causes about 80% of the mortality in type 2 DM.4 Hyperglycemia is a weak hazard factor for CVD. The significant advantage of lessening plasma glucose levels in type 2 DM is the reduction of long-term microvascular complications and, to a lesser degree, macrovascular complications that may occur only in young patients with short diabetes duration, no prevalent CVD, and followed-up for more than ten years.5,6 Mediations concentrated on lessening plasma glucose have failed to essentially diminish CV hazard and mortality, especially in the secondary prevention trials.7,8
Cardiometabolic risk refers to a higher lifetime hazard for CVD. The specific factors that can make this expanded hazard include obesity, hyperglycemia, high blood pressure (BP), insulin resistance, and dyslipidemia. When patients are physically inactive or smokers and have at least one cardiometabolic risk factor, this markedly increases the cardiometabolic risk. Also, clustering of these risk factors can markedly increase the risk of CVD. Diseases that frequently share the above risk factors, such as type 2 DM, can also increase cardiometabolic risk.9
Some of the cardiometabolic risk factors that contribute to the development of DM are non-modifiable, as age, sex, race/ethnicity, and family history. However, many other risk factors, as overweight and obesity, smoking, sedentary life, dyslipidemia, high BP, insulin resistance, and inflammation, are modifiable through lifestyle changes and treatment.10
A multifactorial intervention to improve CV hazard factors decreased cardiovascular events and mortality in type 2 DM patients. For example, lowering BP and improving the lipid profile leads to a more prominent CVD risk reduction than lowering plasma glucose in diabetic patients.11
We do not know about the prevalence of CV risk factors in diabetic patients in Upper Egypt. We conducted this study to assess the cardiovascular risk factors in type 2 diabetic patients in Upper Egypt villages.
Methods
Design of the Study
We conducted a cross-sectional study in 12 villages in Upper Egypt. We selected villages randomly at Sohag, Beni Suef, and Qena Governorates and involved 800 patients with type 2 DM. We recruited the participants at primary care unit clinics. We classified the participants into three groups according to the HbA1c level, group 1; Patients have HbA1c below 7, group 2; Patients have HbA1c 7 to less than 9, and group 3; Patients have HbA1c ≥ 9. We conducted the study from May 2018 to March 2020.
All subjects provided written informed consent to participate in the study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The ethics committee at Sohag, Beni Suef, and Qena faculty of medicine approved the study protocol.
We Subjected the Participants to the Following
Detailed history
Detailed history, including age, sex, education level, income, smoking history, hypertension, family history of DM, family history of CAD, duration of DM, history of established CVD, physical activity, and type of anti-glycemic used (oral hypoglycemic, insulin therapy or no anti-glycemic drugs).
We measured BP while the patient in the sitting position after a rest for 5 minutes and used a standardized mercury sphygmomanometer. We considered the average of three readings taken as the estimated systolic and diastolic blood pressures.
The Joint National Committee VIII (JNC VIII) criteria defined systemic hypertension as a BP ≥140/≥90 mmHg and/or on current antihypertensive drugs.12
We calculated BMI by the following equation: -
We estimated weight with the subject delicately shut to the closest 0.5 Kg, and estimated height without shoes to the closest 0.5 cm. BMI was considered elevated ≥ 25 kg/m2.13
Measured using a measuring tape around the bare stomach and just above the right iliac crest parallel to the floor. Abdominal obesity defined as a waist circumference of ≥102 cm for men and≥ 88 cm for women.13
Laboratory Investigation
1- HbA1c level, Complete blood count, and serum creatinine.
2- Lipid profile: we measured 12 hours overnight fasting serum triglycerides (TG), high-density lipoprotein cholesterol (HDL) levels, total serum cholesterol, and low-density lipoproteins (LDL). We considered the TG level elevated as TG of ≥150 mg/dl. HDL cholesterol was considered low <40 mg/dl in males and <50 mg/dl in females. LDL level was considered elevated ≥100 mg/dl.14
3- Resting 12 leads surface Electrocardiogram to identify signs of myocardial ischemia (ST-segment and T wave abnormalities and pathological Q waves.
Diagnosis of Type 2 DM
In this study, we considered the participant as previously diagnosed as being diabetic if (1) A physician once told him/her that he/she had DM in a setting other than during pregnancy or (2) On regular anti-diabetic treatment.
We considered the participants as newly diagnosed diabetics if they had one of the following:
1 - (Fasting plasma glucose > or =126 mg/dl) and/or (Random glucose > or =200 mg/dl) on two successive days.
2 - Hyperglycemia >200 mg/dl plus classic symptoms of diabetes: polyuria, polydipsia, and Loss of weight.
The recommendation of the American Diabetes Association (ADA) to include the use of HbA1c to diagnose diabetes, using a cut point of 6.5%, was not used in this study, as many labs in our area are not adequately standardized.14
----The following formula used to calculate the minimum size of the required sample:
Where n shows the sample size, z demonstrates the level of confidence according to the standard normal distribution (for a level of confidence of 95%, z = 1.96), p shows the estimated proportion of the population that presents the characteristic (about 16%), d demonstrates the tolerated margin of error (for example, we want to know the real proportion within 5%).
Utilizing the past equation for the sample size calculation 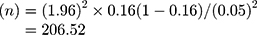 . Therefore, the minimum sample size is 207 participants.
. Therefore, the minimum sample size is 207 participants.
Participant Characteristics
All patients with type 2 diabetes, except those with hematologic diseases (could affect HbA1c values), were selected to participate in the study.
Analysis of Data
Data were analyzed using SPSS version 19. We analyzed the quantitative data using the analysis of variance (ANOVA) to compare the three groups’ means. We compared the qualitative data using the Chi-square test. We used Fisher’s exact correction when the expected cell count is less than five. We used the Pearson correlation to study the correlation between HbA1c level and other variables. We performed univariate and multivariate regression analyses between cardiovascular risk factors and HbA1c groups after adjustment for confounding factors.
We also analyzed the association between clustering of cardiovascular risk factors and HbA1c level using estimated risks (odds ratios (OR)) from the logistic regression analysis. We applied Bonferroni correction, and thus we considered the p-value as significant at or below 0.0167.
The outcome variables were the CV risk factors and the HbA1c level.
Results
Out of the 800 participants in our study, 75% were males, 25.5% were elderly, 60.25% were hypertensives, 60.75% had dyslipidemia, 33.25% were overweight or obese, 19.75% had a family history of CAD, 42.5% were smokers, and only 12.25% were physically inactive. Table 1
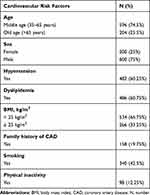 |
Table 1 Distribution of Cardiovascular Risk Factors in Participants |
We found that no one had type 2 DM without any other CV risk factors, and 84% of the participants had ≥ two CV risk factors other than DM. Table 2, Figure 1
 |
Table 2 Distribution of Other Cardiovascular Risk Factors Than DM in Participants |
 |
Figure 1 Distribution of CV risk factors other than DM in participants. Abbreviations: CV, cardiovascular; DM, diabetes mellitus. |
HbA1c level was ≥ 7% in 77% of patients. We found that 446 (55.75%) patients had a history of established CVD. After multivariate regression analysis, both old age and long duration of DM were independent factors associated with a higher HbA1c level, while insulin therapy was an independent factor associated with a lower HbA1c level. Sex, low education level, low income, smoking, positive family history of DM, and history of established CVD were dependent risk factors that affect HbA1c in the presence of other risk factors only (Tables 3 and 4).
 |
Table 3 Distribution of Baseline Sociodemographic Characteristics and Detailed History of DM |
 |
Table 4 Multivariate Regression Analysis of Baseline Sociodemographic Characteristics and Detailed History of DM for HbA1c Level |
HbA1c level was significantly higher in participants with higher systolic BP (OR 1.53, 95% CI 0.88:2.36, p <0.001), higher diastolic BP (OR 1.71, 95% CI 1.09:2.37, p <0.001), higher BMI (OR 1.39, 95% CI 0.74:2.35, p <0.001), and increased waist circumference (OR 1.29, 95% CI 0.65:1.99, p <0.001). (Tables 5–7)
 |
Table 5 Distribution of Cardiometabolic Risk Factors as Regards to HbA1c Level |
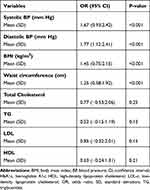 |
Table 6 Adjusted Univariate Regression Analysis of Cardiometabolic Risk Factors Regards to HbA1c Level |
 |
Table 7 Adjusted Multivariate Regression Analysis of Cardiometabolic Risk Factors Regards to HbA1c Level |
Age, systolic BP, diastolic BP, BMI, and waist circumference have shown a statistically significant positive correlation with HbA1c level (p < 0.0167). (Table 8)
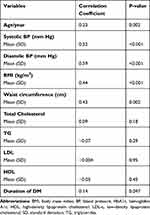 |
Table 8 Correlation Between Continuous Variables and HbA1c Level |
Regression analysis showed that the increased number of clustered CV risk factors is statistically significantly associated with the increased HbA1c levels. (Table 9)
 |
Table 9 Regression Analysis Showing the Association of Clustered Cardiovascular Risk Factors and the HbA1c Level |
Discussion
Out of the 800 diabetic participants in our study, 25.5% were elderly, and 75% were males. Most of them have hypertension (60.25%), dyslipidemia (60.75%), and a history of established CVD (55.75%). 33.25% were overweight or obese, 19.75% had a family history of CAD, 42.5% were smokers, and only 12.25% were physically inactive. The case of being truly active by the majority of subjects is justifiable because the major economic activity in this community is farming, and all residents, irrespective of age and gender, engage in farming daily. The case of being genuinely active by most subjects, together with low economic state and certain dietary habits, may explain the low percentage of overweight and obesity in these communities. Also, we found that no one had type 2 diabetes without any other CV risk factors, and 84% of the participants had ≥ two cardiovascular risk factors other than DM. This finding means that most diabetic patients in Upper Egypt villages are considered at high-risk for CVD.
We found that 77% of the patients had uncontrolled DM (HbA1c ≥ 7%). Old age and longer duration of DM were independent factors associated with a higher HbA1c level, while insulin therapy was an independent factor associated with a lower HbA1c level. Sex, low education level, low income, smoking, positive family history of DM, and history of established CVD were dependent risk factors that affect HbA1c in the presence of other risk factors only. HbA1c level was significantly higher in patients with more elevated systolic BP, higher diastolic BP, higher BMI, and increased waist circumference.
We found a positive correlation between age, systolic BP, diastolic BP, BMI, and waist circumference, and the HbA1c level. Also, we demonstrated that the clustering of cardiovascular risk factors significantly increased with a higher HbA1c level. So, it is required to pay more attention to type 2 diabetic patients in Upper Egypt. We should create periodic screening programs for early detection and multifactorial intervention of associated cardiovascular risk factors besides controlling DM. Patients should use anti glycemic agents, which improve insulin sensitivity and various components of insulin resistance syndrome, ie, hypertension, dyslipidemia, and endothelial dysfunction, that reduce CVD risk in type 2 diabetes, independent of its glucose-lowering action. GLP-1 receptor agonists, SGLT2 inhibitors, and pioglitazone should be considered to reduce CVD risk in type 2 diabetes, independent of its glucose-lowering action, along with metformin, over other agents with similar lowering effect on HbA1c but not reduce CV risk.15–18
In our study, 49.5% of diabetic patients showed an HbA1c level ≥ 9%, while Bogdan et al showed in their study that only 18.3% of the diabetic participants had an HbA1c level > 9%.19 This finding indicates that patients in Upper Egypt villages have a significantly higher rate of poor diabetes control that may be explained by low economic and education levels, higher smoking rates, and CVD besides poor health services.
We found a significant association between higher systolic or diastolic BP and a higher HbA1c level. Bower et al reported a significant association of higher HbA1c values with increased hypertension risk during long-term follow-up.20 In their study, Kathryn A Britton et al concluded that the HbA1c level in non-diabetic women was significantly associated with more risk of hypertension after adjustment for the majority of traditional hypertension and CAD risk factors. However, this association was no longer significant after adjustment for BMI.21 Other studies also reported a significant positive association between HbA1c level and hypertension.22,23 The presence of shared risk factors, especially adiposity, may explain the positive association between hyperglycemia and hypertension. Furthermore, inflammatory processes play a role in both the development of hyperglycemia and hypertension.24 In contrast, some studies have shown a better blood pressure profile among diabetic individuals receiving anti glycemic treatment.25,26
In our study, BMI and waist circumference showed a significant positive correlation with HbA1c level that matches with the results of a large prospective cohort study, conducted on 17,638 individuals by Chris Power and Claudia Thomas in England 2011; they concluded that excessive increase in BMI at all life stages was associated with elevated HbA1c levels.27 Another study conducted by Leah M Lipsky et al showed that the role of greater BMI in diabetes management in youth with type 1 diabetes might relate specifically to increase hyperglycemia excursions.28 Many other studies have shown similar findings.29,30 These findings explained by the accumulation of visceral fat after some time may enhance insulin resistance, potentially through dysregulation of adipokines released from visceral fat, resulting in diabetes.31
Our study did not find a significant relation between HbA1c level and lipid profile, which was different from the results obtained by some other studies showing that dyslipidemia is significantly associated with a higher HbA1c level.19,32 This difference may be due to the effects of other confounding factors and the vast majority of our studied population’s tendency to dyslipidemia and lack of patients with controlled lipid profile among our studied populations.
There was a significant positive correlation between age and HbA1c levels in our study. Dubowitz et al also supported our results. They said that the association of age with higher HbA1c levels was consistent in their research, and age should be taken into consideration when using HbA1c for the diagnosis and management of diabetes and prediabetes.33
We demonstrated that patients receiving insulin showed significantly lower levels of HbA1c compared to other lines of treatment. Ray et al enrolled 33,040 patients and compared standard treatment effects to the intensive one on cardiovascular events and mortality in DM patients. They showed that the average HbA1c was 0.9% lower, the number of cardiovascular events was 15%, and myocardial infarction was 17% lower in intensively treated patients with insulin.34
We found the clustering of cardiovascular risk factors significantly associated with increased HbA1c levels. This finding is consistent with that showed by Peng et al,35 in which subjects with HbA1c ≥6.5% had more unfavorable cardiovascular and metabolic risk profiles than those with HbA1c <6.5%. Also, Okosun et al,36 concluded that cardiometabolic risk factors’ clustering is positively associated with elevated HbA1c. Several kinds of research explained this finding in that long-term uncontrolled DM is associated with endothelial dysfunction,37 systemic inflammation,38 oxidative stress,39 and platelet activation.40 In type 2 diabetic patients at high CV risk, intensive intervention with multiple drug combinations and lifestyle changes had supported beneficial impacts as for vascular complications and on a CV, and any cause mortality rate.11,41 This discovering requires the thought of comorbid cardiovascular risk factors in choosing medical treatments to optimize glycemic control and reduce CV risk in people with type 2 diabetes.
Strengths of Our Study
To our knowledge, no previous study assessed the cardiovascular risk factors in type 2 diabetic patients in Upper Egypt villages.
Limitations of Our Study
We did not consider some crucial cardiovascular risk factors like alcohol consumption, socioeconomic status, dietary habits, and adherence to prescribed drug regimens.
Conclusion
All diabetic patients in Upper Egypt villages have one or more associated CV risk factors, with a high prevalence of hypertension, dyslipidemia, and established CVD. Old age, longer duration of DM, hypertension, high BMI, increased waist circumference, and cardiovascular risk factors’ clustering showed a significant association with higher HbA1c levels. These findings require the thought of associated cardiovascular risk factors in choosing medical treatments to optimize glycemic control in people with type 2 diabetes, together with multifactorial intervention, to improve CV risk.
Abbreviations
BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CVD, cardiovascular disease; DM, diabetes mellitus; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HTN, hypertension; LDL, low-density lipoprotein; TG, triglyceride.
Acknowledgments
We thank all the patients who participated in this study. We thank all the staff of primary care units in Upper Egypt villages.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Bianchi C, Miccoli R, Bonadonna RC, et al. Metabolic syndrome in subjects at high risk for type 2 diabetes: the genetic, physiopathology, and evolution of type 2 diabetes (GENFIEV) study. Nutr Metab Cardiovasc Dis. 2011;21:699–705. doi:10.1016/j.numecd.2010.03.006
2. Shin JA, Lee JH, Lim SY, et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig. 2013;4:334–343. doi:10.1111/jdi.12075
3. Bonadonna RC, Cucinotta D, Fedele D, et al. The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: results from Metascreen, a multicenter diabetes clinic-based survey. Diab Care. 2006;29:2701–2707.
4. Morrish NJ, Wang SL, Stevens LK, et al. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44(Suppl. 2):S14–S21. doi:10.1007/PL00002934
5. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. doi:10.1016/S0140-6736(98)07019-6
6. Prattichizzo F, de Candia P, De Nigris V, et al. Legacy effect of intensive glucose control on major adverse cardiovascular outcome: systematic review and meta-analyses of trials according to different scenarios. Metabolism. 2020;110:154308. doi:10.1016/j.metabol.2020.154308
7. Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559.
8. Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
9. Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2013;51(15):1512–1524. doi:10.1016/j.jacc.2008.02.034
10. Buse JB, Ginsberg HN, Bakris GL, et al.; American Heart Association, American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diab Care. 2007;30:162–172.
11. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi:10.1056/NEJMoa021778
12. Reisin E, Harris RC, Rahman M. Commentary on the 2014 BP guidelines from the panel appointed to the eighth Joint National Committee (JNC 8). J Am Soc Nephrol. 2014;25(11):2419–2424.
13. Kenneth FAS, Harris TB, et al. Overweight, Obesity, and Mortality in a Large Prospective Cohort of Persons 50 to 71 Years Old. N Engl J Med. 2006;355:763–778. doi:10.1056/NEJMoa055643
14. American Diabetes Association: diagnosis and classification of diabetes mellitus. Diab Care. 2010;33:S62–S69. doi:10.2337/dc10-S062
15. Marso SP, Daniels GH, Brown-Frandsen K, et al. LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi:10.1056/NEJMoa1603827
16. Abdul-Ghani M, Del Prato S, Chilton R, et al. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diab Care. 2016;39:717–725.
17. Dormandy JA, Charbonnel B, Eckland DJA, et al. PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
18. Prattichizzo F, La Sala L, Rydén L, et al. Glucose-lowering therapies in patients with type 2 diabetes and cardiovascular diseases. Eur J Prev Cardiol. 2019;26(2_suppl):73–80. doi:10.1177/2047487319880040
19. Bogdan T, Oana A. The relationship between hemoglobin a1c and chronic complications in diabetes mellitus. Romanian J Diab Nutrition Metabolic Diseases. 2012;19:2.
20. Bower JK, Appel LJ, Matsushita K, et al. Glycated hemoglobin and risk of hypertension in the atherosclerosis risk in communities study. Diab Care. 2012;35(5):
21. Britton KA, Pradhan AD, Michael Gaziano J, et al. Hemoglobin A1C, body mass index, and the risk of hypertension in women. Am J Hypertens. 2011;24(3):328–334. doi:10.1038/ajh.2010.233
22. He J, Klag MJ, Caballero B, et al. Plasma insulin levels and incidence of hypertension in African Americans and whites. Arch Intern Med. 1999;159:498–503. doi:10.1001/archinte.159.5.498
23. Liese AD, Mayer-Davis EJ, Chambless LE, et al. Atherosclerosis risk in communities study investigators elevated fasting insulin predicts incident hypertension: the ARIC study. J Hypertens. 1999;17:1169–1177. doi:10.1097/00004872-199917080-00017
24. Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci. 2007;112:375–384. doi:10.1042/CS20060247
25. Imperatore G, Cadwell BL, Geiss L, et al. Thirty-year trends in cardiovascular risk factor levels among US adults with diabetes: national health and nutrition examination surveys, 1971–2000. Am J Epidemiol. 2004;160:531–539. doi:10.1093/aje/kwh232
26. Preis SR, Pencina MJ, Hwang SJ, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation. 2009;120:212–220. doi:10.1161/CIRCULATIONAHA.108.846519
27. Power C, Thomas C. Changes in BMI, duration of overweight and obesity, and glucose metabolism: 45 years of follow-up of a birth cohort. Diab Care. 2011;34(9):1986–1991. doi:10.2337/dc10-1482
28. Lipsky LM, Gee B, Liu A, et al. Glycemic control and variability in association with body mass index and body composition over 18months in youth with type 1 diabetes. Diabetes Res Clin Pract. 2016;120:97–103. doi:10.1016/j.diabres.2016.07.028
29. Wei GS, Coady SA, Reis JP, et al. Duration and degree of weight gain and incident diabetes in younger versus middle-aged black and white adults: ARIC, CARDIA, and the framingham heart study. Diab Care. 2015;38(11):
30. Yabe D, Type SY. 2 diabetes via beta-cell dysfunction in East Asian people. Lancet Diab Endocrinol. 2016;4:2–3. doi:10.1016/S2213-8587(15)00389-7
31. Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi:10.1038/nri2921
32. Bodhe C, Jankar D, Bhutada T, et al. HbA1c: predictor of dyslipidemia and atherogenicity in diabetes mellitus. Int J Basic Med Sci Pharmacy June. 2012;2(1):25–27.
33. Dubowitz N, Xue W, Long Q, et al. Aging is associated with increased HbAlc levels, independently of glucose levels and insulin resistance, and also with decreased HbAlc diagnostic specificity. Diabet Med. 2014;31:927–935. doi:10.1111/dme.12459
34. Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomized controlled trials. Lancet. 2009;373:1765–1772. doi:10.1016/S0140-6736(09)60697-8
35. Peng G, Lin M, Zhang K, et al. Hemoglobin A1c can identify more cardiovascular and metabolic risk profile in OGTT-negative chinese population. Int J Med Sci. 2013;10(8):1028–1034. doi:10.7150/ijms.5905
36. Okosun I, Annor F, Dawodu E, et al. clustering of cardiometabolic risk factors and risk of elevated HbA1c in non-hispanic white, non-hispanic black, and mexican-American adults with type 2 diabetes. Diab Metab Syndrome. 2014;8:88–90. doi:10.1016/j.dsx.2014.04.026
37. Lorbeer R, Empen K, Dorr M, et al. Association between glycosylated haemoglobin A (1c) and endothelial function in an adult non-diabetic population. Atherosclerosis. 2011;217:358–363. doi:10.1016/j.atherosclerosis.2011.04.007
38. Natali A, Toschi E, Baldeweg S, et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133–1140. doi:10.2337/diabetes.55.04.06.db05-1076
39. Zheng F, Lu W, Jia C, et al. Relationships between glucose excursion and the activation of oxidative stress in patients with newly diagnosed type 2 diabetes or impaired glucose regulation. Endocrine. 2010;37:201–208. doi:10.1007/s12020-009-9296-6
40. Shah B, Sha D, Xie D, et al. The relationship between diabetes, metabolic syndrome, and platelet activity as measured by mean platelet volume: the National Health And Nutrition Examination Survey, 1999–2004. Diab Care. 2012;35(5):1074–1078. doi:10.2337/dc11-1724
41. Gaede P, Lund-Andersen H, Parving HH, et al. effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi:10.1056/NEJMoa0706245
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.