Back to Journals » Journal of Pain Research » Volume 11
Assessment and pathophysiology of pain in cardiac surgery
Authors Zubrzycki M, Liebold A , Skrabal C, Reinelt H, Ziegler M , Perdas E, Zubrzycka M
Received 9 January 2018
Accepted for publication 23 May 2018
Published 24 August 2018 Volume 2018:11 Pages 1599—1611
DOI https://doi.org/10.2147/JPR.S162067
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Erica Wegrzyn
Marek Zubrzycki,1 Andreas Liebold,1 Christian Skrabal,1 Helmut Reinelt,2 Mechthild Ziegler,2 Ewelina Perdas,3 Maria Zubrzycka3
1Department of Cardiac Surgery, University of Ulm Medical Center, Ulm, Germany; 2Department of Cardiac Anesthesiology, University of Ulm Medical Center, Ulm, Germany; 3Department of Cardiovascular Physiology, Medical University of Lodz, Lodz, Poland
Abstract: Analysis of the problem of surgical pain is important in view of the fact that the success of surgical treatment depends largely on proper pain management during the first few days after a cardiosurgical procedure. Postoperative pain is due to intraoperative damage to tissue. It is acute pain of high intensity proportional to the type of procedure. The pain is most intense during the first 24 hours following the surgery and decreases on subsequent days. Its intensity is higher in younger subjects than elderly and obese patients, and preoperative anxiety is also a factor that increases postoperative pain. Ineffective postoperative analgesic therapy may cause several complications that are dangerous to a patient. Inappropriate postoperative pain management may result in chronic pain, immunosuppression, infections, and less effective wound healing. Understanding and better knowledge of physiological disorders and adverse effects resulting from surgical trauma, anesthesia, and extracorporeal circulation, as well as the development of standards for intensive postoperative care units are critical to the improvement of early treatment outcomes and patient comfort.
Keywords: cardiac surgery, postoperative pain, pain intensity, analgesics
Introduction
Each surgical intervention is associated with the patient’s perception of pain. Postoperative pain is due to intraoperative damage to tissues/organs, and its intensity and extent are generally proportional to the extent of the surgery.1 In cases of a large trauma, in addition to superficial and deep somatic pain, the visceral component of postoperative pain is also involved, triggered both by smooth-muscle contraction, caused by compression and tension of the visceral structures, and by inflammatory lesions.2
The severity of pain experienced by the patient is significantly influenced by various factors that increase or decrease the nociceptive threshold. These include the location of the surgery, its extent, the degree of tissue traumatization, the direction of skin incision, preoperative anxiety level, and the analgesic techniques used in the perioperative period.3–5 Postoperative pain is a major factor aggravating the general condition of the patient. Its systemic sequels are disorders of the respiratory and cardiovascular systems, stimulation of the sympathetic nervous system, and impairment of muscular mobility, general mobility, and physical fitness of the patient.1,6 Severe pain is also burdensome for the patient’s psyche.7
Pain after cardiac surgery is most severe during the first 24 hours and decreases on subsequent days, because it is a “self-limiting” phenomenon.8,9 Pain is the most severe in patients after open thoracic surgery.4,10 Patients undergoing surgery with the use of cardiopulmonary bypass report slightly higher pain intensity than those in whom extracorporeal circulation is not used.11 Extracorporeal circulation is essentially associated with the induction of the systemic inflammatory response syndrome, with potential end-organ dysfunctions.12 It has been shown that women after cardiac surgery report higher pain intensity and a significantly higher number of pain areas than men, and that elderly patients have a higher pain threshold.13–15
Assessment of the intensity of postoperative pain should be based on standardized and documented assessment scales. The established tools are the visual analog scale (VAS) and numeric rating scale (NRS).16,17 Postoperative pain management is crucial, as inadequately controlled pain delays rehabilitation, prolongs the duration of treatment and worsens the patient’s quality of life.18
Despite the tremendous progress made in medicine in the field of pain pathophysiology and its treatment, patients still experience pain after surgery. An appropriate method of alleviating pain and observing the patient for the occurrence of complications associated with the administration of certain analgesics play a key role in postoperative pain control.19,20
This paper is a review of pain-related problems associated with cardiac surgery procedures. It presents the pathomechanism and factors of postoperative pain. It explores the factors affecting the intensity and perception of pain in patients undergoing cardiac surgery, including the preoperative ones. To our knowledge, no similar comprehensive reviews concerning this topic have been published recently.
Commonest conditions in adults requiring cardiac surgery
Coronary artery bypass grafting (CABG) and heart valve surgery are the most commonly performed procedures in cardiac surgery worldwide. The further development of surgical skills and technical settings enables complex surgery in increasingly old and morbid patients with poor left ventricular function and a multitude of comorbidities.21 To minimize as far as possible the pain experienced by cardiac surgery patients, surgical procedures should be performed using minimally invasive techniques. Minimally invasive surgery has grown in popularity, and the potential benefits of reducing surgical trauma include decreased postoperative bleeding, reduced incidence of sternal wound infections, and shortened recovery period after surgery.22
There are also less invasive procedures, like minimally invasive direct CABG or the totally endoscopic CABG, where the sternum is not opened. Here, the bypass grafting is performed by minithoracotomy or totally endoscopically by inserting two or three ports, which may contribute to less postoperative pain to improve the patients’ quality of life in the postoperative period. The shortcomings of both techniques are that only one or two target vessels at the anterior wall of the heart can be achieved.23,24
As for heart-valve surgery, minimally invasive techniques are well established for atrioventricular and aortic valve surgery, atrial septal defect, and radiofrequency ablation with closure of the left atrial appendage. With the introduction of a 3-D totally endoscopically functioning system into minimally invasive cardiac surgery, further reduction of skin incisions and trauma have become possible.21,25
Now, specific valve failures can be treated with percutaneous access techniques. There is growing evidence of the effectiveness of such procedures. In particular, transcatheter aortic valve implantation is considered an effective therapeutic alternative to surgical treatment for patients at high risk or for those who are not suitable or willing for open-heart surgery.26–28
Postoperative pain can be reduced additionally by the elimination of intraoperative use of extracorporeal circulation, thus reducing the incidence of inflammatory response syndrome, eg, using off-pump CABG. This is an alternative technique, where no heart–lung machine is used and anastomoses are sewn on a beating heart. This technique may be favorable in avoiding complications resulting from extracorporeal circulation.11,29
Surgical trauma and its consequences: acute postoperative pain
The incidence of acute postoperative pain is associated with surgical trauma to tissue or organs, as well as the presence of a surgical wound. It occurs after the cessation of action of the analgesic agents administered intraoperatively. Pain of this type decreases day by day with healing of organic tissue and skin. It usually lasts a few or >10 days, but may also persist for up to 3 months.30,31
Tissue damage leads to the development of so-called neurogenic inflammation at the site of the trauma. The site of the injury is edematous, red, and painful. These symptoms result from the release of potassium ions, bradykinin, prostanoids, and numerous inflammatory mediators, such as substance P, serotonin, histamine, cytokines, and leukotrienes, from cells.32,33 This leads to a change in the properties and sensitivity of primary afferent nerve terminals (peripheral sensitization). Changes in the environment also cause the activation of so-called sleeping nociceptors. These processes are accompanied by changes in the central nervous system (central sensitization). These phenomena are expressed in the form of excessive response to painful stimuli within the postoperative wound (primary hyperalgesia) and/or in the tissue surrounding the site of injury (secondary hyperalgesia), pain perception, even after an innocuous nonnociceptive stimulus (allodynia), and spontaneous and projected pain. This type of postoperative pain, occurring as a consequence of tissue injury and induction of sensitization mechanisms, is referred to as clinical pain. In contrast to physiological pain, clinical pain is characterized by slower conduction, lasts longer than desired than the noxious stimulus, is diffuse in character and difficult to locate, and aggravates the patient’s attempts to move.34
Acute postoperative pain is also classified as receptor pain.33,35 Just as physiological pain (caused by a stimulus innocuous to tissue), it is due to irritation of pain receptors (nociceptors). In the transduction process, in nociceptors located in the peripheral terminals of Aδ and C fibers, conversion of the energy of a noxious stimulus (mechanical, thermal, chemical) into electrical impulses takes place. Then, the nociceptive information is conducted along the Aδ and C fibers to ganglia of the posterior roots or ganglia of the cranial nerves (V, VII, IX, and X), and subsequently to the dorsal horn of the spinal cord. From the dorsal horn, pain stimuli are transmitted via lateral and medial spinohypothalamic, spinomesencephalic, and spinoreticular pathways to the thalamus, reticular formation, pons, hypothalamus, and periaqueductal gray matter. Then, nociceptive information is transmitted to the cerebral cortex and the limbic system. In the transmission process, nociceptive stimulation is inhibited or enhanced, owing to the involvement of (among others) endogenous opioids and noradrenergic, cholinergic, serotonergic, and γ-aminobutyric acid-ergic systems. The final perception of pain takes place in the cerebral cortex. Its presence is acknowledged there and its severity assessed, as well as the decision concerning the action to be taken being made31,36 (Figure 1).
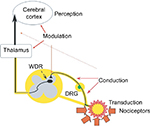  | Figure 1 Pain sensation process – nociception. Abbreviations: DRG, dorsal root ganglia; WDR, wide dynamic range. |
Adverse effects of nociceptive stimulation
Postoperative pain is a side effect of a planned injury. As such, unlike physiological pain, it does not play the role of warning and defense. Instead, it leads to the development of a sequence of unfavorable pathophysiological processes triggered by nociceptive stimulation.37 This has been expressed in the definition of pain proposed by the International Association for the Study of Pain, according to which severe pain is an unpleasant, sensual, and emotional sensation caused by existing or potentially threatening damage to tissue, a sensation accompanied by autonomic and behavioral systemic response.38
Postoperative pain is associated with increased susceptibility of the patient to the development of many complications. A particularly dangerous sequel of severe pain after cardiosurgical procedures are dysfunctions of the respiratory system.6,39 Pain is the cause of reflex muscular tension and impairs the patient’s activity or even immobilizes them. It leads to a shortness of breath and consequently to reduction in tidal volume, vital capacity, functional residual capacity, and pulmonary compliance.40,41 The fear of pain also impairs expectoration of secretions accumulating in the bronchial tree, which may result in atelectasis, pulmonary infections, and hypoxemia of arterial blood.42
Activation of the sympathetic nervous system has a significant effect on the cardiovascular system and gastrointestinal and urinary tracts. This leads to acceleration in heart rate, increase in contractility and tone of the heart walls, and elevation in arterial blood pressure. Peripheral blood flow is reduced, which predisposes to venous stasis (potentiated by immobilization) and the development of deep-vein thrombosis. Stimulation of the sympathetic system also impairs the motility of the gastrointestinal tract (spasm of the sphincters), the urinary bladder, and the urethra.1,43–46
Changes in the endocrine system are also observed.44,47 The release of cortisol, catecholamine, antidiuretic hormone, corticotropic hormone, renin, angiotensin, and aldosterone is increased. In contrast, insulin level decreases. The catabolic phase develops. If nociceptive stimulation lasts longer, it leads to suppression of the immune system, with increased risk of infection and possible disturbances in the wound-healing process. Additionally, increased ability of blood platelets to aggregate and a systemic tendency to retain sodium and water is observed.48 These processes are mainly due to activation of the sympathoadrenal–hypothalamic–pituitary system and release of inflammatory mediators at the site of tissue damage.43 Severe pain has a devastating impact on the patient’s psyche. It causes anxiety and malaise, which may manifest in sleep disorders, fear, and in extreme cases depression.7,31
Factors influencing the perception of pain
Pain is a subjective sensation, and every surgical patient may feel it in a different way, despite the same type of surgical intervention. The perception of pain is influenced by factors associated not only with the surgical trauma and the method of anesthesia but also by biological, psychological, and sociological ones. The extent of the incision and duration of the surgical procedure are among the essential factors influencing the intensity of pain.1 With the aim of minimizing postoperative pain, surgeons modify the traditional approaches and search for solutions sparing thoracic wall structures.21,49,50
The presence of postoperative drainage intensifies nociceptive impulse from the parietal pleura innervated by the intercostal and phrenic nerves.51 Exacerbation of postoperative pain is also influenced by the extent of traumatization of the intercostal nerve when the intercostal space is opened. It is particularly vulnerable to damage when the intercostal space is opened. This maneuver increases the risk of damage to the nerve inflicted by the rib in cases of fracture or by compression exerted by the dilator during surgery.51,52 As demonstrated in animal model studies, intercostal nerve compression may lead to its degeneration and demyelination.53 Intercostal nerve damage is regarded as one the factors inducing both acute and chronic pain.54 Chronic pain after thoracic surgery is a serious problem in 20%–80% of adult patients.51,55 It is characterized by the occurrence of allodynia and/or hyperalgesia, which suggests its neuropathic origin.2,51 As noted by Allama in a randomized study of adult patients, the use of intercostal sutures significantly reduces the severity of pain in the early postoperative period.56 It should be mentioned that research conducted by Steegers et al demonstrated that the neuropathic pain component after thoracic surgery was present only in 53% of patients with chronic pain, while in 47% of patients the pain was not neuropathic.2
It should not be forgotten that psychological components of pain, in addition to factors related to the surgical intervention itself, have a significant impact on the sensation of pain. Personality traits, such as high levels of anxiety, emotional lability, and pessimistic attitude, can increase pain sensations.5,57 Cardiac surgery with extracorporeal circulation is a very strong physical stressor. The specificity of this type of surgical treatment results in a significant load on the mental mechanisms of adjustment. In addition, the cardiac surgery procedure is associated with a sense of considerable threat, and exaggeration of the risks and postoperative suffering stimulates anxiety reactions.58,59 Elevated preoperative anxiety increases the sensation of pain in patients in the postoperative period.5,60,61 It has been noted that anxiety influences pain perception more in men than in women.62 Also, the intensive care unit is regarded as a stress-generating place. The most stressful issues reported by the patients in the intensive care unit include “being thirsty”, “having tubes/probes in the nose and/or mouth”, and “not being able to sleep”, as well as pain.63 Not without significance for the perception of pain may be the influence of ethnic and cultural conditions, social status, and severity of the disease. Data concerning perception of pain also take into account the subject’s age and previous experiences associated with pain.13,57
Location of acute pain after cardiac surgery
As shown by studies conducted in the USA and Norway, 77%–85% of patients experience postoperative pain within 2 weeks after cardiac surgery.9,13,25 In a study of patients undergoing CABG, patients were evaluated for 4 postoperative days. Pain evaluation results were higher than expected: severe pain at rest in 49% of patients, severe pain when coughing in78%, and during movement in 62%.64 Another prospective study carried out on 705 patients undergoing cardiac surgery assessed activity-related pain daily until the sixth postoperative day. The most severe pain was associated with coughing, movements, turning around, getting up from bed, and deep breathing. Although pain scores were high immediately after the operation, the mean pain score reported by the patients when coughing and on the sixth day after the surgery was 4.33.42 The location of the most intense pain was also noted to change over time to the shoulder on postoperative day 7. The median duration of postoperative pain was 5 days for patients who had undergone bypass surgery and 6 days for patients who had had valvular surgery.65
The location of pain in patients after cardiac surgery varies from day to day. In the early postoperative period (within the first 24 hours), areas directly related to thoracotomy (the chest and mediastinal and pleural drain-placement sites) and forced position due to intubation (the back and buttocks) are predominant. On following days, pain in these areas decreases gradually, probably due to the removal of drains and some possibility to move within the bed. However, patients suffer from pain in the shoulders and lower legs that have undergone surgical intervention (vein extraction), which in turn may be associated with increased motor activity and spasticity of shoulder muscles stretched by thoracic dilatation during surgery.13,66 The pain is described as chest discomfort of noncardiac origin in up to 65% of cases, and can coexist with pain in the upper extremities, neck, head, and mid-back area.67
Like the number of painful areas, pain intensity is significantly higher in women than men and on the first day after cardiac surgery (4.57 vs 3.70 on VAS).15,68,69 Maximum pain intensity does not change significantly during the first 2 postoperative days, but decreases from the third day.13,65.68,70,71 Mueller et al reported significantly higher pain intensity in younger patients (<60 years of age) than older ones (≥60 years of age) on the second day after surgery.13 Overweight or obese (body mass index ≥25) patients reported higher pain intensity than those of normal weight on all days,13,72–74 and greater pain sensitivity was demonstrated in obese patients. Preventive approaches to postoperative pain in cardiac surgery have resulted in a reduction in risk of delirium and psychosis, which was lower than reported in the wider literature: <1% in patients in whom such approaches were applied.75,76
Chronic pain after cardiac surgery
If pain persists longer than necessary for postoperative wound healing or regression of the disease, it may take a chronic form. In contrast to acute pain, chronic postoperative pain (CPOP) loses its warning function, thus becoming a pathologic condition in itself. CPOP is a potential adverse effect of an otherwise-successful surgical procedure.14 Recently, Werner and Kongsgaard proposed the following diagnostic criteria for CPOP based on current understanding: the pain develops after a surgical procedure or increases in intensity after the surgical procedure, the pain is of at least 3–6 months’ duration and significantly affects quality of life, the pain is a continuation of acute postsurgery pain or develops after an asymptomatic period, the pain is localized to the surgical field, projected to the innervation territory of a nerve situated in the surgical field, or referred to a dermatome, and other causes of pain should be excluded.77
CPOP after cardiac surgery should be differentiated from myocardial ischemia, sternal instability, and mediastinitis. Pain may be present at the site of the sternotomy or in the legs, due to vein-graft harvesting.78 It can manifest as neuropathic pain, visceral pain, somatic pain, or mixed pain. This pain affects all areas of human life. Also, other factors, such as psychological (eg, anxiety, depression, and catastrophizing), demographics (eg, female sex and younger age), surgical (eg, open approach and procedure duration >3 hours), allergy to the osteosynthesis wire used for sternum closure, CABG, history of previous surgery, and intensity of pain in the immediate postoperative period (ie, the first few days), can influence the development of CABG.79–81 Explanation of the pathophysiology of chronic sternal pain after surgery has been poorly described in the literature. It may be related to structural changes in peripheral nerve endings, which would send altered afferent impulses to the spinal cord and central nervous system, leading to allodynia.54,82 In addition, epigenetic analysis may lead to identification of mechanisms critical to the development of chronic pain after an injury, thus providing new pathways and target mechanisms for future drug development and individualized medicine.83
Severe pain duration during the initial 24 postoperative hours is a predictor of the probability of developing CPOP. For every 10% increase in time spent in severe pain, the risk of developing CPOP goes up by 30%.14,84 According to the adopted average incidence patterns of CPOP, it has been shown to occur after cardiac surgery (30%–55%) and thoracotomies (5%–65%).4,85
CPOP after cardiac surgery is a significant clinical problem. The prevalence of CPOP reported in different studies is varied, but severe CPOP is present in <12% of patients.84 In some patients, CPOP lasts for many years or suddenly reappears a long time after sternotomy. Recent studies reported a 43% incidence of CPOP at 3 months,81 but only 11% at 12 months and 3.8% at 5 years after sternotomy.80 In 2016, Setälä et al demonstrated that the area of hyperalgesia around the sternotomy wound on postoperative day 4 was not associated with CPOP at 4–6 months.86 Among cardiac surgery patients, 37% suffer from persistent postoperative pain in the first 6 postoperative months, and in 17% it remains present >2 years after surgery.87
Sternotomy pain is described as pain of medium intensity affecting daily activities by 56% of patients, while 38% report unbearable pain. Adverse effects of CPOP on quality of life after cardiac surgery can be observed even a year after the procedure, manifesting as sleep disturbances in a third of patients with chronic pain.88–90 Pain has been observed even 28 months after surgery, with reported frequency up to 40% in patients over 60 years of age. In female patients, pain can persist for over a year, requiring in many cases treatment by a physician or physiotherapist.72,89,91
The incidence of CPOP has been observed to be similar after thoracotomy: 57% at 3 months,92 39%–56% at 6 months,55,92,93 and 50% at 1 year.94 In a 2014 meta-analysis, Bayman and Brennan determined that the incidence had been stable from the 1990s until the time of their study.92 Findings from studies show consistently that preoperative pain is a predominant predictor of CPOP,55,94 as well as poor pain control during the first 72 hours after surgery,93 whereas “dispositional optimism” has a protective effect.94 In 2016, Wildgaard et al showed a lower incidence of CPOP after video-assisted thoracic surgery (11%–30%, depending on the definition) compared to open thoracotomies.95
Assessment of patients with postoperative pain
Pain measurement is the first important step in effective treatment. A thorough assessment requires selection of validated measurement tools, which are adjusted to perceptual abilities and allow the detection of pain and assessment of its intensity, as well as the efficiency of treatment.17,19,96 Preferred scales, recognized by clinicians as the most precise evaluation tools, are based on self-assessment.16,97 The scales most commonly used in the postoperative period include the verbal rating scale (VRS), NRS, VAS, and picture scales, eg, based on the representation of facial expressions (Figure 2).
  | Figure 2 Pain scales – assessment of pain intensity. |
The VRS is ordinal in character, comprising a sequentially arranged set of digits with descriptions of pain intensity, eg, 0 = no pain, 1 = low pain, 2 = moderate pain, 3 = severe pain, and 4 = very severe pain. The NRS is an interval scale evaluating pain on the basis of 0- to 10-point scores, where 0 means no pain and 10 maximum pain. The VAS and NRS are interval scales and graphic in character. The length of a section of a horizontal line within the 0–10 cm range, described on the left as “no pain” and on the right as “unbearable pain”, is the measure of pain intensity.98,99
The VAS is the most difficult to use for patients, particularly those with cognitive disorders. In contrast, the VRS is well understood by patients, but the least sensitive.98 Patients rarely choose extreme values on this scale, which reduces its suitability for statistical comparisons. The NRS has a sensitivity similar to the VAS, is easy to use, and recommended for pain assessment by many authors.16,97,99
It is noteworthy while assessing pain that both behavioral and physiological parameters may be indicators of various stressful stimuli and not necessarily of pain. Additionally, pain reported by the patient is not always reflected in increased pulse rate or blood pressure or changes in facial expression. A facial expression scale can be used both for patients unable to communicate verbally and patients with tracheostomy.1,100
When pain is assessed, the frequency and regularity of measurements should be adjusted to the individual needs of the patient, taking into account his/her sleep and rest. It is postulated that pain should be estimated not only at rest but also under dynamic conditions. Pain assessment at rest is important to ensure the patient’s comfort in bed, whereas that conducted under dynamic conditions is intended to reduce the risk of postoperative complications, mainly circulatory and respiratory ones.99 As such, the Prince Henry Hospital Pain Score has become more widely used in patients after thoracic surgery. It evaluates painful symptoms during basic physical activity: 0 = no pain on cough, 1 = pain on cough, no pain while taking deep breaths, 2 = pain on deep breathing, no pain at rest, 3 = slight pain at rest, and 4 = severe pain at rest (Figure 3). The scale used in the original version does not include differentiation of mild and severe pain at rest.101
  | Figure 3 Prince Henry Hospital Pain Score. |
Measurement of pain under dynamic conditions has one additional advantage: it is easier to detect possible differences in pain perception when analgesic methods are compared.99 Observations should be recorded in the patient’s documentation to “visualize” the pain to medical staff. Pain-measurement results provide at the same time assessment of postoperative care, enabling an insight into the knowledge, skills, and commitment of the therapeutic team.
Use of drawings to assess pain perceptions in cardiac surgery patients
Perception of the disease constitutes an important factor affecting patients’ emotional state and outcomes of therapy. Drawing is one of the methods of assessing the perception of pain.102 The use of a graphic representation of the location of pain has become an occasion to learn about patient experiences that have passed undetected in a conventional examination.103 It allows one to go beyond the schema of clinical assessment, which seems especially important in work with cardiac surgery inpatients. Patients referred for CABG surgery declare particularly intense pain and anxiety at the decision-making phase.104,105 Information provided in a skillful and empathic way by a physician and/or psychologist may be an important factor in decreasing patient pain and anxiety levels (evaluated using the VAS), which affects decisions concerning treatment, as well as acceptance of the disease and the therapeutic methods proposed.
Pain assessment questionnaires
Multidimensional scales in the form of questionnaires assessing both the intensity of pain and the effect of chronic pain on various aspects of the patient’s functioning, physical activity, well-being, and health-related quality of life have been developed. Such scales are more complex and take more time to complete; therefore, they have been used in selected patients only. They are usually adjusted to a specific type of pain, and then the questions they contain are profiled to address precisely the selected pain syndrome. The most commonly used questionnaires of this type include the McGill Pain Questionnaire. It consists of a diagram of pain, the NRS, and 74 adjectives divided into four groups concerning the location, character, frequency, and intensity of pain. Subjects select the words that describe their current sensations most adequately. Additionally, the questionnaire allows the monitoring of the course of treatment of patients with chronic pain, including also emotional changes.106 The SF36 is a validated questionnaire widely used in medical practice and research, and has been used with cardiac surgery patients.104,107 It consists of 36 items measuring eight domains of quality of life: physical functioning, social functioning, role limitations due to physical problems, role limitations due to emotional problems, mental health, energy, vitality, bodily pain, and general health. Two summary scores are calculated from the eight domains’ scores.
Assessment of postoperative analgesia efficacy
In 2012, the American Society of Anesthesiologists published guidelines for treating perioperative pain based on the World Health Organization’s analgesic ladder.108 They were intended to increase the efficacy of acute perioperative pain management, improve patients’ quality of life during the perioperative period, and prevent the development of side effects and chronic pain due to inappropriate analgesia.
The guidelines were implemented in a number of studies on the quality of postoperative pain management conducted in different countries to improve postoperative care. For example, in the USA, Apfelbaum et al showed that 80% of patients experienced pain after the procedure. Among these, 86% had moderate, severe, or extremely severe pain, which in many cases occurred after discharge from hospital.3
Pain treatment in German hospitals was analyzed by Maier et al during a 3-year study based on interviewing patients undergoing surgical and nonsurgical procedures about the intensity of their pain and efficiency of treatment received:109 55% of patients from the surgical group and 57% from the nonsurgical group reported dissatisfaction with their pain treatment. Furthermore, 39% of nonsurgical and 15% of surgical patients did not receive any analgesia, despite pain. Pain therapy was considered inadequate for 46% of nonsurgical and 30% of surgical patients. In conclusion, the authors pointed out that pain after surgical procedures is still treated inadequately.109
A similar study conducted in China by Liu et al reported postoperative pain relief obtained within 3 days in 83% of patients.110 However, 20% of patients interviewed expressed dissatisfaction with their pain treatment, whereas 52% reported that they did not receive any analgesia, even though 91% of patients reported pain. The authors concluded that despite the availability of effective methods of pain treatment, additional education of hospital staff, patients, and their families about pain treatment is needed, as well as better communication with patients in the postoperative period.110 Poorer response to analgesic medication is also observed in patients qualifying for CABG surgery demonstrating high levels of preoperative anxiety (state) and stronger anxiety (trait) than in patients with low levels. Therefore, actions undertaken to reduce patients’ anxiety may reduce their need for analgesic medication.61
The treatment of pain after thoracic surgery is a challenge, and takes place in individual clinics mostly according to clinic internal standards. There are currently no valid guidelines for the treatment of acute perioperative and posttraumatic pain. For effective pain treatment, individual pain experience and pain intensity of the various thoracic surgical procedures must be considered. Regular pain assessment with appropriate methods and their documentation form the basis of adequate and well-adjusted pain therapy. There are a number of pain-therapy methods – nonpharmacological and drug-based – whose effectiveness has been described in the literature. For the treatment of acute postoperative pain after thoracic surgery, mainly drug-related procedures are used, except for physiotherapy as a nonpharmacological method.19,111
Methods of postoperative analgesia
The fundamental aim of effective postoperative analgesia is to provide the patient with subjective comfort, facilitate the recovery process, and reduce the risk of complications, including the development of persistent postoperative pain.112,113 Effective treatment of postoperative pain should be multidimensional and based on three main therapeutic principles: administration of analgesics, multimodal analgesia, and regional anesthesia techniques.112
Pharmacological therapy
Pharmacological therapy utilizes nonopioid and opioid analgesics. The most commonly used nonopioids include nonsteroidal anti-inflammatory drugs (NSAIDs), metamizole, and paracetamol. A particularly good analgesic effect can be obtained by combining these with the use of an intravenous opioid. This allows reduction of opioid doses by about 40%–50%, which in turn results in a decrease in incidence of adverse reactions associated with their use. Opioid analgesic agents are frequently used to manage postoperative pain; however, adverse effects, including drowsiness and respiratory depression, can delay extubation and prolong the patient’s stay in intensive care.114,115 NSAID use may be limited by increased risk of renal dysfunction and bleeding in cardiac surgery patients.46,116,117
Multimodal (or balanced) analgesia
In cases of extensive cardiac and thoracic surgery procedures, the most frequently recommended type of analgesia is multimodal, involving simultaneous use of a few analgesic agents with different mechanisms of action (combined pharmacotherapy), together with selected regional analgesia techniques. In practice, balanced analgesia involves administration of paracetamol and/or NSAIDs with opioids or local analgesia techniques, depending on the individual indications.117 The use of patient-controlled analgesia (PCA) is very important.
Patient-controlled analgesia
This method involves the administration of parenteral analgesics, most frequently opioids, through a microprocessor-controlled infusion pump used by patients at the moment they begin to feel pain. It is based on the premise that only the patient can assess the intensity of pain and the potency of the analgesic drug used. At the onset of pain, the patient activates the dosing system and receives a dose of medicine programmed by the doctor, which is followed by activation of a PCA protection system (lockout time), ie, a temporary blockade of the dispensing system, which allows avoidance of overdose. PCA determines maintaining a steady drug concentration in the serum, which allows for more effective relief of postoperative pain in comparison with other methods of parenteral administration of opioids.118,119 PCA is superior to nurse-controlled analgesia in poststernotomy patients.120 In postcardiac surgical patients, PCA increases cumulative 24- and 48-hour morphine consumption and improves 48-hour VAS scores compared with nurse-controlled analgesia.121
Techniques of regional anesthesia
In the postoperative period, regional anesthesia techniques are also applied, but their use is dependent on the general condition of the patient, as well as the location and extent of the surgical procedure.114 Such anesthesia techniques as thoracic epidural analgesia (TEA), continuous unilateral thoracic paravertebral block, and intercostal nerve block are recommended and frequently used.122
Thoracic epidural analgesia
Continuous TEA used in cardiac and thoracic surgery ensures effective control of intra- and postoperative pain. It is particularly important in patients with contraindications for high-dose analgesics. Additionally, a favorable effect of TEA on coronary flow, stabilization of the cardiovascular system, reduction of postoperative respiratory dysfunctions, humoral response to surgery, and lower tendency to activation of the coagulation system has been emphasized. However, there are many concerns with respect to the use of this technique, due to its invasiveness and high cost.123–125
Paravertebral blockade
The technique recommended after thoracic surgery is paravertebral block, ensuring most effective pain control during both cough and at rest, lower demand for opioids, improvement in ventilation, lower incidence of postoperative nausea and vomiting, and stability of arterial blood pressure compared with TEA.126,127
Other regional anesthesia techniques
Regional anesthesia methods based on peripheral nerve blocks have developed considerably, mainly owing to modern techniques of imaging neural structures, including ultrasound in particular. Mastering the techniques of effective and safe blockade of neural conduction, including that of sensory stimuli at the level of plexuses, ganglia, specific nerves, or infiltration anesthesia of the wound margins provide the basis for the use and popularization of these methods in postoperative analgesia.128 So-called continuous techniques are also used, such as administration of local analgesics through implanted subcutaneous catheters and continuous blocks of neural plexuses by transdermal catheters introduced into these structures. An example of such target neural structures are intercostal nerves after thoracic surgery and rib fractures.128
Transcutaneous electrical nerve stimulation
Transcutaneous electrical nerve stimulation is also used in postoperative analgesia in light of its high effectiveness, reduction in demand for analgesic drugs, lower incidence of complications in the postoperative period, and the possibility of earlier ambulation, rehabilitation, and reduction in hospitalization time in patients undergoing cardiac surgery.129 It is a comfortable, noninvasive, and nonpharmacological method that can be applied easily.
Additionally, nonpharmacological methods of treatment of postoperative pain, including relaxation, massage, osteopathic manipulative treatment, heat therapy, cryotherapy, acupuncture, hypnosis, psychoeducation, and behavioral therapy, are used.18,130–132 Nutritional support as a necessary therapy for critically ill cardiac surgery patients is also important.133,134 Also, sleep and rest are indicated to support pain management. It should be borne in mind that even patients under general anesthesia receive stimuli from the environment; therefore, positive reinforcements (such as the use of music therapy in the operating room or postanesthesia care unit) can contribute to faster recovery.135,136
Conclusion
It should be remarked that progress in surgical techniques alone would not have had such importance had it not been for modern anesthesia and postoperative management of patients after cardiac surgery. Significant progress has also been observed in this area not only as a result of introduction of modern drugs or devices, such as modern respirators, precision infusion pumps, or cardiovascular support equipment. First and foremost, understanding and better knowledge of physiological disorders resulting from surgical trauma, anesthesia, and adverse effects of extracorporeal circulation, as well as the development of standards for intensive postoperative care units, are critical to improvements in early treatment outcomes.
Acknowledgment
This work was supported by grant 503/1-079-03/503-16-001-003 from the Medical University of Lodz.
Disclosure
The authors report no conflicts of interest in this work.
References
Mazzeffi M, Khelemsky Y. Poststernotomy pain: a clinical review. J Cardiothorac Vasc Anesth. 2011;25(6):1163–1178. | ||
Steegers MA, Snik DM, Verhagen AF, van der Drift MA, Wilder-Smith OH. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain. 2008;9(10):955–961. | ||
Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–540. | ||
Cogan J. Pain management after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2010;14(3):201–204. | ||
Vaughn F, Wichowski H, Bosworth G. Does preoperative anxiety level predict postoperative pain? AORN J. 2007;85(3):589–604. | ||
Sasseron AB, Figueiredo LC, Trova K, et al. Does the pain disturb respiratory function after heart surgeries? Rev Bras Cir Cardiovasc. 2009;24(4):490–496. | ||
Freynhagen R, Baron R, Gockel U, Tölle TR. Pain DETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. | ||
Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118(4):934–944. | ||
Bjørnnes AK, Rustøen T, Lie I, Watt-Watson J, Leegaard M. Pain characteristics and analgesic intake before and following cardiac surgery. Eur J Cardiovasc Nurs. 2016;15(1):47–54. | ||
Darr C, Cheufou D, Weinreich G, Hachenberg T, Aigner C, Kampe S. Robotic thoracic surgery results in shorter hospital stay and lower postoperative pain compared to open thoracotomy: a matched pairs analysis. Surg Endosc. 2017;31(10):4126–4130. | ||
Fot EV, Izotova NN, Yudina AS, Smetkin AA, Kuzkov VV, Kirov MY. Automated weaning from mechanical ventilation after off-pump coronary artery bypass grafting. Front Med (Lausanne). 2017;4:31. | ||
Träger K, Fritzler D, Fischer G, et al. Treatment of post-cardiopulmonary bypass SIRS by hemoadsorption: a case series. Int J Artif Organs. 2016;39(3):141–146. | ||
Mueller XM, Tinguely F, Tevaearai HT, Revelly JP, Chioléro R, von Segesser LK. Pain location, distribution, and intensity after cardiac surgery. Chest. 2000;118(2):391–396. | ||
Kalso E, Mennander S, Tasmuth T, Nilsson E. Chronic post-sternotomy pain. Acta Anaesthesiol Scand. 2001;45(8):935–939. | ||
Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, et al. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology. 2014;120(5):1237–1245. | ||
van Dijk JF, van Wijck AJ, Kappen TH, Peelen LM, Kalkman CJ, Schuurmans MJ. Postoperative pain assessment based on numeric ratings is not the same for patients and professionals: a cross-sectional study. Int J Nurs Stud. 2012;49(1):65–71. | ||
Rothaug J, Zaslansky R, Schwenkglenks M, et al. Patients’ perception of postoperative pain management: validation of the International Pain Outcomes (IPO) questionnaire. J Pain. 2013;14(11):1361–1370. | ||
Braun LA, Stanguts C, Casanelia L, et al. Massage therapy for cardiac surgery patients: a randomized trial. J Thorac Cardiovasc Surg. 2012;144(6):1453–1459. | ||
Hendrix H, Kamlak V, Prisadov G, Welcker K. [Pain treatment after thoracic surgery]. Zentralbl Chir. 2017;142(3):337–347. German. | ||
Mcmahon SR, Ades PA, Thompson PD. The role of cardiac rehabilitation in patients with heart disease. Trends Cardiovasc Med. 2017;27(6):420–425. | ||
Doenst T, Essa Y, Jacoub K, et al. Cardiac surgery 2016 reviewed. Clin Res Cardiol. 2017;106(11):851–867. | ||
Easterwood RM, Bostock IC, Nammalwar S, Mccullough JN, Iribarne A. The evolution of minimally invasive cardiac surgery: from minimal access to transcatheter approaches. Future Cardiol. 2018;14(1):75–87. | ||
Soylu E, Harling L, Ashrafian H, et al. A systematic review of the safety and efficacy of distal coronary artery anastomotic devices in MIDCAB and TECAB surgery. Perfusion. 2016;31(7):537–543. | ||
Kofler M, Schachner T, Reinstadler SJ, et al. Comparative analysis of perioperative and mid-term results of TECAB and MIDCAB for revascularization of anterior wall. Innovations (Phila). 2017;12(3):207–213. | ||
Roediger L, Larbuisson R, Lamy M. New approaches and old controversies to postoperative pain control following cardiac surgery. Eur J Anaesthesiol. 2006;23(7):539–550. | ||
Melidi E, Latsios G, Toutouzas K, et al. Cardio-anesthesiology considerations for the trans-catheter aortic valve implantation (TAVI) procedure. Hellenic J Cardiol. 2016;57(6):401–406. | ||
Bauernschmitt R, Bauer S, Liewald C, et al. First successful transcatheter double valve replacement from a transapical access and nine-month follow-up. EuroIntervention. 2017;12(13):1645–1648. | ||
Sokoloff A, Eltchaninoff H. [TAVI: history and perspectives]. Presse Med. 2017;46(7–8 Pt 1):772–776. French. | ||
Kowalewski M, Pawliszak W, Malvindi PG, et al. Off-pump coronary artery bypass grafting improves short-term outcomes in high-risk patients compared with on-pump coronary artery bypass grafting: meta-analysis. J Thorac Cardiovasc Surg. 2016;151(1):60–77. | ||
Watt-Watson J, Stevens B. Managing pain after coronary artery bypass surgery. J Cardiovasc Nurs. 1998;12(3):39–51. | ||
Swarm RA, Karanikolas M, Kalauokalani D. Pain treatment in the perioperative period. Curr Probl Surg. 2001;38(11):835–920. | ||
Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain. 2008;9(2):122–145. | ||
Yaksh T, Woller S, Ramachandran R, Sorkin L. The search for novel analgesics: targets and mechanisms. F1000Prime Rep. 2015;7:56. | ||
Mao J. Current challenges in translational pain research. Trends Pharmacol Sci. 2012;33(11):568–573. | ||
Moloney N, Rabey M, Nijs J, Hush J, Slater H. Support for extended classification of pain states. Pain. 2017;158(7):1395. | ||
Sorkin LS, Wallace MS. Acute pain mechanisms. Surg Clin North Am. 1999;79(2):213–229. | ||
Offner KA, Loop T. [Pathophysiology and epidemiology of pain in thoracic surgery]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2012;47(9):568–575. German. | ||
[No authors listed]. Pain terms: a list with definitions and notes on usage. Pain. 1979;6(3):249–252. | ||
Ergün A, Sirlak M. [Pulmonary function test before and after operation of coronary artery by-pass surgery]. Tuberk Toraks. 2003;51(1):17–22. Turkish. | ||
Shenkman Z, Shir Y, Weiss YG, Bleiberg B, Gross D. The effects of cardiac surgery on early and late pulmonary functions. Acta Anaesthesiol Scand. 1997;41(9):1193–1199. | ||
Roncada G, Dendale P, Linsen L, Hendrikx M, Hansen D. Reduction in pulmonary function after CABG surgery is related to postoperative inflammation and hypercortisolemia. Int J Clin Exp Med. 2015;8(7):10938–10946. | ||
Milgrom LB, Brooks JA, Qi R. Pain levels experienced with activities after cardiac surgery. Am J Crit Care. 2004;13(2):116–125. | ||
Bartoloni A, Polati E, Finco G, Facchin S, Rigo V, Gottin L. [The neuroendocrine and metabolic responses to surgical stress]. Chir Ital. 1995;47(6):3–11. Italian. | ||
Saito H. [Endocrine response to surgical stress]. Nihon Geka Gakkai Zasshi. 1996;97(9):701–707. Japanese. | ||
Malberg H, Wessel N, Kopp B, Bauernschmitt R. [Analysis of cardiovascular regulation after heart operation]. Biomed Tech. 2002;47 Suppl 1 (Pt 2):541–542. German. | ||
Liu SS, Wu CL, Cl W. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesth Analg. 2007;104(3):689–702. | ||
Schultz CH, Rivers EP, Feldkamp CS, et al. A characterization of hypothalamic-pituitary-adrenal axis function during and after human cardiac arrest. Crit Care Med. 1993;21(9):1339–1347. | ||
Karkouti K, Dattilo KM. Perioperative hemostasis and thrombosis. Can J Anaesth. 2006;53(12):1260–1262. | ||
Smit PJ, Shariff MA, Nabagiez JP, Khan MA, Sadel SM, McGinn JT. Experience with a minimally invasive approach to combined valve surgery and coronary artery bypass grafting through bilateral thoracotomies. Heart Surg Forum. 2013;16(3):E125–E131. | ||
Uymaz B, Sezer G, Coskun PK, Tarcan O, Ozleme S, Aybek T. Clinical outcome, pain perception and activities of daily life after minimally invasive coronary artery bypass grafting. Anadolu Kardiyol Derg. 2014;14(2):172–177. | ||
Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin. 2008;26(2):355–367. | ||
Dualé C, Ouchchane L, Schoeffler P, et al. Neuropathic aspects of persistent postsurgical pain: a French multicenter survey with a 6-month prospective follow-up. J Pain. 2014;15(1):24.e1–e20. | ||
Buvanendran A, Kroin JS, della Valle CJ, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010;110(1):199–207. | ||
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. | ||
Kampe S, Geismann B, Weinreich G, Stamatis G, Ebmeyer U, Gerbershagen HJ. The influence of type of anesthesia, perioperative pain, and preoperative health status on chronic pain six months after thoracotomy: a prospective cohort study. Pain Med. 2017;18(11):2208–2213. | ||
Allama AM. Intercostal muscle flap for decreasing pain after thoracotomy: a prospective randomized trial. Ann Thorac Surg. 2010;89(1):195–199. | ||
Shaw A, Keefe FJ. Genetic and environmental determinants of postthoracotomy pain syndrome. Curr Opin Anaesthesiol. 2008;21(1):8–11. | ||
Underwood MJ, Firmin RK, Jehu D. Aspects of psychological and social morbidity in patients awaiting coronary artery bypass grafting. Heart. 1993;69(5):382–384. | ||
Fitzsimons D, Parahoo K, Richardson SG, Stringer M. Patient anxiety while on a waiting list for coronary artery bypass surgery: a qualitative and quantitative analysis. Heart Lung. 2003;32(1):23–31. | ||
Nelson F, Zimmerman L, Barnason S, Nieveen J, Schmaderer M. The relationship and influence of anxiety on postoperative pain in the coronary artery bypass graft patient. J Pain Symptom Manage. 1998;15(2):102–109. | ||
Greszta E, Siemińska MJ. [Relationship of preoperative anxiety-state and anxiety-trait in patients qualified for coronary artery bypass graft surgery to the perception of postoperative pain and other pain complaints]. Ann Acad Med Stetin. 2008;54(1):157–163. Polish. | ||
Thibodeau MA, Welch PG, Katz J, Asmundson GJ. Pain-related anxiety influences pain perception differently in men and women: a quantitative sensory test across thermal pain modalities. Pain. 2013;154(3):419–426. | ||
Dessotte CA, Rodrigues HF, Furuya RK, Rossi LA, Dantas RA. Stressors perceived by patients in the immediate postoperative of cardiac surgery. Rev Bras Enferm. 2016;69(4):741–750. | ||
Lahtinen P, Kokki H, Hendolin H. Pain after cardiac surgery: prospective cohort study of 1-year incidence and intensity. Anesthesiology. 2006;105(4):794–800. | ||
Reimer-Kent J. From theory to practice: preventing pain after cardiac surgery. Am J Crit Care. 2003;12(2):136–143. | ||
Carle C, Ashworth A, Roscoe A. A survey of post-sternotomy chronic pain following cardiac surgery. Anaesthesia. 2009;64(12):1387. | ||
van Leersum NJ, van Leersum RL, Verwey HF, Klautz RJ. Pain symptoms accompanying chronic poststernotomy pain: a pilot study. Pain Med. 2010;11(11):1628–1634. | ||
Meehan DA, Mcrae ME, Rourke DA, Eisenring C, Imperial FA. Analgesic administration, pain intensity, and patient satisfaction in cardiac surgical patients. Am J Crit Care. 1995;4(6):435–442. | ||
Yorke J, Wallis M, Mclean B. Patients’ perceptions of pain management after cardiac surgery in an Australian critical care unit. Heart Lung. 2004;33(1):33–41. | ||
Puntillo K, Weiss SJ. Pain: its mediators and associated morbidity in critically ill cardiovascular surgical patients. Nurs Res. 1994;43(1):31–36. | ||
Kianfar A, Shadvar K, Mahoori A, Azarfarin R. Pain after cardiac surgery. Critical Care. 2007;11 Suppl 2:P429. | ||
Bruce J, Drury N, Poobalan SA, Jeffrey RR, Smith SW, Chambers AW. The prevalence of chronic chest and leg pain following cardiac surgery: a historical cohort study. Pain. 2003;104(1):265–273. | ||
Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of obesity and pain in a general population: results from the Southern Pain Prevalence Study. J Pain. 2007;8(5):430–436. | ||
Cadish LA, Hacker MR, Modest AM, Rogers KJ, Dessie S, Elkadry EA. Association between body mass index and pain following transobturator sling. J Obstet Gynaecol. 2017;37(6):766–769. | ||
Leacche M, Carrier M, Bouchard D, et al. Improving neurologic outcome in off-pump surgery: the “no touch” technique. Heart Surg Forum. 2003;6(3):169–175. | ||
Kazmierski J, Kowman M, Banach M, et al. Incidence and predictors of delirium after cardiac surgery: results from the IPDACS study. J Psychosom Res. 2010;69(2):179–185. | ||
Werner MU, Kongsgaard UE. I. Defining persistent post-surgical pain: is an update required? Br J Anaesth. 2014;113(1):1–4. | ||
Myles PS, Daly DJ, Djaiani G, Lee A, Cheng DC. A systematic review of the safety and effectiveness of fast-track cardiac anesthesia. Anesthesiology. 2003;99(4):982–987. | ||
Fine PG, Karwande SV. Sternal wire-induced persistent chest pain: a possible hypersensitivity reaction. Ann Thorac Surg. 1990;49(1):135–136. | ||
Gjeilo KH, Stenseth R, Wahba A, Lydersen S, Klepstad P. Chronic postsurgical pain in patients 5 years after cardiac surgery: a prospective cohort study. Eur J Pain. 2017;21(3):425–433. | ||
Guimarães-Pereira L, Farinha F, Azevedo L, Abelha F, Castro-Lopes J. Persistent postoperative pain after cardiac surgery: incidence, characterization, associated factors and its impact in quality of life. Eur J Pain. 2016;20(9):1433–1442. | ||
van Gulik L, Janssen LI, Ahlers SJ, et al. Risk factors for chronic thoracic pain after cardiac surgery via sternotomy. Eur J Cardiothorac Surg. 2011;40(6):1309–1313. | ||
James SK. Chronic postsurgical pain: is there a possible genetic link? Br J Pain. 2017;11(4):178–185. | ||
Fletcher D, Stamer UM, Pogatzki-Zahn E, et al. Chronic postsurgical pain in Europe: an observational study. Eur J Anaesthesiol. 2015;32(10):725–734. | ||
Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101(1):77–86. | ||
Setälä P, Kalliomäki ML, Järvelä K, Huhtala H, Sisto T, Puolakka P. Postoperative hyperalgesia does not predict persistent post-sternotomy pain: observational study based on clinical examination. Acta Anaesthesiol Scand. 2016;60(4):520–528. | ||
Guimares-Pereira L, Rejs P, Abelha F, Azevedo L, Castro-Lopes J. Persistent postoperative pain after cardiac surgery: a systematic review with meta-analysis regarding incidence and pain intensity. Pain. 2017;158(10):1869–1885. | ||
Eisenberg E, Pultorak Y, Pud D, Bar-El Y. Prevalence and characteristics of post coronary artery bypass graft surgery pain (PCP). Pain. 2001;92(1):11–17. | ||
Meyerson J, Thelin S, Gordh T, Karlsten R. The incidence of chronic post-sternotomy pain after cardiac surgery: a prospective study. Acta Anaesthesiol Scand. 2001;45(8):940–944. | ||
Routledge FS, Tsuyuki RT, Hervas-Malo M, Leblanc P, Mcfetridge-Durdle JA, King KM. The influence of coronary artery bypass graft harvest site on women’s pain, functional status, and health services utilization throughout the first post-operative year: a longitudinal study. Int J Nurs Stud. 2009;46(8):1054–1060. | ||
Bjørnnes AK, Parry M, Lie I, et al. Pain experiences of men and women after cardiac surgery. J Clin Nurs. 2016;25(19–20):3058–3068. | ||
Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain. 2014;15(9):887–897. | ||
Niraj G, Kelkar A, Kaushik V, et al. Audit of postoperative pain management after open thoracotomy and the incidence of chronic postthoracotomy pain in more than 500 patients at a tertiary center. J Clin Anesth. 2017;36:174–177. | ||
Hetmann F, Kongsgaard UE, Sandvik L, Schou-Bredal I. Prevalence and predictors of persistent post-surgical pain 12 months after thoracotomy. Acta Anaesthesiol Scand. 2015;59(6):740–748. | ||
Wildgaard K, Ringsted TK, Hansen HJ, Petersen RH, Kehlet H. Persistent postsurgical pain after video-assisted thoracic surgery: an observational study. Acta Anaesthesiol Scand. 2016;60(5):650–658. | ||
Janssen KJ, Kalkman CJ, Grobbee DE, Bonsel GJ, Moons KG, Vergouwe Y. The risk of severe postoperative pain: modification and validation of a clinical prediction rule. Anesth Analg. 2008;107(4):1330–1339. | ||
Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth. 2011;107(4):619–626. | ||
Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804. | ||
Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101(1):17–24. | ||
McCaffery M, Beebe A. Pain Clinical Manual for Nursing Practice. Maryland (MO): Mosby; 1989. | ||
Torda TA, Hann P, Mills G, de Leon G, Penman D. Comparison of extradural fentanyl, bupivacaine and two fentanyl-bupivacaine mixtures for pain relief after abdominal surgery. Br J Anaesth. 1995;74(1):35–40. | ||
Guillemin M. Understanding illness: using drawings as a research method. Qual Health Res. 2004;14(2):272–289. | ||
Egloff N, Cámara RJ, von Känel R, Klingler N, Marti E, Ferrari ML. Pain drawings in somatoform-functional pain. BMC Musculoskelet Disord. 2012;13:257. | ||
al-Ruzzeh S, Athanasiou T, Mangoush O, Modine T, George S, Amrani M. Predictors of poor mid-term health related quality of life after primary isolated coronary artery bypass grafting surgery. Heart. 2005;91(12):1557–1562. | ||
Nowicka-Sauer K, Jaroszewicz K, Szyndler K, Beta S, Siebers J. How to express pain and illness? Using drawing to assess pain and illness perceptions in patients awaiting coronary artery by-pass grafting surgery: preliminary report. Forum Med Rodz. 2015;9(3):288–290. | ||
Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. | ||
Simchen E, Galai N, Braun D, et al. Sociodemographic and clinical factors associated with low quality of life one year after coronary bypass operations: the Israeli Coronary Artery Bypass study (ISCAB). J Thorac Cardiovasc Surg. 2001;121(5):909–919. | ||
Thompson M, Tiwari A, Fu R, Moe E, Buckley DI. A framework to facilitate the use of systematic reviews and meta-analyses in the design of primary research studies. 2012. Available from: https://www.ncbi.nlm.nih.gov/sites/books/NBK83621. Accessed July 10, 2018. | ||
Maier C, Nestler N, Richter H, et al. The quality of pain management in German hospitals. Dtsch Arztebl Int. 2010;107(36):607–614. | ||
Liu WR, Zhang L, Woo SM, et al. A study of patient experience and perception regarding postoperative pain management in Chinese hospitals. Patient Prefer Adherence. 2013;7:1157–1162. | ||
Boitor M, Gélinas C, Richard-Lalonde M, Thombs BD. The effect of massage on acute postoperative pain in critically and acutely ill adults post-thoracic surgery: systematic review and meta-analysis of randomized controlled trials. Heart Lung. 2017;46(5):339–346. | ||
Myles PS, Daly DJ, Djaiani G, Lee A, Cheng DC. A systematic review of the safety and effectiveness of fast-track cardiac anesthesia. Anesthesiology. 2003;99(4):982–987. | ||
Stasiowska MK, Ng SC, Gubbay AN, Cregg R. Postoperative pain management. Br J Hosp. Med (Lond). 2015;76(10):570–575. | ||
Kruger M, McRae K. Pain management in cardiothoracic practice. Surg Clin North Am. 1999;79(2):387–400. | ||
White PF, Rawal S, Latham P, et al. Use of a continuous local anesthetic infusion for pain management after median sternotomy. Anesthesiology. 2003;99(4):918–923. | ||
Hynninen MS, Cheng DC, Hossain I, et al. Non-steroidal anti-inflammatory drugs in treatment of postoperative pain after cardiac surgery. Can J Anaesth. 2000;47(12):1182–1187. | ||
White PF. Multimodal analgesia: its role in preventing postoperative pain. Curr Opin Investig Drugs. 2008;9(1):76–82. | ||
Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87(1):36–46. | ||
Walder B, Schafer M, Henzi I, Tramèr MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain: a quantitative systematic review. Acta Anaesthesiol Scand. 2001;45(7):795–804. | ||
Pettersson PH, Lindskog EA, Owall A. Patient-controlled versus nurse-controlled pain treatment after coronary artery bypass surgery. Acta Anaesthesiol Scand. 2000;44(1):43–47. | ||
Bainbridge D, Martin JE, Cheng DC. Patient-controlled versus nurse-controlled analgesia after cardiac surgery: a meta-analysis. Can J Anaesth. 2006;53(5):492–499. | ||
Steinthorsdottir KJ, Wildgaard L, Hansen HJ, Petersen RH, Wildgaard K. Regional analgesia for video-assisted thoracic surgery: a systematic review. Eur J Cardiothorac Surg. 2014;45(6):959–966. | ||
Clemente A, Carli F. The physiological effects of thoracic epidural anesthesia and analgesia on the cardiovascular, respiratory and gastrointestinal systems. Minerva Anestesiol. 2008;74(10):549–563. | ||
Mehta Y, Vats M, Sharma M, Arora R, Trehan N. Thoracic epidural analgesia for off-pump coronary artery bypass surgery in patients with chronic obstructive pulmonary disease. Ann Card Anaesth. 2010;13(3):224–230. | ||
Caputo M, Alwair H, Rogers CA, et al. Thoracic epidural anesthesia improves early outcomes in patients undergoing off-pump coronary artery bypass surgery: a prospective, randomized, controlled trial. Anesthesiology. 2011;114(2):380–390. | ||
Piraccini E, Pretto EA, Corso RM, Gambale G. Analgesia for thoracic surgery: the role of paravertebral block. HSR Proc Intensive Care Cardiovasc Anesth. 2011;3(3):157–160. | ||
d’Ercole F, Arora H, Kumar PA. Paravertebral block for thoracic surgery. J Cardiothorac Vasc Anesth. 2018;32(2):915–927. | ||
Wahal C, Kumar A, Pyati S. Advances in regional anaesthesia: a review of current practice, newer techniques and outcomes. Indian J Anaesth. 2018;62(2):94–102. | ||
Ozturk NK, Baki ED, Kavakli AS, et al. Comparison of transcutaneous electrical nerve stimulation and parasternal block for postoperative pain management after cardiac surgery. Pain Res Manag. 2016;2016:4261949. | ||
Hattan J, King L, Griffiths P. The impact of foot massage and guided relaxation following cardiac surgery: a randomized controlled trial. J Adv Nurs. 2002;37(2):199–207. | ||
Asmussen S, Przkora R, Maybauer DM, et al. Meta-analysis of electroacupuncture in cardiac anesthesia and intensive care. J Intensive Care Med. Epub 2017 Jan 1. | ||
Racca V, Bordoni B, Castiglioni P, Modica M, Ferratini M. Osteopathic manipulative treatment improves heart surgery outcomes: a randomized controlled trial. Ann Thorac Surg. 2017;104(1):145–152. | ||
Keith M, Mokbel R, San Emeterio M, Song J, Errett L. Evaluation of taste sensitivity in patients undergoing coronary artery bypass graft surgery. J Am Diet Assoc. 2010;110(7):1072–1077. | ||
Stoppe C, Goetzenich A, Whitman G, et al. Role of nutrition support in adult cardiac surgery: a consensus statement from an international multidisciplinary expert group on nutrition in cardiac surgery. Crit Care. 2017;21(1):131. | ||
Voss JA, Good M, Yates B, Baun MM, Thompson A, Hertzog M. Sedative music reduces anxiety and pain during chair rest after open-heart surgery. Pain. 2004;112(1–2):197–203. | ||
Bradt J, Dileo C, Potvin N. Music for stress and anxiety reduction in coronary heart disease patients. Cochrane Database Syst Rev. 2013;28(12):CD006577. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
