Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Assessing the Usefulness of the Prevexair Smartphone Application in the Follow-Up High-Risk Patients with COPD
Authors Rodríguez Hermosa JL , Fuster Gomila A, Puente Maestu L , Amado Diago CA , Callejas González FJ , Malo De Molina Ruiz R, Fuentes Ferrer ME, Alvarez-Sala JL, Calle Rubio M
Received 2 September 2020
Accepted for publication 11 December 2020
Published 8 January 2021 Volume 2021:16 Pages 53—65
DOI https://doi.org/10.2147/COPD.S279394
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Richard Russell
Juan Luis Rodríguez Hermosa,1,2 Antonia Fuster Gomila,3 Luis Puente Maestu,4 Carlos Antonio Amado Diago,5,6 Francisco Javier Callejas González,7 Rosa Malo De Molina Ruiz,8 Manuel E Fuentes Ferrer,9,10 Jose Luis Alvarez-Sala,1,2 Myriam Calle Rubio1,2
1Pulmonology Department, Hospital Clínico San Carlos, Madrid, Spain; 2Department of Medicine, Universidad Complutense de Madrid, Madrid, Spain; 3Pulmonology Department, Hospital U. Son Llátzer, Palma De Mallorca, Balearic Islands, Spain; 4Pulmonology Department, Hospital U. Gregorio Marañón, Madrid, Spain; 5Pulmonology Department, Hospital U. Marqués de Valdecilla, Santander, Cantabria, Spain; 6Department of Medicine, Universidad de Cantabria, Santander, Spain; 7Pulmonology Department, Complejo Hospitalario U. de Albacete, Albacete, Spain; 8Pulmonology Department, Hospital U. Puerta de Hierro de Majadahonda, Madrid, Spain; 9Department of Medicine Preventive, San Carlos Health Research Institute (IdISSC), Madrid, Spain; 10Department of Medicine, Universidad Alfonso X El Sabio, Madrid, Spain
Correspondence: Myriam Calle Rubio
Pulmonology Department Hospital Clínico San Carlos, C/Martin Lagos s/n., Madrid 28040, Spain
Tel +34 91 3303477
Fax +34 91 3303374
Email [email protected]
Introduction: This manuscript analyzes the exacerbations recorded by the Prevexair application through the daily analysis of symptoms in high-risk patients with COPD and explores its usefulness in assessing clinical stability with respect to that reported in visits.
Patients and Methods: This study is a multi-centre cohort of COPD patients with the exacerbator phenotype who were monitored over 6 months. The Prevexair application was installed on the patients’ smartphones. Patients used the app to record symptom changes, use of medication and use of healthcare resources. It is not established a recommended action plan when worsening of symptoms. At their clinical visit during the follow-up period, patients were asked about exacerbations suffered during these 6 months of monitoring. The investigators who conducted the visit were blinded about the Prevexair app records.
Results: The patients experienced a total of 185 exacerbations according to daily records in the app whereas only 64 exacerbations were recalled during medical visits. Perception became more accurate for severe exacerbations (kappa 0.6577), although we found no factors that predicted poor recall. The proportion of 72.5% patients were classified as unstable if the exacerbations captured by Prevexair were used to define stability, versus 47.8% if the exacerbations recall in visit was used. Two-thirds of the exacerbations recorded in the Prevexair application were not reported to doctors during their clinical visits. Almost half were treated with oral corticosteroids and/or antibiotics and more than one-quarter of the exacerbations treated did not seek medical attention.
Conclusion: The findings of this cohort study confirm that patients do not always remember the exacerbations suffered during their medical visit. The prevexair application is useful in monitoring COPD patients at high risk, in order to a better assessment of exacerbations of COPD during medical visits. Further research must be carried out to evaluate this strategy in clinical practice.
Keywords: chronic obstructive pulmonary disease, telemonitoring, mobile health, exacerbations, clinical prediction, management, electronic patient record
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic and complex disease that requires a multidimensional assessment including risk evaluation. Identifying the level of risk is highly relevant, as it helps to determine the likelihood of future complications, disease progression, higher consumption of healthcare resources or higher mortality.
In recent years, clinical best practice guides that take into account the need to offer more personalized medicine for patients with COPD recommend identifying risk level as a tool to adapt the levels of both diagnostic and therapeutic intervention.1–3 The correct assessment of COPD exacerbations will be decisive in evaluating the risk,1,2 degree of clinical control in COPD4 and to plan therapeutic interventions.5
Some individuals appear more susceptible to developing exacerbations and are termed frequent exacerbators or COPD exacerbator phenotype.6,7 In these patients, correctly identifying exacerbations will be very important as this group has a higher risk of morbidity and mortality8 since they have a higher risk of hospitalization and are able to benefit from more specific therapies and action plans in order to help prevent exacerbations and improve their quality of life.9 In regular clinical practice, the exacerbator phenotype is based on clinical records and/or patient recall. It is the standard method for assessing exacerbation risk and so determining exacerbation frequency according to the recommendations of the guidelines of good clinical practice. Clinical studies have shown that this diagnosis based on the patient’s statement about the history of exacerbations is reliable.10 Nevertheless, unreported exacerbations are common and important events.11,12 Unreported exacerbations that are not treated by healthcare professionals have been associated with a worsened quality of life12 and increased risk of subsequent hospitalization.13 Therefore, new strategies are needed to help evaluate clinical stability or the level of control of the disease during the medical visit and thus provide a better diagnosis and management of COPD exacerbator phenotype.
Recently, health-related applications running on mobile devices have been developed to optimize manage chronic disease.14 In patients with COPD, such technologies could permit early detection of COPD exacerbations. However, the value of monitoring physiological parameters to predict exacerbations15 and also the effectiveness of mobile health applications compared with usual care are controversial.16
In recent years, a greater insight of exacerbations has emerged, largely from the use of manual or electronic symptom diary cards.17,18 The electronic diaries have shown foster the adoption of patient-reported outcomes and symptom-based measures in clinical studies in COPD.19 However, despite the evidence on the use of electronic symptom diary cards in clinical studies in COPD, the evaluation of mHealth applications in real life as a complementary tool to provide a better diagnosis of exacerbator patients are very few. In patients with COPD suffering frequent exacerbations, mHealth apps could be considered a support tool complement to personal health records to improve management. The monitoring of a patient’s condition and symptoms through mHealth apps may be useful in identifying and correctly assessing COPD exacerbations, reducing the number of unreported exacerbations and facilitating the implementation of personalized therapy and action plans in high-risk patients in regular clinical practice.
This manuscript analyses the frequency and characteristics of exacerbations recorded by the Prevexair application through the daily analysis of symptoms in a population with an exacerbator phenotype, and explores its usefulness in assessing clinical stability with respect to that reported in visits.
Patients and Methods
Study Design and Patient Evaluation
The methodology of the Prevexair study has been extensively described elsewhere.20 This study was a multi-centre prospective cohort with a 6-month follow-up period. Patients were recruited in outpatient respiratory clinics. The inclusion/exclusion criteria described in Table 1. Ethical approval was obtained from the Ethics Committee at the Hospital Clínico San Carlos (Madrid, Spain; internal code 14/124-E), and all patients gave their written informed consent prior to inclusion. This study was conducted in accordance with the Declaration of Helsinki.
 |
Table 1 The Inclusion Criteria and Exclusion Criteria |
Patient assessment included a complete medical history (height, weight, smoking history, drug history, diagnosis of comorbid conditions), spirometry, health-related quality of life using the COPD Assessment Test TM (CAT), dyspnea using the modified Medical Research Council (mMRC) questionnaire, and the number of moderate/severe exacerbations in the last year. Additional information gathered at the scheduled research visits during the follow-up period at 3 months and at 6 months included changes in medication, current smoking habits, CAT and a medical history review regarding healthcare resource utilization. In addition, patients were asked about exacerbations suffered during follow-up. The investigators conducted the visit were blinded and not informed about the Prevexair app records.
Data Collection and Monitoring
The Prevexair app was developed for IOS and Android systems by Virtual Ware http://www.virtualwareco.com/. At recruitment, the Prevexair application was installed on the patients’ smartphones and they were instructed how to use the app. The information recorded in the app included symptom changes, use of medication and use of healthcare resources. The following symptoms were included in the app: dyspnea, sputum colour and amount, wheeze, cough, colds and sore throat. Besides, patients were instructed to use the app to record whether they increased their inhalation medication, started corticosteroids or antibiotics as well as any medical assistance received messages about healthy lifestyle behaviours and disease education, as well as a record of their cumulative symptoms in a graph through the app. No regular contact was established and no mentoring process to increase compliance was implemented. Participants were informed that the data submitted would only be consulted by the research group while their physicians were blinded; they were not informed about the Prevexair app records. Is not established an action plan recommended when worsening of symptoms. Thus, if patients felt ill, they should contact their regular physician for advice as usual and not make decisions based on the information provided during the study. Figure 1 shows several screenshots from the Prevexair app on a smartphone.
 |
Figure 1 Screenshots from the Prevexair app on a smartphone. |
Data Management
Data were collected using two different methods: (1) scheduled visits and (2) daily records in the app.
An exacerbation recorded in the app was defined as an increase in respiratory symptoms for two consecutive days that was recorded in the app, with at least one major symptom (dyspnea, sputum purulence or sputum volume) plus either another major or a minor symptom (wheeze, cold, sore throat, or cough) according to the previously validated criteria.21 This exacerbation definition has been validated against changes in quality of life,22 inflammatory markers21 and FEV1 declive.23 The symptom questions had dichotomous response options, with a positive response indicating that the symptom was worse than at baseline. The patient had to be symptom-free for at least 7 days before defining a new exacerbation.
In order for patients to recall exacerbation events over this period, medical investigators reviewed the medical history and the patients were asked a standardized question at each scheduled visit during follow-up: “How many exacerbations have you had since the last visit? By this, I mean infections, bad chest attacks or worsening of symptoms?” The number of exacerbations and treatments they recalled was recorded. This question avoided asking about treatment and so permitted capture of information on treated and untreated exacerbations. The medical investigators who made the visits during the follow-up were blinded and not informed about the Prevexair app records.
In these analyses, in order to compare the frequency and characteristics of exacerbations recorded via the Prevexair smartphone app and the exacerbations recalled by the patient during the visit, the patients included in the analysis were participants with more than 60% overall compliance and less than 10 days (sequential) of missing records in the app. There were 180 available days for each participant, including the periods of hospitalization. The overall compliance was defined as the percentage of available days that data was recorded in the app.
Exacerbation frequency was determined in the two following ways, depending on the analysis: 1) counting the events recorded in the app or 2) using patients’ estimated number of exacerbations at the visit during the follow-up period.
Exacerbation treatment in the study represents the usual practice of the primary care or respiratory specialist treating the patient. An exacerbation was considered treated if there was a change in at least one medication (ie, antibiotics, corticosteroids and bronchodilators) for the worsened symptoms. The exacerbation was considered mild if it was treated only with increased bronchodilators, moderate if it required outpatient treatment with antibiotics and/or systemic steroids and severe exacerbation if it required hospitalisation or an emergency room visit for more than 24 hours.
Patients were defined as unstable if they had one or more exacerbations treated with oral corticosteroids and/or antibiotics during the six months of the study.
Statistical Analysis
Sample size was calculated on the estimates of the number of exacerbation events recorded on diary cards and the number of events patient recall in the London cohort.10 Given the exploratory nature of our study, we recruited patients with frequent exacerbations and we followed them for 6 months; Accepting an α error of 0.05 and a ß error of 0.1, we estimated that we needed to recruit a minimum of 65 patients including an expected drop-out rate of 15%.
Qualitative variables were summarized by their frequency distribution and quantitative variables by their mean and standard deviation (± SD). Continuous non-normally distributed variables were summarized by the median and interquartile range (IQR: P25-P75). The evaluation of normality was performed by graphical inspection of the histogram, box plot and normal Q-Q plots of each quantitative variable.
Comparison of the number of exacerbations (mild, moderate and severe) recorded by the app and estimated by the patient during the scheduled visits was done using the non-parametric Wilcoxon test, and the correlation analysis between these two variables was done using Spearman’s Rank (rho) non-parametric correlation coefficient. For the agreement analysis between information provided by the app and the visits regarding the number of exacerbations, overall agreement and the quadratic weighted kappa statistic were used.
The comparisons for the characteristics of stable and unstable exacerbators were made using the chi-square test for qualitative variables and the Student’s t-test for quantitative variables.
All analyses were performed using STATA 15.0 software. Statistical significance was assumed as p < 0.05.
Results
Patient Characteristics
A total of one hundred and twenty-six (126) patients were recruited. Of these, 10 patients were excluded for having had one exacerbation at the time of enrolment. Of this cohort, only 69 patients with more than 60% overall compliance were included in this analysis. The baseline characteristics of the analyzed cohort (69 patients) are reported in Table 2. The mean (SD) FEV1 was 1.21 (0.51) L, and the percentage predicted FEV1 was 43.5 (15.7) %. 59.4% of participants had experienced at least one severe exacerbation in the last year and 73.9% had a degree of dyspnea ≥2 mMRC.
 |
Table 2 Baseline Characteristics of Analyzed Population |
Exacerbations
Exacerbations Detected Through App Records
During the study, the patients experienced a total of 185 cases of symptom worsening satisfying all conditions for an exacerbation according to daily records in the app. Of the 69 patients analyzed, only six (8.7%) had no exacerbations. The overall estimated rate of exacerbations recorded in the app was median 3.0 (IQR 1.0–4.0) and mean 2.68 (1.75) per patient in six months. Of this total, 120 (64.8%) were treated and 65 (35.1%) were not treated. Of the 120 treated exacerbations, 35% were treated with only an increase in bronchodilators and 36% only with antibiotics (Figure 2).
 |
Figure 2 Exacerbation frequency and characteristics as recorded via the Prevexair app or according to visit records. |
With regard to reported healthcare utilization for exacerbations, 8.8% of exacerbations resulted in hospitalization or urgent attention for more than 24 hours, 63.3% led to an unscheduled doctor visit and 27.8% were self-treated by the patient and no contact was made with the doctor, illustrated in Figure 3.
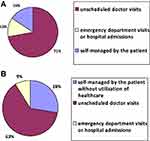 |
Figure 3 Distribution of exacerbations according to the healthcare utilization for their treatment detected in the app (A) or recorded in visit (B). |
Exacerbations Recalled by the Patient During the Visit
A total of 64 exacerbations were recalled during medical visits in the six months of follow-up. The overall estimated rate of exacerbations recorded during visits was median 1.0 (IQR 0.0–1.0) and mean 0.93 (0.94). Concerning treatment, all exacerbations received medical attention and were treated. 65.6% were treated with antibiotics and/or oral corticosteroids (Figure 2). With regard to healthcare utilization for exacerbations, 15.6% were self-treated by the patient and no contact was made with the doctor, 71.9% led to an unscheduled doctor visit and 12.5% required hospitalization or urgent attention more than 24 hours as shown in Figure 3.
Comparison of Patients’ Estimated Number of Exacerbations During the Visit and the Number of Exacerbation Events Recorded in the App
A comparison of these exacerbation numbers according to severity is shown in Table 3. There was no significant difference between the number of severe exacerbations recorded in the app and patient estimates for their number of severe exacerbations (median: 0.0 [IQR 0.0–0.0] for both groups; p=0.257; mean ± SD: 0.15±0.40 and 0.12±0.36, respectively). This correlation was significantly strong for severe exacerbations (rho 0.558; p<0.01). If patient estimates for the number of severe exacerbations and the genuine number of exacerbations over this same period were random, they agreed by 89.8% (kappa 0.6577; p<0.001). There was a significant difference between the number of exacerbations treated with systemic antibiotics and/or corticosteroids recorded in the app and patient estimates over the same period (medians: 1.0 [IQR 0.0–2.0] and 0.0 [IQR 0.0–1.0], respectively; mean ± SD: 1.11±0.91 and 0.60±0.75, respectively; p<0.001). For treated exacerbations, there was only a moderate correlation for exacerbations treated with systemic antibiotics and/or corticosteroids (rho 0.449; p<0.01), with overall agreement being 46.3% (kappa 0.3908; p<0.001). In mild exacerbations, there was a significant difference between the number of mild exacerbations recorded in the app and patient estimates over the same period (medians: 0.0 [IQR 0.0–1.0] and 0.0 [IQR 0.0–0.0], respectively; mean ± SD: 0.62±1.01 and 0.32±0.65, respectively; p<0.01). There was a weak correlation (rho 0.257; p<0.05), with 17.3% overall agreement (kappa 0.2424; p<0.001).
 |
Table 3 Relationship Between Exacerbation Frequency Determined Using App Records and Patient Recall |
Assessment of the Stability of COPD Using Patient Recall During Visit or App Records
The proportion of patients classified as unstable increased significantly from 47.8% to 72.5% when classified according to the number of exacerbations recorded in the app.
Characteristics of stable and unstable patients according to classification by app records or recalled by a patient during visit are shown in Table 4. Unstable patients had a worse quality of life and a higher degree of dyspnea, whether they were identified by the records in the app or by what the patient referred to in the visit.
 |
Table 4 Characteristics of Stable and Unstable Exacerbator Patients by App Records or Recalled by Patient During Visit |
There were no factors (age, sex, smoking history, FEV1, dyspnea scale, comorbid conditions or CAT score) that were predictive of an individual having poor recall of their number of exacerbations (p>0.05 for all).
Discussion
This study provides prospective information on the usefulness of a mobile phone application as a tool to assist during clinical visits to identify exacerbations of COPD. The results show that the application allowed us to record a large number of exacerbations, which were not referred to during the medical visit. In our study, the proportion of patients classified as unstable for had one or more exacerbations treated with oral corticosteroids and/or antibiotics increased significantly if the exacerbations captured by Prevexair were used to define stability than if patient recall was used. In our analysis the unstable patient has a worse quality and greater dyspnea whether it was defined according to the records of the app or recalled by patient during visit. The results show that two-thirds of the exacerbations recorded in the Prevexair application were not reported to doctors during their clinical visits. Almost half were treated with oral corticosteroids and/or antibiotics and more than one-quarter of the exacerbations treated did not seek medical attention. COPD is a disease with high prevalence, characterized by frequent decompensations associated with significant morbidity and mortality.24 There is ample evidence that frequent exacerbations have important consequences for patients due to their negative impact on the quality of life,25 greater disease progression26 and decreased survival.8 As a result, current clinical best practice guides recommend exacerbation evaluation as a key step in evaluating the prognosis and planning therapeutic interventions.1–3
Patients with the exacerbator phenotype are a group with an elevated risk of hospitalization8 and consequently a high risk of hospital readmission, with reported rates ranging from 10% to 20% after 30 days.26 In this higher-risk population, monitoring through mHealth apps may be useful in correctly assessing COPD exacerbations and health status, facilitating more information during follow-up. The monitoring of a patient’s condition and symptoms through Prevexair application has shown to capture changes in the clinical status of the patients not referred to in the visit that could be indicative of future risks and that may have prognostic implications. However, further research must be carried out to evaluate the value of this strategy for the management of COPD in clinical practice.
The Global Initiative for Chronic Obstructive Lung Disease defines an exacerbation as “an acute worsening of respiratory symptoms that results in additional therapy”. Therefore, it is valuable to include information gathered directly from patients in the detection and quantification of exacerbations.27 The EMA 2012 guideline on clinical investigation of medical products in the treatment of COPD specifically recommends the use of questionnaires or diary cards to enable the capture of both reported and unreported exacerbations and to evaluate symptoms in order to provide patient-relevant outcomes.28 Thus, instruments to monitor exacerbations and symptoms in COPD have been developed in recent years on clinical investigation. An important instrument used for exacerbation recognition is the Exacerbations of Chronic Obstructive Pulmonary Disease Tool (EXACT).29 It captures the frequency, severity, and duration of exacerbations in clinical trials of COPD.30 One of the strengths is its ability to recognize unreported events when patients experience a deterioration in their symptoms but do not seek or receive additional therapy. Studies using the EXACT have confirmed the high prevalence and clinical significance of these events.31
In the last few years symptom diary cards have been transitioning from paper-based methods to electronic approaches for its advantages to transmission of data to research and healthcare and incorporate alerts to overcome missing diary transmissions. Recently, mobile health (mHealth)—defined as the use of mobile and wireless technologies for healthcare32 —has become a new treatment approach which can empower self-management and enhance proactive clinical interventions.33.Studies exploring patient experience and acceptability of apps have shown promise,34 suggesting that such technology may be able to complement current clinical care. However, the evidence base to support this approach is insufficient. Several systematic reviews have showed that mobile device applications are effective for the self-management of COPD over usual care although there is insufficient evidence to date due to marked variation in methodology and reporting of outcome measures.15,16 Therefore, further clinical validation remains essential to use mHealth in improving COPD care.
Prevexair is a simple smartphone application in which the patient records their daily symptoms and offers general recommendations in an open observational study. The level of satisfaction with the functionality of the Prevexair app and daily use was high although no strategy was implemented to continue using the app.20 In our study, there was not a process of tutoring or phone contacts between the research team and the patient, and no strategy was implemented to continue using the app. The patient did not make decisions based on information provided by the app during the study. The use of diaries to capture the reality of subjects´ lives in chronic disease is quite problematic. Patient participation in a controlled trial has achieved near-perfect compliance of around 90%. However, in other observational studies in COPD, only 40% of participants achieved 70% compliance,35 or overall compliance of 53% over 12 months with mentoring process, with regular phone calls from the assigned community nurse.36 Simplicity and motivation seem to be the key factors for accepting and using mobile phone apps. It is important to be able to personalize the app to the patient’s needs and to offer specific messages and personalized self-management plans. The participants will tolerate the burden of diary-keeping if they feel it will help them.37
The results of our study show that mHealth also has the potential to allow health professionals to monitor patients and define current health status with more information during follow-up visits. A fact that was noted in our study, where two-thirds of the exacerbations recorded in the Prevexair application were not reported to doctors during their clinical visits. Previous studies have found that COPD patients do not report all their exacerbations to health professionals.11,38,39 Failure to seek medical attention has consequences, such as a worsening of quality of life12 and an increased risk of subsequent hospitalisation.13 In our study, a large majority of exacerbations not recalled during clinical visits were exacerbations that were self-treated by the patient without using healthcare resources. Therefore, it appears that unrecalled exacerbations may be considered by patients as unimportant events, even if treated. This may be more relevant in patients who frequently exacerbate, as they may be more likely to receive emergency treatment (antibiotics and steroids) at home and to have a perception that the episode is self-limiting. Patients have different thresholds for initiating treatment and often depend on their knowledge and experience of previous episodes.39 These results would support the use of mHealth in the follow-up of COPD especially in exacerbation patients, taking into account that despite being highly symptomatic, patients may often fail to recognize small daily variations in their respiratory symptoms.
Another remarkable result was a low concordance between what was recorded in the application and what was remembered in the medical visit in relation to the moderate exacerbations that required treatment. This is a relevant result if we consider that the current recommendation for risk assessment is based on the exacerbations reported by the patient and/or collected from the clinical history.6,10 In this sense, previous studies have also found that frequent exacerbators underestimate the number of exacerbations,10,39 which could be related to being more symptomatic patients and the possibility of several exacerbations overlapping into one, which would favour recall of fewer exacerbations.38 In relation to this determinant of perception, it should be mentioned that the patients who participated in our study were patients with frequent decompensations of their disease and more than half of the patients evaluated had a history of hospitalization for COPD in the previous year. Other sociodemographic characteristics (age, level of education), psychic (anxiety, depression, or cognitive disorder) play a large role in the likelihood recognize or recall symptoms and to seek help.40
Correct assessment of exacerbations of COPD is a critical step in assessing the clinical management of the disease, and in turn, in establishing therapeutic decisions. In our study, the proportion of patients classified as unstable increased significantly when classified by the number of exacerbations recorded on the application. Patients who were not stable whether they were identified by the records in the app or by what the patient referred to in the visit showed a worsening of the degree of dyspnea and a greater loss of quality of life. These results support the use of mobile technologies as an aid in the monitoring of COPD on frequent exacerbators, as more complete and comprehensive information on decompensations and the level of disease control is available so that therapeutic interventions can be better planned.
Several potential limitations should be considered in the interpretation of results. This is a pilot study examining a relatively small prospective group, 69 patients monitored for only 6 months with good compliance in daily use of the application, taking into account that the main objective was to assess the usefulness of detecting exacerbations through daily symptom recording by application and to compare their frequency with that reported during visits. In this sense, we should mention that, although the level of daily use of the Prevexair application limited in this analysis the sample size of the population evaluated, the daily use of the Prevexair application was higher than that reported by previous open observational studies that have evaluated the level of compliance with the daily symptom register, and that have shown compliance of around 50%35,36 with telephone contacts and different strategies to maintain the daily use of the application, unlike our study, where the patient does not maintain any back-up contact with the research team during the 6 months. It should also be mentioned that in the analysis of factors related to the level of compliance with the Prevexair application, it does not seem to be affected by demographic factors or the severity of the disease.20 However, we probably included the most motivated patients in terms of a selection bias that limits the generality of the results of our study. The fact that, by inclusion criteria, the sample analysed was patients with exacerbating phenotype, with a higher risk of exacerbations during follow-up, may have influenced the level of daily use. Other limitation, subjects would have recalled previous exacerbations better if they had been asked about increased treatment or visits to emergency departments. However, this question deliberately avoided asking about treatment and so permitted capture of information on treated and untreated exacerbations. This standard question was the same one used in London COPD study to assess predictive accuracy of patient-reported exacerbation frequency in COPD.10 However, one of the strengths of this study is that it evaluates the usefulness of an application for detecting exacerbations not reported during the visit in a population at high risk of suffering decompensation, which is attributed to greater consumption of health resources and higher morbidity and mortality. Also, our study capture the reality of subjects´ lives as there was not a process of tutoring or phone contacts between the research team and the patient, and no strategy was implemented to continue using the app. These results give us confidence and encourage us to assess their long-term role and open up new possibilities for developing applications in response to the requirements and needs of certain groups of COPD patients.
Conclusions
The use of the Prevexair smartphone application in the follow-up of COPD patients with frequent decompensations helps to detect exacerbations not referred at the doctor’s visit. In the near future, mHealth apps will be a natural complement to health telematics and personal health records. However, further research must be carried out to evaluate this strategy for the management of COPD in clinical practice.
Summary
What was already known about the topic
- The level of risk in COPD to predict exacerbations in the next year is based on clinical records and/or patient recall.
- Unreported exacerbations are common and important events.
What this study added to our knowledge
- Two-thirds of the exacerbations recorded in the Prevexair app were not reported to healthcare professionals.
- Perception is less accurate in frequent exacerbator patients, there is low concordance between treated exacerbations recorded in the app and exacerbations recalled by patients at a clinical visit.
Abbreviations
COPD, chronic obstructive pulmonary disease; AECOPD, acute exacerbations of COPD; CATTM, COPD Assessment Test; mMRC, modified Medical Research Council; BMI, body mass index; mHealth apps, health-related mobile applications; IQR, interquartile range; LAMA, long-acting antimuscarinic agents; LABA, long-acting beta-2 agonists; ICS, inhaled corticosteroids.
Acknowledgments
The authors thank the investigators Francisco Javier Agustín Martínez, Pulmonology Department, Complejo Hospitalario U. de Albacete and Walther Ivan Giron Matute, Pulmonology Department, Hospital U. Gregorio Marañón Madrid, Spain, that participated in the PREVEXAIR study. We thank AstraZeneca for its financial support to carry out the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published.
Funding
This study has been promoted and sponsored by the Spanish Society of Pneumology and Thoracic Surgery (SEPAR). We thank AstraZeneca for its financial support with a donation to carry out the study. The financers had no role in study design, data collection, analysis, decision to publish or in the preparation of this manuscript. This does not alter our adherence to the Journal of Medical Internet Research policies on sharing data and materials.
Disclosure
Juan Luis Rodríguez Hermosa report personal fees from Gebro and Boehringer Ingelheim, outside the submitted work; and has received speaking fees from Boehringer Ingelheim and Gebro Pharma: this does not have a real or perceived conflict of interest between all these sources and the present paper.
Francisco Javier Callejas González reports has received lecture fees, consultancy fees and/or support for conference attendance from various pharmaceutical companies, such as GlaxoSmithKline, Chiesi, Boehringer Ingelheim, Mundipharma, Menarini, Pfizer, Novartis, Laboratorios Esteve, Teva Pharmaceutical, Ferrer, Rovi, Roche, Astra Zeneca, Bial, Actelion, Grifols, CSL Behring, Faes Farma and Gebro Pharma, outside the submitted work; and has received speaking fees from GlaxoSmithKline, Chiesi, Boehringer Ingelheim, Mundipharma, Menarini, Pfizer, Novartis, Esteve, Teva Pharmaceutical, Ferrer, Rovi, Astra Zeneca, Bial, Actelion and Gebro Pharm: this does not have a real or perceived conflict of interest between all these sources and the present paper.
Myriam Calle Rubio reports personal fees from Boehringer Ingelheim, Gebro, AstraZeneca, GlaxoSmithKline, Menarini, from Novartis, outside the submitted work, and has received speaking fees from Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, Menarini, and Novartis and consulting fees from GlaxoSmithKline, Gebro Pharma and Novartis: This does not have a real or perceived conflict of interest between all these sources and the present paper.
Luis Puente Maestu has received travel coverings, funds for educative activities, research grants, paid advisories and participated as site PI in private companies sponsored RCT or observational studies from Air Liquide, Astra-Zeneca, Boston Scientific, Boehringer Ingelheim, Chiesi, ESTEVE, GSK Menarini, MSD, Novartis, Sanofi, Spanish Scientific Societies and Government and the European Regional Cooperation Fund. This does not have a real or perceived conflict of interest between all these sources and the present paper.
Carlos Antonio Amado Diago has received speaking fees from Menarini, GSK, Novartis, Chiesi, Teva, Ferrer. This does not have a real or perceived conflict of interest between all these sources and the present paper.
The authors report no other potential conflicts of interest for this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of COPD. Available from: Http://www.goldcopd.com.
2. Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Guía española de la enfermedad pulmonar obstructiva crónica (GesEPOC) 2017. Tratamiento farmacológico en fase estable. Arch Bronconeumol. 2017;53:324–335. doi:10.1016/j.arbres.2017.03.018
3. Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49:1600791. doi:10.1183/13993003.00791-2016
4. Miravitlles M, Sliwinski P, Rhee CK, et al. Evaluation of criteria for clinical control in a prospective, international, multicenter study of patients with COPD. Respir Med. 2018;136:8–14. doi:10.1016/j.rmed.2018.01.019
5. Calle Rubio M, Rodríguez Hermosa JL, Soler-Cataluña JJ, et al. Medical care according to risk level and adaptation to Spanish COPD Guidelines (Gesepoc): the Epoconsul Study. Arch Bronconeumol. 2018;54:270–279. doi:10.1016/j.arbres.2017.11.015
6. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi:10.1056/NEJMoa0909883
7. Miravitlles M, Calle M, Soler-Cataluña JJ. Fenotipos clínicos de EPOC: identificación, definición e implicaciones para las guías de tratamiento. Arch Bronconeumol. 2012;48:86–98. doi:10.1016/j.arbres.2011.10.007
8. Esteban C, Castro-Acosta A, Alvarez-Martínez CJ, Capelastegui A, López-Campos JL, Pozo-Rodriguez F. Predictors of one-year mortality after hospitalization for an exacerbation of COPD. BMC Pulm Med. 2018;18:18. doi:10.1186/s12890-018-0574-z.
9. Yang F, Wang Y, Yang C, Hu H, Xiong Z. Mobile health applications in self-management of patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis of their efficacy. BMC Pulm Med. 2018;18:147. doi:10.1186/s12890-018-0671-z
10. Quint JK, Donaldson GC, Hurst JR, Goldring JJP, Seemungal TR, Wedzicha JA. Predictive accuracy of patient-reported exacerbation frequency in COPD. Eur Respir J. 2011;37(3):501–507. doi:10.1183/09031936.00035909
11. Langsetmo L, Platt RW, Ernst P, Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med. 2008;177(4):396–401. doi:10.1164/rccm.200708-1290OC
12. Boer LM, Bischoff EW, Borgijink X, et al. ‘Exacerbation-free time’ to assess the impact of exacerbations in patients with chronic obstructive pulmonary disease (COPD): a prospective observational study. NPJ Prim Care Respir Med. 2018;28(1):12. doi:10.1038/s41533-018-0079-5
13. Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169:1298–1303. doi:10.1164/rccm.200310-1443OC
14. Elbert NJ, van Os-medendorp H, van Renselaar W, et al. Effectiveness and cost-effectiveness of ehealth interventions in somatic diseases: a systematic review of systematic reviews and meta-analyses. J Med Internet Res. 2014;16:e110. doi:10.2196/jmir.2790.
15. Ahmed M, Hurst JR. Monitoring of physiological parameters to predict exacerbations of chronic obstructive pulmonary disease (COPD): a systematic review. J Clin Med. 2016;5:108. doi:10.3390/jcm5120108
16. Shaw G, Whelan ME, Armitage LC, et al. Are COPD self-management mobile applications effective? A systematic review and meta-analysis. Primary Care Respiratory Medicine. 2020;30:11. doi:10.1038/s41533-020-0167-1
17. Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, doubleblind, parallel-group study. Lancet Respir Med. 2013;1:199–209. doi:10.1016/S2213-2600(13)70052-3
18. Wedzicha JA, Banerji D, Chapman KR, et al. FLAME Investigators. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374:2222–2234. doi:10.1056/NEJMoa1516385
19. Vestbo J, Woodcock A. Clinical trial research in focus: time to reflect on the design of exacerbation trials in COPD. Lancet Respir Med. 2017;5:466–468. doi:10.1016/S2213-2600(17)30177-7
20. Rodriguez Hermosa JL, Fuster Gomila A, Puente Maestu L, et al. Compliance and utility of a smartphone app for the detection of exacerbations in patients with chronic obstructive pulmonary disease: cohort study. JMIR Mhealth Uhealth. 2020;8(3):e15699. doi:10.2196/15699
21. Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55:114–120. doi:10.1136/thorax.55.2.114
22. Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi:10.1164/ajrccm.157.5.9709032
23. Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi:10.1136/thorax.57.10.847
24. Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc. 2014;11:404–406. doi:10.1513/AnnalsATS.201311-405PS
25. Rubinsztajn R, Przybyłowski T, Maskey-Warzechowska M, Karwat K, Chazan R. Exacerbations of Chronic Obstructive Pulmonary Disease and quality of life of patients. Adv Exp Med Biol. 2016;884:69–74. doi:10.1007/5584_2015_178
26. Seemungal TA, Wedzicha JA. Exacerbation frequency and FEV1 decline of COPD: is it geographic? Eur Respir J. 2014;43:1220–1222. doi:10.1183/09031936.00046014
27. Harries TH, Thornton H, Crichton S, Schofield P, Gilkes A, White PT. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27:31. doi:10.1038/s41533-017-0028-8
28. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report: GOLD executive summary. Arch Bronconeumol. 2017;53:128–149. doi:10.1016/j.arbres.2017.02.001
29. European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment of chronic obstructive pulmonary disease (COPD). 21 June 2012. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-chronic-obstructive-pulmonary-disease_en.pdf.
30. Wedzicha JA, Singh D, Vestbo J, et al. FORWARD Investigators. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respir Med. 2014;108:1153–1162. doi:10.1016/j.rmed.2014.05.013
31. Leidy NK, Wilcox TK, Jones PW, Roberts L, Powers JH, Sethi S, EXACT-PRO Study Group. Standardizing measurement of chronic obstructive pulmonary disease exacerbations: reliability and validity of a patient-reported diary. Am J Respir Crit Care Med. 2011;183:323–329. doi:10.1164/rccm.201005-0762OC.
32. Jones PW, Lamarca R, Chuecos F, et al. Characterisation and impact of reported and unreported exacerbations: results from ATTAIN. Eur Respir J. 2014;44:1156–1165. doi:10.1183/09031936.00038814
33. Agarwal S, LeFevre AE, Lee J, et al. WHO mHealth Technical Evidence Review Group. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ. 2016;352:i1174. doi:10.1136/bmj.i1174
34. World Health Organisation. MHealth: new horizons for health through mobile technologies. 2011. doi:10.4258/hir.2012.18.3.231
35. Williams V, Price J, Hardinge M, Tarassenko L, Farmer A. Using a mobile health application to support self-management in COPD: a qualitative study. Br J Gen Pract. 2014;64:e392–400. doi:10.3399/bjgp14X680473
36. O´Reilly JF, Williams AE, Holt K, Defining RL. COPD exacerbations: impact on estimation of incidence and burden in primary care. Prim Care Respir J. 2006;15:346–353. doi:10.1016/j.pcrj.2006.08.009
37. Walters EH, Walters J, Wills KE, Robinson A, Wood-Baker R. Clinical diaries in COPD: compliance and utility in predicting acute exacerbations. Int J Chron Obstruct Pulmon Dis. 2012;7:427–435. doi:10.2147/COPD.S32222
38. Johnston NW, Lambert K, Hussack P, et al. Detection of COPD exacerbations and compliance with patient-reported daily symptom diaries using a smart phone-based information system. Chest. 2013;144:507–514. doi:10.1378/chest.12-2308
39. Mackay AJ, Donaldson GC, Patel AR, Singh R, Kowlessar B, Wedzicha JA. Detection and severity grading of COPD exacerbations using the exacerbations of chronic pulmonary disease tool (EXACT). Eur Respir J. 2014;43:735–744. doi:10.1183/09031936.00110913
40. Scioscia G, Blanco I, Arismendi E, et al. Different dyspnoea perception in COPD patients with frequent and infrequent exacerbations. Thorax. 2017;72:117–121. doi:10.1136/thoraxjnl-2016-208332
41. Iyer AS, Bhatt SP, Garner JJ, et al. Depression is associated with readmission for acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(2):197–203. doi:10.1513/AnnalsATS.201507-439OC
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
