Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 15
Assessing the Burden of Osteoarthritis in Africa and the Middle East: A Rapid Evidence Assessment
Authors Al Saleh J , Almoallim H , Elzorkany B , Al Belooshi A, Batouk O , Fathy M, Vainstein N, Kaki AM
Received 28 September 2022
Accepted for publication 28 February 2023
Published 15 March 2023 Volume 2023:15 Pages 23—32
DOI https://doi.org/10.2147/OARRR.S390778
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Jamal Al Saleh,1 Hani Almoallim,2 Bassel Elzorkany,3 Ali Al Belooshi,4 Omar Batouk,5 Mohamed Fathy,6 Nora Vainstein,7 Abdullah M Kaki8
1Department of Rheumatology, Dubai Hospital, Dubai Health Authority, Dubai, United Arab Emirates; 2Department of Medicine, Medical College, Umm Al-Qura University, Makkah, Saudi Arabia; 3Rheumatology Department, Cairo University, Cairo, Egypt; 4Mediclinic City Hospital, Department of Surgery, UAE University, Dubai, United Arab Emirates; 5King Saud bin Abdulaziz University for Health Sciences, National Guard Health Affairs, Jeddah, Saudi Arabia; 6Pfizer, Dubai, United Arab Emirates; 7Pfizer, Buenos Aires, Argentina; 8Department of Anesthesia and Critical Care, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
Correspondence: Abdullah M Kaki, MD, FRCPC, Professor of Anesthesiology and Pain Medicine, Faculty of Medicine, King Abdulaziz University, PO Box 2907, Jeddah, 21461, Saudi Arabia, Tel/Fax +966126408335, Email [email protected]; [email protected]
Introduction/Objectives: This rapid evidence assessment (REA) was conducted to assess the burden of weight-bearing joint osteoarthritis in the developing countries of Africa and the Middle East.
Methods: Our REA methodology used a standardized search strategy to identify observational studies, published between January 1, 2010, and April 23, 2020, reporting on outcomes pertaining to the epidemiology and humanistic or economic burden of weight-bearing osteoarthritis. Relevant data from the included studies were used for qualitative analysis.
Results: Among the 20 publications reporting on knee osteoarthritis in 10 countries in Africa and the Middle East, 2 also reported on hip, and 1 on foot osteoarthritis. Prevalence of symptomatic/radiographic knee OA was 9– 14% among rheumatology outpatients and 31– 34% among those with mixed etiology osteoarthritis. Prevalence of knee OA diagnosed by magnetic resonance imaging was 70% among patients ≥ 40 years of age attending a hospital in Saudi Arabia. Quality-of-life outcomes were reported in 16 publications and suggested a substantial humanistic burden of osteoarthritis, including worse pain, function, and quality of life, and more depression; comparisons between studies were hampered by the variety of tools and scoring scales used, however. No studies reported on economic outcomes.
Conclusion: This REA indicates a substantial burden of osteoarthritis in weight-bearing joints in Africa and the Middle East, consistent with publications from other regions of the world.
Keywords: Africa, burden of disease, Middle East, osteoarthritis, quality of life
Introduction
The weight-bearing joints of the knee and hip are commonly affected by degenerative changes leading to osteoarthritis (OA). The pathophysiologic processes involved in OA affect the whole joint and include synovial inflammation, degradation of cartilage, remodeling of bone, and formation of osteophytes; this results in symptoms of pain, stiffness, and impaired joint function.1,2 Additionally, newer evidence suggests association of OA and low-grade intestinal inflammation. The studies also elude that alteration of the gut microbiota and impairment of the epithelial barrier can be related to mechanisms responsible for OA.3 Patients with OA therefore often have reduced health-related quality of life, and chronic OA may lead to long-term disability and the need for joint replacement.1 The 2019 American College of Rheumatology (ACR) Guidelines for the management of OA have made strong recommendations for including following non-pharmacological approaches: exercise, weight management, tai chi, cane use, self-efficacy and self-management programs, hand orthoses for first carpometacarpal (CMC) joint OA, tibiofemoral bracing for tibiofemoral knee OA. The guidelines recommend use of topical nonsteroidal anti-inflammatory drugs (NSAIDs) for knee OA, oral NSAIDs, and intraarticular glucocorticoid injections (knee OA) as a part of pharmacological approach.2 Additional studies have supported use of manual therapy for symptomatic improvement of pain and for reducing the temporal summation in older adults.4 A randomized controlled study by Romero et al explored use of dry needling in conjunction with exercise to reduce pain associated with knee OA, and further studies to assess its clinical effectiveness can be devised.5 Patient education helps in managing pain and improving functionality in elderly OA patients.6
The incidence and prevalence of radiographic and symptomatic OA typically increase with increasing age.1,7 In general, women have a higher incidence of OA than men, especially after menopause.1 In postmenopausal women with OA, strengthening exercises have been proven to result in favorable clinical outcomes and improve quality of life.8 The Global Burden of Disease 2017 study described hip and knee OA contributions to global disability and the overall increasing burden of OA.9 To date, there has been limited evidence regarding the burden of OA in developing countries. A rapid evidence assessment (REA) was conducted to investigate the burden of OA in weight-bearing joints, including the epidemiology and the humanistic and economic burdens of OA, in 3 geographic areas: Africa and the Middle East, Asia, and Latin America. This article presents the results from Africa and the Middle East.
Methods
The current REA used a standardized methodology to identify observational (non-interventional) studies reporting outcomes pertaining to the epidemiology and humanistic or economic burden of weight-bearing OA. This has been described previously.10 The protocol was prospectively registered with PROSPERO, an international database of systematic reviews in health and social care, welfare, public health, education, crime, justice, and international development: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=180225. Searches were conducted on April 23, 2020, in MEDLINE® via Ovid®, Embase® via Ovid®, and PubMed, for publications dated from January 1, 2010, onwards. In addition, hand-screening of reference lists for relevant reviews was also done to identify publications missed in the database searches. This approach was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol (PRISMA-P) guidelines.11 Prespecified inclusion/exclusion criteria were based on the PICOS (population, intervention, comparator, outcome, study type) framework and were used to design the search strategies (Online Resource 1).
Following an initial screening by a single reviewer of publication titles and abstracts for inclusion and exclusion criteria, a quality check of 10% of the screened publications was performed by a second reviewer. This was followed by another round of screening by the first reviewer of the full text of the selected publications, and then a further quality check by the second reviewer of 10% of the selected items. The relevant data from the included studies were used to populate bespoke data extraction tables for qualitative analysis. The modified Newcastle-Ottawa Scale for observational cross-sectional studies was used to systematically assess the included studies for risk of bias. Studies were first categorized by geographic region (Africa and the Middle East, Asia, and Latin America) and were secondarily categorized by outcomes reported (epidemiology, quality of life, economic), joint location (hip, knee), diagnosis (symptomatic, radiographic, or symptomatic/radiographic [using X-ray imaging in combination with either clinical criteria or self-reported pain]), population source, and severity.
Results
Database searches initially identified 10,245 publications; after removal of duplicates, 5854 publications were screened by titles/abstracts and 5267 were excluded (Figure 1). Subsequently, 587 of these publications were screened by full text, 468 were excluded, and 1 additional publication identified through hand-screening was added; this yielded a total of 120 included publications from all 3 geographic areas (Figure 1). Of these, 20 publications reported studies from 10 countries in Africa and the Middle East (Figure 2).12–31
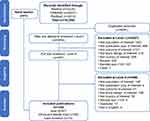 |
Figure 1 Search and screening flow chart. Note: Reproduced from Ciampi de Andrade D, Saaibi D, Sarria N, Vainstein N, Cano RL, Espinosa R. Assessing the burden of osteoarthritis in Latin America: a rapid evidence assessment. Clin Rheumatology. 2021;41(5):1285–1292. Creative Commons.10 |
 |
Figure 2 Countries included in 20 publications. Note: Adapted from Ciampi de Andrade D, Saaibi D, Sarria N, Vainstein N, Cano RL, Espinosa R. Assessing the burden of osteoarthritis in Latin America: a rapid evidence assessment. Clin Rheumatology. 2021;41(5):1285–1292. Creative Commons.10 |
Categorizing the studies by outcomes, 7 reported on epidemiology15–17,19,24,25,28 (prevalence data only) and 16 reported on humanistic burden.12–14,18–23,25–31 No studies reported on economic burden/outcomes, and no studies were identified that reported incidence, survival/death rates, or all-cause mortality rates. All 20 publications were rated as satisfactory or higher quality for risk of bias (Online Resource 2) and all reported knee OA. Two (10%) studies also included hip OA17,31 and 1 (5%) publication included foot OA.17
The prevalence of physician-diagnosed symptomatic knee OA was reported in 3 publications (Table 1).15,24,28 Among men and women ≥18 years of age attending primary health care clinics, the overall prevalence was 11.5% in a study from Nigeria; it was also over 2-fold higher in women than men.28 In a study of patients (77% were women) attending primary health care clinics in Dubai, the prevalence was 25.8%.15 Among the general population of 35–70-year-olds in Iran, 1 publication reported that the prevalence of symptomatic knee OA was 47.7%.24 The same publication reported that prevalence was generally higher in women than men and was highest among those aged ≥60 years.24
 |
Table 1 Overall Prevalence of Physician-Diagnosed Symptomatic Knee OA in 3 Publications |
The prevalence of symptomatic/radiographic knee OA among those with either musculoskeletal complaints or OA of mixed etiology was reported in 3 publications (Table 2).17,19,25 None of these publications reported the prevalence of symptomatic/radiographic knee OA within the general population. Among rheumatology outpatients ≥40 years of age in Cameroon, prevalence of grade ≥2 symptomatic/radiographic knee OA was 9%.19 In a study from the Democratic Republic of Congo, the total prevalence of symptomatic/radiographic knee OA in patients ≥30 years of age was 14.1% in rheumatology outpatients and 31.4% in a subgroup with OA of mixed etiology. Within this symptomatic/radiographic knee OA population, 94% had grade ≥2 OA and more than 70% had severe, grade 3/4, OA.25 Asokan et al reported a 33.9% prevalence of symptomatic/radiographic knee OA among a population of female patients ≥40 years of age with mixed etiology OA in Bahrain.17
 |
Table 2 Prevalence of Symptomatic/Radiographic Knee OA Within a Population of Patients with OA (Mixed Etiology) or Musculoskeletal Complaints in 3 Publications |
The prevalence of knee OA as diagnosed by magnetic resonance imaging (MRI) was reported in a sample of 137 randomly selected patients and their relatives attending a hospital in Saudi Arabia.16 Among those ≥40 years of age, the total prevalence was 70.0% and was greater in women (90.4%) than in men (54.1%). This was higher than the prevalence of symptomatic (11.9%) and symptomatic/radiographic (14.1%) knee OA in similarly aged populations in Iran24 and the Democratic Republic of Congo,25 respectively. In the Bahrain study, cross-sectional analysis of data from 398 women ≥40 years of age with OA of mixed etiology found that the prevalence of symptomatic/radiographic OA was 1.8% in the hip, 37.9% in the hip/knee, and 0.5% in the foot.17
Quality of life outcomes, reflecting the humanistic burden of OA, were reported in 16 publications.12–14,18–23,25–31 All reported on patients with knee OA and 1 publication also included patients with hip OA.31 Five publications12–14,28,29 used the OA-specific tool, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC;© 1996 Nicholas Bellamy. WOMAC® is a registered trademark of Nicholas Bellamy (CDN, EU, USA)), for their evaluations, some versions of which use a visual analog pain scale. Ogunbode et al reported that WOMAC scores among 46 men and women ≥18 years of age with symptomatic knee OA were significantly higher/worse (p<0.0001) than those among 354 men and women ≥18 years of age without knee OA.28 Results from 4 publications12–14,29 presenting WOMAC scores in patients with symptomatic/radiographic knee OA are shown in Table 3. In 2 separate studies of Egyptian men and women ≥40 years of age with symptomatic/grades 1–4 radiographic knee OA, mean WOMAC scores were 53.612 and 44.2.29 Two studies from Nigeria reported the same patient population of men and women ≥18 years of age with knee OA who were assessed according to whether they had depression13 or poor sleep quality.14 WOMAC pain subscale scores were significantly worse in patients with knee OA and depression (p<0.0001),13 neuropathic pain (p<0.001),29 or poor sleep quality (p<0.001),14 compared to those with knee OA with no depression, no neuropathic pain, or normal sleep quality.
 |
Table 3 Results from Studies Reporting WOMAC Scores in Men and Women with Symptomatic/Radiographic Knee OA in 4 Publications |
In addition, 3 studies reported Lequesne Algofunctional Index of Disease Severity mean scores in patients with symptomatic/radiographic knee OA (Table 4).19,21,30 Among the population in Tunisia, Hana et al reported a mean severity score of 11, indicating very severe disability, and scores significantly higher in those ≥65 years of age compared with those <65 years of age.21 In a Cameroon study, Bija et al found that the proportion of patients within higher score ranges increased with severity of radiographic grade of knee OA and 40% of patients with grade 4 symptomatic/radiographic knee OA had a score indicating extremely severe disability.19 Rezakhani Moghaddam et al reported on a population of men and women ≥18 years of age in Iran with knee OA. Comparisons with the other studies could not be made, however, because a different scoring scale was used.30
 |
Table 4 Results from Studies Reporting Lequesne Algofunctional Index of Severity and OAKHQoL Scores in Men and Women with Symptomatic/Radiographic Knee OA in 4 Publications |
The Osteoarthritis Knee and Hip Quality of Life (OAKHQoL) tool was used to measure disease severity in men and women ≥30 years of age with symptomatic/radiographic knee OA in Egypt (Table 4).26 Women had significantly worse scores for most parameters compared with men (p<0.05), and men and women 51–75 years of age had significantly poorer scores than subjects 36–50 years of age (p<0.001). The worst (lowest) scores were reported within the pain and social function domains, especially among older men and women. Two publications reported use of the 36-item Short Form Health Survey questionnaire (SF-36)20 or the abbreviated 12-item version (SF-12),31 where higher scores indicate better quality of life. Among men and women ≥55 years of age in Saudi Arabia, those with severe symptomatic/radiographic knee OA had SF-36 scores indicating significantly worse quality of life than those with mild/moderate knee OA.20 In a population of men and women ≥40 years of age in Iran with grade ≥1 symptomatic/radiographic knee or hip OA, SF-12 Physical Component Summary scores appeared to be more affected than Mental Component Summary scores.31
Results using a visual analog scale (VAS) for pain in patients were reported in 6 publications.19–22,25,29 Among men and women ≥55 years of age in Saudi Arabia, VAS pain scores were significantly higher (indicating worse pain) for those with severe versus mild/moderate radiographic knee OA.20 Among patients in Tunisia with symptomatic/radiographic grade ≥2 knee OA, mean (standard deviation [SD]) VAS scores were significantly higher in those ≥65 years of age (65.2 [SD 18]) than in those <65 years of age (58.3 [SD 12]; p=0.02).21 Lukusa et al found that VAS scores were similar across all radiographic severity grades in men and women ≥30 years of age in the Democratic Republic of Congo.25 In contrast, a study from Cameroon showed a trend toward higher VAS scores with increasing grade of radiographic severity.19
Depression in patients with knee OA was assessed using the Patient Health Questionnaire (PHQ-9)13,14 and Beck Depression Inventory (BDI);12 with both instruments, the higher the scores, the more severe the depression. Akintayo et al found that 42% of patients ≥18 years of age with symptomatic knee OA in Nigeria reported evidence of depression on the PHQ-9, which was significantly associated with WOMAC pain score (p<0.025) and poor sleep quality (p<0.001) but not with Kellgren-Lawrence grade severity.13 A further study in the same population also showed significant associations between PHQ-9 depression scores, WOMAC scores, and sleep quality.14 Another publication reported that 30% of patients ≥40 years of age in Egypt with symptomatic/radiographic knee OA had BDI scores consistent with depression, compared with 5% of healthy controls (p=0.03). Further, there was a significant correlation between BDI depression and WOMAC score (p=0.005).12
Discussion
In 2019, the age-standardized prevalence of OA in the Middle East and North Africa (MENA) was 5,342.8 per 100,000 (95% UI: 4,815.9–5,907.8) and the age-standardized annual incidence of OA per 100,000 was 430.4 (382.2–481.9).32 A systematic review by Yahaya et al calculated the pooled prevalence of OA to be 14.2% (CI 7.95–21.89%) in Sub Saharan Africa.33
This REA found that prevalence of OA in Africa and the Middle East was reported in 7 epidemiologic studies.15–17,19,24,25,28 Among the 3 studies reporting symptomatic knee OA, the prevalence in men and women 35–70 years of age in Iran was 47.7%,24 and among those ≥18 years of age, it ranged from 11.5% in Nigeria28 to 25.8% in Dubai.15 In the Dubai study, 77% were female and prevalence of OA was similar between men and women;15 this is in contrast to the other 2 studies24,28 as well as other reports1 that indicated higher prevalence in women versus men. Among the 3 studies reporting symptomatic/radiographic knee OA, prevalence ranged from 9% in rheumatology outpatients in Cameroon19 to 34% in women ≥40 years of age with OA of mixed etiology in Bahrain.17 The prevalence of knee OA as diagnosed by MRI among those ≥40 years of age in Saudi Arabia was 70%,16 which was considerably higher than the prevalence of symptomatic (12%)24 and symptomatic/radiographic (14%)25 OA in populations of similar ages in other countries. The authors commented that despite the high ability of MRI to visualize lesions, its sensitivity was 61% and specificity was 82% for OA diagnosis, suggesting that MRI is less accurate than symptomatic and symptomatic/radiographic diagnostic methods for estimating prevalence of knee OA, and that it may be more useful for monitoring progression in high-risk individual patients.16 Lack of availability of MRI scanners may also limit its use for diagnostic purposes, especially in developing countries with limited resources.
The humanistic burden of OA was examined in 16 studies reporting quality of life outcomes.12–14,18–23,25–31 The OA-specific WOMAC questionnaire was reported in 5 publications.12–14,28,29 In the 1 publication comparing adults with symptomatic knee OA to those without, all 3 subdomains (pain, stiffness, and physical function) were significantly worse in those with versus those without knee OA.28 The other 4 publications investigated the quality of life of patients with symptomatic/radiographic knee OA.12–14,29 Patients with knee OA plus another condition (depression,13 poor sleep quality,14 or neuropathic pain29) had significantly worse WOMAC pain scores than patients with knee OA alone.
Differences in methods of reporting limited comparisons across 3 publications using the Lequesne Algofunctional Index of Disease Severity, with the majority of patients having scores indicating severe or very severe disability.19,21,30 Older patients had significantly more disability than younger patients.21 These results suggest that knee OA has a significant impact on quality of life, especially for those with more severe disease.
The effect of symptomatic/radiographic knee OA assessed by OAKHQoL in Egyptian adults ≥30 years of age also showed significantly worse scores in older versus younger patients and in women versus men.26 The limited results from 2 studies using SF-36 and SF-12 to assess symptomatic/radiographic knee OA20 and knee or hip OA,31 respectively, were consistent with those from a study by the Osteoarthritis Initiative in US adults that found health-related quality of life losses due to radiographic knee OA measured by the Short Form Six Dimensions (SF-6D) tool increased with radiographic severity in domains related to physical but not mental or emotional health.34 In general, VAS pain scores were worse with disease severity,19,20 age,21 and in women compared to men.22 The findings that 42% of Nigerian adults with symptomatic knee OA reported depression according to PHQ-913 and 30% of patients ≥40 years of age in Egypt with symptomatic/radiographic knee OA reported depression according to the BDI12 are in agreement with a study from the UK that found clinically significant depression in 40% of patients with lower limb OA.35
There are several limitations to this REA analysis. There was a large degree of heterogeneity between studies, and this complicated direct comparisons of epidemiologic outcomes data. Between-study variability occurred mainly in the diagnostic criteria used and the populations investigated, in addition to sex and age. Most of the included studies examined patients within primary or secondary health care settings; only Kolahi et al24 reported prevalence in the general population of Iran. Prevalence in populations recruited from health care settings, especially rheumatology clinics, may overestimate the situation within the general population. Further, comparisons between studies reporting humanistic burden outcomes may have been complicated by cultural differences and the use of different versions of the WOMAC.
In conclusion, the available evidence in publications from January 1, 2010, to April 23, 2020, suggests that the burden of OA in weight-bearing joints in Africa and the Middle East is substantial, especially regarding the impact of pain. These findings are consistent with publications from the United States and Europe.34,36 OA has a substantial impact on the quality of life of the patients, especially if they are also suffering from mental health issues. There is a lack of data on economic burden of OA in the region. This REA identified the need to generate more evidence on epidemiology, HRQoL and socioeconomic outcomes. Further studies will enable a better understanding of the holistic burden of OA in the region thus supporting better healthcare services and resource allocation.
Research Involving Human Participants and/or Animals
The manuscript does not contain clinical studies or patient data.
Data Sharing Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Acknowledgments
Sara Lucas, PhD, and Catherine Rolland, PhD, of CURO (part of the Envision Pharma Group) were involved in the design and development of the rapid evidence assessment, which was funded by Pfizer. Medical writing support was provided by Margit Rezabek, DVM, PhD, of Engage Scientific Solutions and was funded by Pfizer and Eli Lilly and Company. Additional editorial support was provided by Vaidehi Wadhwa (Medical Excellence, Emerging Markets, Pfizer).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by Pfizer.
Disclosure
Bassel Elzorkany has received consulting fees, speakers’ honoraria, and research grants from Abbott, AbbVie, Amgen, Aspire, BMS, Eva, Hekma, Janssen, Lilly, MSD, New Bridge, Novartis, Pfizer, Roche, Sanofi-Aventis, and Servier (none of these was related to this manuscript). Mohamed Fathy and Nora Vainstein are employees of and own stock/options in Pfizer. Jamal Al Saleh, Hani Almoallim, Ali Al Belooshi, Omar Batouk, and Abdullah M. Kaki declare that they have no conflicts of interest in this work.
References
1. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–199. doi:10.1093/bmb/lds038
2. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, Hip, and knee. Arthritis Rheumatol. 2020;72(2):220–233. doi:10.1002/art.41142
3. Sanchez Romero EA, Melendez Oliva E, Alonso Perez JL, et al. Relationship between the gut microbiome and osteoarthritis pain: review of the literature. Nutrients. 2021;13(3). doi:10.3390/nu13030716
4. Sánchez-Romero EA, González-Zamorano Y, Arribas-Romano A, et al. Efficacy of manual therapy on facilitatory nociception and endogenous pain modulation in older adults with knee osteoarthritis: a case series. Appl Sci. 2021;11(4):1895. doi:10.3390/app11041895
5. Sanchez Romero EA, Fernandez-Carnero J, Calvo-Lobo C, Ochoa Saez V, Burgos Caballero V, Pecos-Martin D. Is a combination of exercise and dry needling effective for knee OA?. Pain Med. 2020;21(2):349–363. doi:10.1093/pm/pnz036
6. Sinatti P, Sanchez Romero EA, Martinez-Pozas O, Villafane JH. Effects of patient education on pain and function and its impact on conservative treatment in elderly patients with pain related to hip and knee osteoarthritis: a systematic review. Int J Environ Res Public Health. 2022;19(10):6194. doi:10.3390/ijerph19106194
7. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160–167. doi:10.1097/BOR.0000000000000479
8. Alonso Perez JL, Martin Perez S, Battaglino A, Villafane JH, Alonso-Sal A, Sanchez Romero EA. An up-date of the muscle strengthening exercise effectiveness in postmenopausal women with osteoporosis: a qualitative systematic review. J Clin Med. 2021;10(11):2229. doi:10.3390/jcm10112229
9. Safiri S, Kolahi AA, Smith E, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819–828. doi:10.1136/annrheumdis-2019-216515
10. Ciampi de Andrade D, Saaibi D, Sarria N, Vainstein N, Cano RL, Espinosa R. Assessing the burden of osteoarthritis in Latin America: a rapid evidence assessment. Clin Rheumatology. 2021;41(5):1285–1292. doi:10.1007/s10067-022-06063-9
11. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi:10.1136/bmj.g7647
12. Abd El Monaem SM, Hashaad NI, Ibrahim NH. Correlations between ultrasonographic findings, clinical scores, and depression in patients with knee osteoarthritis. Eur J Rheumatol. 2017;4(3):205–209. doi:10.5152/eurjrheum.2017.160097
13. Akintayo RO, Yerima A, Olaosebikan HB, Uhunmwangho C, Akpabio AA. How much gloom is in groans? Depression and its determinants in Nigerian patients with knee osteoarthritis: a multi-center cross-sectional study. Clin Rheumatol. 2019;38(7):1971–1978. doi:10.1007/s10067-019-04497-2
14. Akintayo RO, Yerima A, Uhunmwangho C, Olaosebikan H, Akpabio AA. Tossing and turning with degenerative arthropathy: an assessment of poor sleep quality in knee osteoarthritis. Reumatologia. 2019;57(4):207–213. doi:10.5114/reum.2019.87615
15. Al Saleh J, Sayed ME, Monsef N, Darwish E. The prevalence and the determinants of musculoskeletal diseases in Emiratis attending primary health care clinics in Dubai. Oman Med J. 2016;31(2):117–123. doi:10.5001/omj.2016.23
16. Alrowaili MG. Magnetic resonance evaluation of knee osteoarthritis among the Saudi population. Pak J Med Sci. 2019;35(6):1575–1581. doi:10.12669/pjms.35.6.874
17. Asokan G, Hussain MS, Ali EJ, Awate RV, Khadem ZK, Al-Safwan ZA. Osteoarthritis among women in Bahrain: a public health audit. Oman Med J. 2011;26(6):426–430. doi:10.5001/omj.2011.108
18. Ateef M, Alqahtani MM, Alzhrani M, Alshewaier S. Physical function and quality of life and modification of authentic Islamic prayer procedure by osteoarthritis knee patients in Saudi Arabia: a cross-sectional study. J Relig Health. 2021;60(2):764–773. doi:10.1007/s10943-019-00878-8
19. Bija MD, Luma HN, Temfack E, Gueleko ET, Kemta F, Ngandeu M. Patterns of knee osteoarthritis in a hospital setting in sub-Saharan Africa. Clin Rheumatol. 2015;34(11):1949–1953. doi:10.1007/s10067-014-2702-3
20. Bindawas SM, Vennu V, Alfhadel S, Al-Otaibi AD, Binnasser AS. Knee pain and health-related quality of life among older patients with different knee osteoarthritis severity in Saudi Arabia. PLoS One. 2018;13(5):e0196150. doi:10.1371/journal.pone.0196150
21. Hana S, Aicha BT, Selim D, Ines M, Rawdha T. Clinical and radiographic features of knee osteoarthritis of elderly patients. Curr Rheumatol Rev. 2018;14(2):181–187. doi:10.2174/1573397113666170425150133
22. Hawamdeh ZM, Al-Ajlouni JM. The clinical pattern of knee osteoarthritis in Jordan: a hospital based study. Int J Med Sci. 2013;10(6):790–795. doi:10.7150/ijms.5140
23. Ilori T, Ladipo MM, Ogunbode AM. Functional health of patients with knee osteoarthritis in a family medicine clinic in Ibadan. Afr J Med Med Sci. 2016;45(3):269–274.
24. Kolahi S, Khabbazi A, Malek Mahdavi A, et al. Prevalence of musculoskeletal disorders in Azar cohort population in Northwest of Iran. Rheumatol Int. 2017;37(4):495–502. doi:10.1007/s00296-017-3661-1
25. Lukusa A, Malemba JJ, Lebughe P, Akilimali P, Mbuyi-Muamba JM. Clinical and radiological features of knee osteoarthritis in patients attending the University Hospital of Kinshasa, Democratic Republic of Congo. Pan Afr Med J. 2019;34:29. doi:10.11604/pamj.2019.34.29.11283
26. Mahmoud GA, Moghazy A, Fathy S, Niazy MH. Osteoarthritis knee Hip quality of life questionnaire assessment in Egyptian primary knee osteoarthritis patients: relation to clinical and radiographic parameters. Egypt Rheumatol. 2019;41:65–69. doi:10.1016/j.ejr.2018.05.001
27. Mirmaroofi N, Ghahramanian A, Behshid M, et al. Relationship between self-efficacy and pain control in Iranian women with advanced knee osteoarthritis. Niger J Clin Pract. 2019;22(4):460–468. doi:10.4103/njcp.njcp_437_17
28. Ogunbode AM, Adebusoye LA, Olowookere OO, Alonge TO. Physical functionality and self-rated health status of adult patients with knee osteoarthritis presenting in a primary care clinic. Ethiop J Health Sci. 2014;24(4):319–328. doi:10.4314/ejhs.v24i4.7
29. Radwan A, Borai A. Neuropathic pain in Egyptian patients with primary knee osteoarthritis: relationship with functional status and radiological severity. Egypt Rheumatol. 2019;41:261–264. doi:10.1016/j.ejr.2018.12.005
30. Rezakhani Moghaddam H, Nadrian H, Abbagolizadeh N, Babazadeh T, Aghemiri M, Fathipour A. Mental health-ill health differences in disease severity and its sociodemographic biobehavioral predictors among patients with knee osteoarthritis. Clin Nurs Res. 2019;28(7):886–904. doi:10.1177/1054773817751527
31. Saffari M, Meybodi MKE, Ghanizadeh G, Koenig HG. Determinants of health status among patients with knee or Hip osteoarthritis: the role of demographic, clinical and health related quality of life variables. J Musculoskelet Res. 2014;17(1):1450004. doi:10.1142/S0218957714500043
32. Shamekh A, Alizadeh M, Nejadghaderi SA, et al. The burden of osteoarthritis in the middle east and North Africa Region From 1990 to 2019. Front Med. 2022;9:881391. doi:10.3389/fmed.2022.881391
33. Yahaya I, Wright T, Babatunde OO, et al. Prevalence of osteoarthritis in lower middle- and low-income countries: a systematic review and meta-analysis. Rheumatol Int. 2021;41(7):1221–1231. doi:10.1007/s00296-021-04838-y
34. Wilson R, Blakely T, Abbott JH. Radiographic knee osteoarthritis impacts multiple dimensions of health-related quality of life: data from the Osteoarthritis Initiative. Rheumatology. 2018;57(5):891–899. doi:10.1093/rheumatology/key008
35. Axford J, Butt A, Heron C, et al. Prevalence of anxiety and depression in osteoarthritis: use of the Hospital Anxiety and Depression Scale as a screening tool. Clin Rheumatol. 2010;29(11):1277–1283. doi:10.1007/s10067-010-1547-7
36. Jackson J, Iyer R, Mellor J, Wei W. The burden of pain associated with osteoarthritis in the Hip or knee from the patient’s perspective: a multinational cross-sectional study. Adv Ther. 2020;37(9):3985–3999. doi:10.1007/s12325-020-01445-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
