Back to Journals » Adolescent Health, Medicine and Therapeutics » Volume 10
Assent and consent in pediatric and adolescent research: school children’s perspectives
Authors Al-Sheyab NA , Alomari MA , Khabour OF , Shattnawi KK , Alzoubi KH
Received 28 August 2018
Accepted for publication 10 January 2019
Published 11 February 2019 Volume 2019:10 Pages 7—14
DOI https://doi.org/10.2147/AHMT.S185553
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Alastair Sutcliffe
Nihaya A Al-Sheyab,1 Mahmoud A Alomari,2,3 Omar F Khabour,4 Khulood K Shattnawi,1 Karem H Alzoubi5
1Faculty of Nursing, Maternal and Child Health Department, Jordan University of Science and Technology, Irbid, 22110, Jordan; 2Division of Physical Therapy, Department of Rehabilitation Sciences, Jordan University of Science and Technology, Irbid, 22110, Jordan; 3Department of Physical Education, Qatar University, Doha 2713, Qatar; 4Department of Medical Laboratory Sciences, Jordan University of Science and Technology, Irbid, 22110, Jordan; 5Department of Clinical Pharmacy, Jordan University of Science and Technology. Irbid, 22110, Jordan
Background: School students’ views and perceptions of informed parental consent and child assent about child participation in research in the Middle East are not known.
Methods: Focus group interviews were conducted to understand high school students’ perspectives toward child and adolescent assents and consents in research including the importance of, and depth of information needed in consent and assent, and perception toward written vs verbal consent and assent.
Results: The majority of students agreed that it is necessary to take parental approval and that they would not participate in research if their parents refused. Furthermore, the majority of male students agreed that if the research requires only questionnaires to be completed, then child’s approval is sufficient whereas measures, such as blood sugar screening required approval from both the parent and child. Females believed it is enough to provide parental consent to participate in research unless information provided is adequate, then child approval is enough. All students stressed the importance of including detailed information; however, parental consent needs to have more detailed information than child assent.
Conclusion: Parts of the students’ perceptions were congruent, whereas other views were not congruent with proper conduct of pediatric research. Such a situation warrants further research and actions.
Keywords: informed consent, adolescent, child, Jordan, Middle East
Introduction
Child involvement in scientific research requires parent/guardian approval, which is termed parental consent, and child’s approval, which is termed assent.1,2 Depending on the child’s developmental level, child assent involves independent approval to participate in research, given parental approval had been sought and granted.3,4 Protecting children’s and adolescents’ rights and welfare is the main reason to seek both parental consent and child assent when participating in scientific research. Thus, adhering to ethical guidelines,5 which are usually followed in developed countries.6–8 These rules are set within the international guidelines of ethical conduct in research in human such as the Belmont report, the Helsinki declaration and its amendments, and the International Ethical Guidelines for Health-related Research Involving Humans.9–11
In developing countries, adherence to ethical conduct of child and adolescent research is limited for various reasons including, but not limited to, lack of existing relevant rules and regulations, unawareness12 of such regulations, or lack of enforcement of these regulations.2,13–15 For example, a study found that faculty members from four health colleges in Egypt had varying awareness and perception about several parts of ethical conduct in human research.16 Yet, another regional study found that health care professionals from 12 medical disciplines believed in the importance of obtaining informed consent/assent from children participating in medical research.15,17
Findings within adult research regarding consent cannot be generalized to youth perceptions due to differences in brain development, life experience, age, and educational level. For example, from a neuroscience point of view, the gap between development of the brain’s reward system and of the control system diminishes decision-making competence in adolescents in specific contexts.18 Therefore, additional support should be offered to adolescents in order to create a context in which they can make competently informed decisions.18 In this case, parents have a substantial role in children’s decisions, and thus how families come to provide consent.19 In some Western countries, there is specific legislation that specifies the age at which children should be involved in decisions about medical treatment or scientific research. For example, in the Netherlands and the UK, children aged 16 and above may make treatment decisions independently.18,20 In the USA, a child of at least 7 years old is eligible by law for assent.21
Findings that arise from adult research cannot be applied to children due to the differences in physiological functions,1,22 highlighting the fact that research involving children needs to be ethically acceptable.23 Informed consent is a critical aspect of ethical conduct in research involving human beings.24,25
In the Middle East, children younger than 15 years old comprise 100,000,000 (>35%) of the population in 2025, and behavioral choices are generally dominated by a complex interaction among religious, sociocultural, and individual factors in the region.15 Similarly, in Jordan, youth population is expected to increase by 28% to reach ~1.5 million (a fifth of the population),26 making pediatric research particularly important in the region. Schools are common and essential venues for conducting pediatric research, particularly epidemiological studies or those aimed at promoting good health. School students play an important role in the recruitment process of medical research. In Western countries, several studies have examined the attitudes and perceptions of children toward informed consent process and scientific research in normal setting and during emergencies.19,27–29
However, students’ perceptions toward informed parental consent as well as child assent for participation in research have not been investigated in the Middle East region. Thus, the current study aimed at exploring high school students’ perception toward obtaining parental consent and child assent using in-depth focus group interview approach.
Methods
Participant characteristics
The students were from homogenous age range (13–17 years old) and socioeconomic status (middle).
Study approach and procedure
The current study used a descriptive qualitative design aimed at exploring school children’s perception and insights toward parental consent and child assent in pediatric research. Several schools in the North of Jordan, which were part of a larger concurrent study at the time of data collection, were invited to participate in this study. In brief, five schools were randomly selected – using a simple random technique – from a list of all public high schools in Irbid and Ramtha districts after being stratified for gender. These five schools were invited to participate in the study, of which one male and one female school were selected to carry out one focus group in each school. Selection of these two schools was because they were the first that showed interest in participation. The two focus groups were conducted by the same researcher. Both parental consent and child assent were sought before recruiting children into the focus groups.
Within each focus group, eight students in seventh, eighth, and ninth grades voluntarily agreed to participate. The number of focus groups conducted was two, thus the total sample size was 16 students (8 boys and 8 girls). All eight students participated in each respective focus group. The focus groups were conducted in an unoccupied room using a round table setting. Only participating students – with no parents present – and the researchers were in the room to ensure confidentiality. Some of the recruited students who took part in the study actually participated in research requiring them to make decisions related to assent/consent of children/adolescents; noting that their participation involved only completing surveys, not invasive medical procedures. In addition, when asked about whether they had filled in an assent or consent form, these students said that they did not recall completing a written informed consent prior to their participation. Ethical approval was obtained from the Institutional Research Committees of Jordan University of Science and Technology and the Ministry of Education to conduct this study.
Written parental informed consent as well as written child assent was obtained from all students participating in the two focus groups. The study purpose and procedures were explained to the students and their parents, and permissions were obtained from all students/parents to audio-record the focus group interviews and to transcribe them later. Subsequently, an open discussion, focusing on three aspects with probing questions, was initiated and continued until a saturation stage for each theme/question was reached. Saturation of data was defined as reaching a point in data analysis whereby further sampling leads to no new information related to their research questions. Intergroup discussions and interactions were allowed and encouraged; however, the researchers intervened when the interactions deviated from the purpose of the study. A pilot study was not conducted for this research. In fact, in qualitative research, the use of a pilot is less common or even unnecessary.30 However, to achieve the benefits of a pilot study, the recommendations of Breen were followed by extending the length of each focus group to two hours so that questions were checked for their meaning and clarity during the focus group itself and to ensure saturation was reached.31
Focus groups questions
The focus groups questions are presented in Table 1. The questions focused on four aspects including the importance of consent and assent, depth of information needed in consent and assent, and perception toward written vs verbal consent and assent during pediatric research. The predetermined aspects and questions were used to guide the discussion, thereby facilitating consistent and systematic data collection during the focus groups. The interviews were led and moderated by one researcher while another was present for taking field notes and helping in the discussion when required. Discussion moderation included presenting predetermined questions, encouraging all participants to be involved, reframing, repetition for validating participants’ point of views, and expansion of the questions as required, thus enhancing the credibility of the obtained information.32,33 The discussion questions and responses were kept confidential.
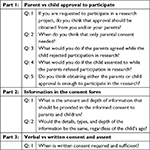  | Table 1 Questions presented in the focus group interviews |
Probing questions were selected based on the available literature in this topic as well as the field experience of the researchers. In particular, the researcher and the facilitator conducted preliminary, informal interviews with school students and teachers from the five schools that were part of a larger concurrent study to get some ideas and perspectives about students’ awareness about the child consent process in research. Based on the findings of these informal interviews, the researchers directed the relevant conversations and discussions on consent and assent of children and adolescent participation in research.
Data analysis
The two focus group interviews were transcribed verbatim in Arabic, and then translated in full into English by the principal investigator of the study who is a bilingual researcher fluent in both English and Arabic (mother tongue). The accuracy of the transcripts was validated by the focus group researchers. A content thematic analysis approach was undertaken. Transcripts were read in their entirety by the two researchers independently. Important statements were highlighted and assigned to categories. Subsequently, the transcripts and translations were confirmed by exchanging notes, categories, and themes to ensure credibility of data. In the case of controversy or discrepancies, researchers further deliberated until issues were resolved. Indeed, this deliberation process included further coding and re-coding of data until final identified codes and themes had been agreed upon.
Results
Theme 1: parent vs child approval to participate
Participating students were asked: “If you are requested to participate in a research project, do you think that approval should be obtained from you and/or your parents?” All students (males and females) agreed that it is necessary to take the approval from the parents, except one male student who thought it is not necessary to take parents’ approval in research participation except for a research that requires blood withdrawal test. Additionally, all female students agreed that parental consent is necessary, but that largely depends on the nature of the survey/questionnaire.
One male student (FG1) said:
If I’m asked to be part of a research study, I would certainly ask my parents for approval […] they surely know better than me.
Another male student said (FG1):
Seeking approval from my father is important, I guess […] sometimes, participation in research might result in detecting unwanted health disorders that we are unaware of.
One male student (FG21) said:
Regardless of the type of research or measurement, parental consent is a must and they should be informed. However, if it is only a questionnaire, seeking parental consent is not necessary. But after filling out the questionnaire/survey, we will tell our parents.
When students were asked specifically about the importance of their approval in participating in research studies, some agreed that child participation is very important but others perceived their parental consent is more important and sometimes could be enough in certain types of research.
One female student (FG2) added:
No, child approval is not necessary because participation in research might be beneficial, such as discovering diseases we’re not aware of such as optometry […] However, invasive procedures, such as blood withdrawals require my approval.
One male student said (FG1):
There is a need to ask for children’s approval/consent because it fosters a sense of responsibility, and gives him a role to make decisions, thus can help boosting the student’s personality.
Another male student (FG2) said:
If I’m not convinced to participate, I won’t participate even if my parents agree.
The researcher then asked a more specific question: “What do you do, as a student, if you agreed to participate but your parents refused?”
Unanimously, all students (regardless of gender) agreed that they would not participate in research if their parents refused. However, only one student said that he would discuss it with his parents to persuade them to agree but if they insist on refusing, he will not participate in research.
One female student (FG2) said:
We will not participate in the research in spite of our desire to join the study because parents’ disapproval reflects their knowledge and experience toward our benefit.
One male student (FG1) said:
I will participate; but generally as long as the father agreed […] it’s important to have an open dialog and have a debate to try and understand each other’s point of view.
Another probing question was asked by the researcher: “What do you do if the parents agreed but the child refused to participate?”
Most male students said they would agree after several attempts from parents to persuade them. However, some male students stressed the point that they will not participate in research studies in spite of parental approval.
One male student (FG1) said: “No, I won’t join the research, because parents may get upset”. He further elaborated: “They’re more aware of stuff and have more life experience than me […] they care about my own benefit that’s why I’ll join only if they feel that I should join”.
Moreover, the researchers further investigated this aspect by asking the following question: “Do you think that obtaining consent from one of the parties (child or parent) is enough for the child to participate in research and it replaces the other party’s consent?”
The responses varied between female vs male students. The majority of male students agreed that it depends on the type of research. They explained that if the research requires only questionnaires/survey to be completed, then child’s approval is sufficient. However, when some of the measures are required such as blood sugar screening, then seeking consent /approval is required from both the parent and child. Females, on the other hand, had different opinions compared to male students. Female students believed it is enough to provide parental consent to participate in research unless information provided is adequate/detailed enoough to make decisions, then child approval is enough.
One male student (FG1) answered:
Yes, it’s normal to fill out a survey that’s clear and the objective of the study are also obvious without the consent of parents.
Another female student (FG2) replied:
Sure, approval from me and my parents is important in all cases especially in cases where an invasive procedure is required such as in blood sugar tests where the role of the parents is important.
Theme 2: depth of information in the consent and assent forms
The researcher asked students: “What do you think of the extent of information/content and depth of details that need to be provided in the consent/assent forms?”
All students (males and females) stressed the importance of including detailed information without excluding any important or relevant aspect that might help them and their parents to take an appropriate decision.
One male student (FG1) answered:
... it’s very important that the researcher tells us everything about the research and what’s required from us, so we can make a decision.
Another probing question was asked by the researcher: “Do you think that there should be a different level of information in terms of quality, details and depth in the consent vs assent?”
All female students agreed that parental consent needs to include more and deeper details than assent form.
One female student (FG2) said:
... both consent and assent should be different because parents have more experience […] and they know better than me […] so their consent should have deeper and more details.
However, few boys thought that both parental consent and child assent should have the same level of information.
One boy said (FG1):
I need to know all about the study before I decide to take part in it […] and yes, what’s written in the consent form for parents need to be in the child’s as well.
Theme 3: age of providing child assent
The researcher asked students the following question: “Beside parental consent, what is the age of the child that you think is appropriate to provide adequate information to the child and thus child can provide assent?”
Most of the students (males and females) agreed that adolescents can provide assent, however, the age at which they can give assent varied. In specific, students believed that children are able to provide and sign assent when they reach puberty, whereas some students (males and females) said that when the child reaches 18 years old they can provide assent.
One female student (FG2) replied:
The age of 18 years old, which is the adulthood age […] because people can recognize right from wrong at this age and they can make informed, independent decisions.
Another female (FG2) student elaborated:
Under 12 years, child’s opinion is important […] but not as important as who’re older than 12 years.
Another male student (FG1) answered,
12 and older […] because being able to decide will boost the child confidence.
The researcher further asked the following question in order to elicit more information: “When does the researcher only need parental consent (no need for child assent)?”
There were some discrepancies in responses among male vs female students. Most female students believed that parents are entitled to provide consent on behalf of their children as long as the child is under the age of puberty (12–13 years). However, several male students believed that it depends on the procedure, suggesting that invasive procedures, such as obtaining blood samples, require only parental consent without child assent.
One female student answered (FG2):
Parental consent is enough if the child is under13 years old […] I assume this is the age of puberty.
One male student said (FG1):
If they’re going to take blood samples from me, then my parents’ decision is enough regardless of my age.
Another male student (FG1) said:
... consents from our parents can be adequate most of the times, but there are cases where their consent alone is not enough […] such as research that involves invasive tests […] in this case, children need to agree too.
Theme 4: type of consent/assent forms (written vs oral)
The researcher asked students the following question to elicit information about the type of consent/assent forms: “when is written consent/assent required and enough?”
There were some variations in students’ responses based on gender. All female students agreed that in almost all research studies, written consent is required and sufficient and even better than verbal consent. However, all male students said that it depends on the type of research and procedure. For example, if blood samples are required, then written consent is needed.
One male student (FG1) said:
... blood withdrawals surely need written consent.
Another male student (FG1) elaborated:
... focus groups don’t need written consent neither vision screening […] but more serious procedures need written consent, I guess.
One female student (FG2) replied:
...written consent is needed in various kinds of research.
Another female student said (FG2):
... written consent is superior to oral consent as it makes sure to protect our rights more than oral consent does.
Discussion
This qualitative study examined high school children’s perceptions and insights on informed parental consent and child assent in pediatric and adolescent research using focus group interviews. The questions presented during the interviews focused on four themes including importance, type, and depth of information in informed parental consent and child assent in pediatric and adolescent research. Overall, some of the students’ perceptions were consistent with the proper conduct of pediatric research. However, some insights were of concern, thus, actions are needed. To the best of our knowledge, no studies have previously investigated school children’s perspective toward informed parental consent and child assent in pediatric and adolescent research in developing countries, particularly the Middle East and Jordan.
Ethical behaviors in any country are affected by social, cultural, education, religion, law, and codes of conduct. In Jordan and the Middle East, where Islam is the predominant religion, the definition of the age of children group may be different from that used in other countries.34 “Fatwa”, an Islamic legal pronouncement issued by the clergy, is also pivotal in forming opinions about proper ethical behaviors.15,35 In addition, social, cultural, religious and educational systems reinforce guardian influence on children’s decision, even beyond legal adulthood age (ie, 18 years).17 Moreover, gender of the child could also be another factor in parental influence and decision to allow his/her participation in research. In some cultures, males and females do not have the same level of independence or societal roles/rights, which may uniquely contribute to female’s perceptions about assent/consent processes. In fact, few of the Middle East countries have ethical guidelines that regulate pediatric research.36,37 Taken together, in Jordan and the region, parental consent and children assent process may be affected by many factors, such as child’s age, parents’ perception, culture, religion, and education, adopted ethical guideline and other personal factors.
Overall, some of current findings were inconsistent with international guidelines and recommendations of conducting appropriate research,1 highlighting the need for educating children and adolescents in the importance of obtaining parental consent in all types of research.6 Moreover, it has been documented that there should be no force on children to participate in research especially in the absence of parents’ attendance, such as in schools.23 Similarly, the child should understand the process of participating in research including the autonomous right to assent despite the parents’ opinion.6 In addition, researchers should be alert to the possibility of divergence in child and parent views and enabling children’s perspectives to be heard.19 Forcing children to participate in research might lead to inaccurate study findings.1,6 Therefore, researchers need to ensure voluntary assent from children to join research without any coercion.4
The majority of research studies involving children usually necessitate obtaining parental consent as well as child assent,8 hence, students’ perceptions and understanding of the process of assent entail focus from researchers. This can be obtained through organizing educational workshops aiming at increased awareness about appropriate and voluntary conduct of research and thus decrease violation.
Clinical studies in Jordan, including those on children or adolescents, are governed by the Jordanian Food and Drug Administration via the Jordanian Clinical Studies Law. Each clinical study, including those on children, is required by law to get institutional review board approval from the researchers’ own institution and the institution where the study will be conducted.38 Additionally, if the study is a drug-related trial, the approval from the Jordanian Food and Drug Administration is also required. These laws are strictly monitored and reinforced in Jordan.39 However, there is no current requirement of special/formal training for those who conduct research on human subjects including children or adolescents.38
The international guidelines of ethical conduct in research emphasize that informed consents need to be simple, comprehensive – but not overwhelming – confidential, and voluntary.13,25,40,41 Furthermore, the informed consent should include all potential benefits, and risks, detailed procedure and data collection strategy. Some of the students’ opinions in the current study are consistent with those guidelines, however, other perceptions indicate that those adolescents are not aware of the correct way of conducting research.13,25,40,41 Verbal consent of children to join research is sufficient in some types of research, however, seeking written consent before recruiting children is important to ensure their rights, safety, and welfare. Thus, it should be sought before recruitment.15,25,40,41
Conclusion
The current study shows that some of the students’ perceptions were congruent with proper conduct of pediatric research. However, other views were of concern, as they do not adhere to international guidelines of conduct of studies in human. For example, some students believe that the decision to participate is solely for the parents whereas others think that children should make their own decision even without parental consent. This is certainly in conflict with the international ethical guidelines, which emphasize the importance of coupling the child assent to participate in a research project with the parental consent. Such a situation warrants further research and action. Indeed, participating children and adolescents have certain perceptions about informed consent and assent that reflects lack of understanding and awareness of the universal guidelines of proper conduct on research on children and adolescents, with certain differences between boys and girls. Interestingly, some students do not recognize the importance of parental approval for procedures deemed simple, such as questionnaires, or beneficial, such as new/expensive treatment. Some students perceived that they could be forced by their parents to join research, especially when parents think the study is useful. In fact, a follow-up study using questionnaire survey approach focusing on these important points from the current study is a future direction.
Researchers need to be aware of the students’ perceptions about informed consent/assent and thus increasing students’ and parents’ awareness about the process of obtaining parental consent and child assent. Importantly, researchers need to ensure that ethical guidelines are applied and practiced appropriately within the relevant institutional review board in the region to ensure children’s welfare and autonomy in making decisions.
Acknowledgments
The authors would like to thank the schools’ principals and teachers who facilitated the study and the students who voluntarily participated in the study. This work was funded by grant number 5R25TW010026-02 from the Fogarty International Center of the US National Institutes of Health.
Disclosure
The authors report no conflicts of interest in this work.
References
Alderson P, Morrow V. The Ethics of Research with Children and Young People: A Practical Handbook. Thousand Oaks (CA): Sage Publications Ltd; 2011. | ||
Hoberman A, Shaikh N, Bhatnagar S, et al. Factors that influence parental decisions to participate in clinical research: consenters vs nonconsenters. JAMA Pediatr. 2013;167(6):561–566. | ||
Wolfenden L, Kypri K, Freund M, Hodder R. Obtaining active parental consent for school-based research: a guide for researchers. Aust N Z J Public Health. 2009;33(3):270–275. | ||
Field MJ, Berman RE. The Ethical Conduct of Clinical Research Involving Children. Washington: National Academies Press; 2004. | ||
Glantz LH. Research with children. Am J Law Med. 1998;24(2–3):213–244. | ||
Sibley A, Pollard AJ, Fitzpatrick R, Sheehan M. Developing a new justification for assent. BMC Med Ethics. 2016;17:2. | ||
Larcher V, Brierley J. Developing guidance for pregnancy testing of adolescents participating in research: ethical, legal and practical considerations. Arch Dis Child. 2016;101(10):980–983. | ||
De Lourdes Levy M, Larcher V, Kurz R; Ethics Working Group of the Confederation of European Specialists in Paediatrics (CESP). Informed consent/assent in children. Statement of the ethics Working Group of the Confederation of European specialists in paediatrics (CESP). Eur J Pediatr. 2003;162(9):629–633. | ||
International Ethical Guidelines for Health-related Research Involving Humans, Fourth Edition. Geneva. Council for International Organizations of Medical Sciences (CIOMS); 2016. | ||
van Delden JJ, van der Graaf R. Revised CIOMS international ethical guidelines for health-related research involving humans. JAMA. 2017;317(2):135–136. | ||
WMA Declaration of Helsinki. [webpage on the Internet] WMA Declaration of Helsinki – ethical principles for medical research involving human subjects. 2018. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed January 25, 2019. | ||
Allegri RF, Guekht A. Cerebrolysin improves symptoms and delays progression in patients with Alzheimer’s disease and vascular dementia. Drugs Today (Barc). 2012;48(Suppl A):25–41. | ||
Okello G, Jones C, Bonareri M, et al. Challenges for consent and community engagement in the conduct of cluster randomized trial among school children in low income settings: experiences from Kenya. Trials. 2013;14(1):142. | ||
Mystakidou K, Panagiotou I, Katsaragakis S, Tsilika E, Parpa E. Ethical and practical challenges in implementing informed consent in HIV/AIDS clinical trials in developing or resource-limited countries. SAHARA J. 2009;6(2):46–57. | ||
Alahmad G, Al Jumah M, Dierickx K. Confidentiality, informed consent, and children’s participation in research involving stored tissue samples: interviews with medical professionals from the Middle East. Narrat Inq Bioeth. 2015;5(1):53–66. | ||
Kandeel N, El-Nemer A, Ali NM, et al. A multicenter study of the awareness and attitudes of Egyptian faculty towards research ethics: a pilot study. J Empir Res Hum Res Ethics. 2011;6(4):99–108. | ||
Alahmad GH, Dierickx K. Confidentiality, informed consent and children’s participation in the Saudi Biobank governance: a comparative study. East Mediterr Health J. 2014;20(11):681–689. | ||
Grootens-Wiegers P, Hein IM, van den Broek JM, de Vries MC. Medical decision-making in children and adolescents: developmental and neuroscientific aspects. BMC Pediatr. 2017;17(1):120. | ||
Madden L, Shilling V, Woolfall K, et al. Questioning assent: how are children’s views included as families make decisions about clinical trials? Child Care Health Dev. 2016;42(6):900–908. | ||
Mayer RE, Moreno R. Nine ways to reduce cognitive load in multimedia learning. Educational Psychologist. 2003;38(1):43–52. | ||
National Institutes of Health. National Cancer Institute [homepage on the Internet]. Children’s assent to clinical trial participation. 2005. Available from: https://www.cancer.gov/about-cancer/treatment/clinical-trials/patient-safety/childrens-assent. Accessed January 25, 2019. | ||
Beckett C, Taylor H. Human Growth and Development. Thousand Oaks (CA): Sage Publications Ltd; 2016. | ||
Matza LS, Patrick DL, Riley AW, et al. Pediatric patient-reported outcome instruments for research to support medical product labeling: report of the ISPOR pro good research practices for the assessment of children and adolescents Task Force. Value Health. 2013;16(4):461–479. | ||
Doyal L. Informed consent: moral necessity or illusion? Qual Health Care. 2001;10(Supp 1):i29–i33. | ||
Vollmann J, Winau R. Informed consent in human experimentation before the Nuremberg Code. BMJ. 1996;313(7070):1445–1447. | ||
Assaad R, Roudi-Fahimi F. Youth in the Middle East and North Africa: Demographic Opportunity or Challenge. Washington, DC: Population Reference Bureau; 2007. | ||
Grady C, Wiener L, Abdoler E, et al. Assent in research: the voices of adolescents. J Adolesc Health. 2014;54(5):515–520. | ||
Roper L, Sherratt FC, Young B, et al. Children’s views on research without prior consent in emergency situations: a UK qualitative study. BMJ Open. 2018;8(6):e022894. | ||
Swartling U, Helgesson G, Ludvigsson J, Hansson MG, Nordgren A. Children’s views on long-term screening for type 1 diabetes. J Empir Res Hum Res Ethics. 2014;9(4):1–9. | ||
Holloway I. Basic Concepts for Qualitative Research. Oxford: Blackwell Science; 1997. | ||
Breen RL. A practical guide to focus-group research. J Geograp High Educ. 2006;30(3):463–475. | ||
Punch KF. Introduction to Social Research: Quantitative and Qualitative Approaches. Thousand Oaks (CA): Sage Publications Ltd; 2013. | ||
Tobin GA, Begley CM. Methodological rigour within a qualitative framework. J Adv Nurs. 2004;48(4):388–396. | ||
Mobasher M, Salari P, Larijani B. Key ethical issues in pediatric research: Islamic perspective, Iranian experience. Iran J Pediatr. 2012;22(4):435–444. | ||
Alahmad G, Dierickx K. Pediatric research ethics: Islamic perspectives. Br J Med Medic Res. 2015;5(9):1158–1168. | ||
Marzouk D, Abd El Aal W, Saleh A, et al. Overview on health research ethics in Egypt and North Africa. Eur J Public Health. 2014;24(Suppl 1):87–91. | ||
Alahmad G, Al-Jumah M, Dierickx K. Review of national research ethics regulations and guidelines in Middle Eastern Arab countries. BMC Med Ethics. 2012;13(1):34. | ||
Treish IM. Clinical research law in Jordan. Dev World Bioeth. 2009;9(2):97. | ||
Ramahi I, Silverman H. Clinical research law in Jordan: an ethical analysis. Dev World Bioeth. 2009;9(1):26–33. | ||
Macrae DJ. The Council for International Organizations and Medical Sciences (CIOMS) guidelines on ethics of clinical trials. Proc Am Thorac Soc. 2007;4(2):176–179. | ||
Sachs B. Going from principles to rules in research ethics. Bioethics. 2011;25(1):9–20. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
