Back to Journals » International Journal of General Medicine » Volume 16
Recognition and Management of Hospital-Acquired Sepsis Among Older General Medical Inpatients: A Multi-Site Retrospective Study
Authors Barker N, Scott IA , Seaton R , Mehta N, Kalke VR, Redpath L
Received 9 December 2022
Accepted for publication 28 February 2023
Published 21 March 2023 Volume 2023:16 Pages 1039—1046
DOI https://doi.org/10.2147/IJGM.S400839
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Nicholas Barker,1 Ian A Scott,1 Robert Seaton,2 Naitik Mehta,2 Vikrant R Kalke,2 Lyndell Redpath2
1Department of Internal Medicine and Clinical Epidemiology, Princess Alexandra Hospital, Brisbane, Queensland, Australia; 2Patient Quality and Safety Improvement Service, Queensland Health, Brisbane, Australia
Correspondence: Ian A Scott, Department of Internal Medicine and Clinical Epidemiology, Princess Alexandra Hospital, 199 Ipswich Road, Brisbane, 4102, Australia, Tel +61-7-31767355, Fax +61-7-31765214, Email [email protected]
Purpose: To assess accuracy of early diagnosis, appropriateness and timeliness of response, and clinical outcomes of older general medical inpatients with hospital-acquired sepsis.
Methods: Hospital abstracts of inpatient encounters from seven digital Queensland public hospitals between July 2018 and September 2020 were screened retrospectively for diagnoses of hospital-acquired sepsis. Electronic medical records were retrieved and cases meeting selection criteria and classified as confirmed or probable sepsis using pre-specified criteria were included. Investigations and treatments following the first digitally generated alert of clinical deterioration were compared with a best practice sepsis care bundle. Outcome measures comprised 30-day all-cause mortality after deterioration, and unplanned readmissions at 14 days after discharge.
Results: Of the 169 screened care episodes, 59 comprised probable or confirmed cases of sepsis treated by general medicine teams at the time of initial deterioration. Of these, 43 (72.9%) had no mention of sepsis in the differential diagnosis on first medical review, and only 38 (64%) were managed as having sepsis. Each care bundle component of blood cultures, serum lactate, and intravenous fluid resuscitation and antibiotics was only delivered in approximately 30% of cases, and antibiotic administration was delayed more than an hour in 28 of 38 (73.7%) cases.
Conclusion: Early recognition of sepsis and timely implementation of care bundles are challenging in older general medical patients. Education programs in sepsis care standards targeting nurses and junior medical staff, closer patient monitoring, and post-discharge follow-up may improve patient outcomes.
Keywords: early recognition, clinical deterioration, management, care bundle, mortality, readmissions, older adults, frailty
Plain Language Summary
Serious infections in hospitalised patients can be life-threatening unless recognised in a timely fashion and treated with antibiotics and fluid replacement. This study showed that among a group of older general medical patients whose condition was deteriorating and who were eventually proven as having a serious infection, the diagnosis was missed or delayed, and appropriate treatments were not administered in more than two thirds of the group. In older frail patients, the possibility of serious infection must be considered and appropriate care provided when their condition unexpectedly worsens.
Introduction
Despite advances in modern medicine, hospital-acquired sepsis remains a major cause of morbidity and mortality.1 Sepsis constitutes a severe inflammatory reaction to an infectious organism, which causes vital organ dysfunction, and can present in almost any clinical setting and across all ages.2 Undetected and untreated sepsis can quickly lead to major organ dysfunction, resulting in death in 15% to 35% of patients.1 Symptoms and signs of early onset sepsis are variable, insidious and non-specific, delaying its recognition and management and predisposing to poor outcomes.3 This challenge is accentuated in older people in whom commonly recognised clinical features of sepsis, including fever, raised white cell count or elevated C-reactive protein are less frequent, underlying chronic organ dysfunction is common,4 and risk factors of relative immunosuppression, malnutrition and frailty are prevalent.5,6
The multiple, evolving definitions of sepsis2 underscore the difficulty in accurately delineating its features, as evidenced by several tools and algorithms aimed at its early recognition.7,8 Sepsis is a clinical diagnosis, with no physical signs or biomarkers that are diagnostic, and clinical features that overlap with those of other conditions such as major bleeding, myocardial infarction, and pulmonary embolus.9 Consequently, misdiagnosis is common, prompting education programs, implementation of sepsis pathways with care bundles, and national sepsis care standards aimed at improving early diagnosis and management.10
The extent to which older, multi-morbid patients are more vulnerable to delayed recognition and/or management of sepsis, their risk factors for sepsis, and their clinical outcomes, are uncertain. We therefore undertook a review of the early recognition, management and outcomes of sepsis in a cohort of older adult general medical inpatients with probable or confirmed sepsis.
Methods
Study Design
This was a retrospective cohort study of patients admitted acutely to seven public hospitals in Queensland over a two-year period who, at discharge, were coded as having an episode of hospital-acquired sepsis.
Participants and Setting
The Queensland Hospital Admitted Patient Data Collection (QHAPDC), a government data repository of coded inpatient information obtained after discharge or death, was retrospectively screened on November 5, 2020, for discharge abstracts from the seven participating digital hospitals of episodes of care between July 2018 and September 2020 that met the following criteria:
- contained any one of 45 pre-specified “sepsis” codes within the International Classification of Diseases, version 10, Australian Modification (ICD-10-AM) (see eAppendix 1), with a condition onset flag of hospital acquired (and no other onset);
- adult (age >18 years) inpatients;
- admitted on presentation to general medicine units;
- not an inbound interhospital transfer;
- not admitted for terminal palliative care.
Electronic medical records (EMRs) of positively screened patients were retrieved and subject to independent review by a single investigator (NB) and cross-checked by a second investigator (IS), neither of whom were involved in the care of any patient. Records were interrogated to find the time at which clinical deterioration due to possible sepsis met criteria, as recognised by nursing staff, for urgent review by treating teams or, if these teams were unavailable, activation of medical emergency teams (MET). The EMR-embedded systems used for detecting deterioration comprised the Between the Flags (BTF) system11 in one hospital and the Queensland Adult Detection of Deterioration System (QADDS)12 in the remaining hospitals (see eAppendix 2).
Verification of Sepsis
In verifying discharge codes of hospital-acquired sepsis, and to account for variables within the Sepsis-3 definition2 that were not consistently performed or measured at the time of deterioration (serum bilirubin, blood cultures, antibiotics), we pragmatically applied quick Sepsis-Related Organ Failure Assessment (qSOFA) criteria (respiratory rate [RR] ≥ 22 breaths/min, Glasgow Coma Scale [GCS] < 15), and systolic blood pressure < 100 mmHg) and Systemic Inflammatory Response Syndrome (SIRS) criteria (heart rate >90 beats/min, RR > 20 breaths/min), temperature >38 or <36 °C, white blood cells <4000/mm3 or >1200/mm3 or bandemia ≥10%)2 at the time of clinical deterioration. Sepsis was considered probable or confirmed by consensus of two investigators (NB, IS) if two or more of the preceding criteria were met, no alternative non-sepsis diagnoses was confirmed by investigations performed within 3 hours following the alert, and one or more of the following criteria were met: elevated serum lactate; positive microbiological cultures; laboratory investigations confirming a definite source of infection.
Early Recognition of Possible Sepsis
In maximising sensitivity, sepsis was considered possible at the time of clinical deterioration if two or more of the following criteria were met: (i) RR ≥ 20 breaths/min; (ii) heart rate ≥ 100 beats/min; (iii) oxygen saturations ≤ 90%; (iv) temperature ≥ 38°C or ≤ 36°C; (v) white cell count <4000/mm3 or >1200/mm3; and (vi) microbiological samples were taken by treating clinicians.
Ascertainment of Risk Factors
Premorbid risk factors for sepsis were ascertained as: (i) indwelling medical device; (ii) Indigenous and Pacific islander status;13 (iii) immunocompromised state, defined as asplenia, neutropenia, alcohol abuse; (iv) recent trauma or surgery within previous 6 weeks; and (v) malnourishment, as indicated by serum albumen <30g/L. The following co-morbidities were also recorded: asthma/chronic obstructive pulmonary disease (COPD), heart failure, diabetes, chronic kidney disease (defined as baseline estimated glomerular filtration rate [eGFR] <60mL/min), hypertension, active malignancy, ischaemic heart disease, cerebrovascular disease, peripheral vascular disease, and dementia.4 Frailty was retrospectively measured on admission and prior to discharge or death using the Rockwood Clinic Frailty Score, based on documented assessments of allied health, medical and nursing staff.14
Adherence to Sepsis Care Bundle
In cases of probable or confirmed sepsis, the extent to which all components of a recommended sepsis care bundle were enacted, and in recommended timeframes, following initial deterioration was ascertained. The care bundle15 comprised: measurement of serum lactate, taking of two sets of blood cultures, and administration of intravenous antibiotics and fluids (20mL/kg bolus if systolic blood pressure <90mmHg). The recommended timeframe for care bundle implementation was within 30 minutes of suspected meningococcal or neutropenic sepsis, 60 minutes for patients in septic shock, and 3 hours for patients with sepsis otherwise defined.15
Clinical Outcomes
The primary clinical end-points comprised all-cause death, either in-hospital or within 30 days of discharge, and unplanned all-cause readmission within 14 days of discharge. Readmission and in-hospital mortality data were sourced from QHAPDC; out of hospital deaths were captured by probabilistic linkage of QHAPDC with Queensland Death Registry data based on patient variables (eg name, date of birth, address), and associated with high (99.9%) accuracy.
Data Collection and Analysis
Extracted data were entered into a secure online database, Research Electronic Data Capture (REDCap, Vanderbilt University, USA) using a standardised, pilot tested template. Descriptive statistics used proportions and means with standard deviations; group comparisons were made using chi-square and Students t-test respectively, with 2-sided p values <0.05 denoting statistical significance. The study findings are reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.16
Ethical Approval and Funding
The study was approved by The Prince Charles Hospital Human Research Ethics Committee, Prince Charles Hospital, Brisbane: Project ID: 50589-Queensland Sepsis Collaborative. It constituted a sub-study of a larger Queensland Sepsis Collaborative study into the effects of implementing a sepsis clinical pathway into Queensland public hospitals using routinely collected data obtained from medical records of completed episodes of care.17 This primary study was deemed a quality improvement study with no reporting of identifiable information, and a waiver of informed patient consent was approved by the Metro North Human Research Ethics Committee (LNR/2019/QPCH/5089) on behalf of all participating hospitals, including the Prince Charles Hospital situated in the Metro North Hospital and Health Service, and ratified by its own ethics committee. The Collaborative decided to undertake a further study of the care of older patients with discharge code of hospital-acquired sepsis who were admitted to general medicine wards during the same study period, and approval was granted from the Prince Charles Hospital Ethics Committee to add the current study as a supplement to the original Collaborative study protocol and need for patient consent was again waivered. The study complies with the Declaration of Helsinki and this research did not receive any specific funding.
Results
Screening of discharge abstracts identified 169 episodes of care involving 168 unique patients. One hundred and ten episodes were excluded due to incomplete records (n = 43), not meeting any criteria for probable or confirmed sepsis (n = 34), and general medicine not being the treating team at the time of clinical deterioration (n = 33). The remaining 59 episodes met the definition of probable or confirmed sepsis and were used for analysis.
Patient Characteristics
Mean age was 74.3 (± 12.6) years, most cases (42/59; 71.2%) were male, all had one or more co-morbidities, almost half (26/59; 44.1%) were malnourished, and one in ten had another risk factor for sepsis (Table 1).
 |
Table 1 Patient Characteristics (n = 59) |
Source of Sepsis
The source of probable or confirmed sepsis was pneumonia (n = 26), urinary tract infection (n = 15), cellulitis (n = 10), intra-abdominal abscess (n = 6), and unknown (n = 2). Microbiological cultures were positive in 18 (30.5%) cases, negative in 19 cases (32.2%), contaminated in 2 cases (3.4%) and not performed in 20 cases (33.8%).
Screening Criteria for Sepsis
Applying a score of 2 or more, the SIRS criteria were more sensitive than qSOFA criteria in identifying patients with confirmed sepsis (96.7% vs 25.4%; p < 0.001, Table 2). As this was a study of probable or confirmed sepsis cases only, specificity for both criteria were not able to be calculated.
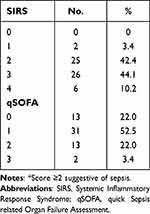 |
Table 2 Scores for SIRS and qSOFA Sepsis Screening Tools Applied at the Initial Deterioration Alert* |
Recognition of Possible Sepsis
In response to clinical deterioration alerts, in 17 (28.8%) cases a MET was activated; in 27 (45.8%) cases, an urgent clinical review was undertaken by the treating medical team; and in 15 (25.4%) cases no action was immediately taken by either nursing or medical staff.
At the time of the alert, sepsis was recorded as the provisional diagnosis in 15 (25.4%) cases, listed in the differential diagnosis for one (1.7%) case and not mentioned in the remaining 43 cases (72.8%).
Adherence to Sepsis Care Bundle
At the time of clinical deterioration, serum lactate levels were measured in 19 (32.2%) cases (Table 3), of which most (13/19; 68.4%) were elevated. Only 18 (30.5%) cases received an intravenous fluid bolus or had two blood cultures taken. Intravenous antibiotics were ordered at the time of initial deterioration in 38 (64.4%) patients, although in 9 of these cases (23.7%), administration was delayed until 3 hours or more after the alert. The most common recorded reasons in explaining the delay in administering antibiotics of more than 60 minutes were a nursing schedule conflict after the order had been entered or delays in patient review by medical teams.
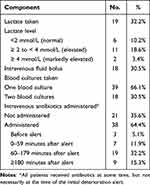 |
Table 3 Adherence to Each Component of the Sepsis Care Bundle at the Initial Deterioration Alert |
Clinical Outcomes
Of the 59 cases, 18 (30.5%) died in the admitting hospital and 8 subsequently died within 30 days of discharge, yielding a total all-cause mortality rate of 44.1%. Three (5.1%) cases required ICU admission for severe sepsis or septic shock, with one requiring inotropic support. Of the 41 cases discharged alive, 8 (19.5%) had a subsequent unplanned readmission within the following 14 days, due to urinary retention (1), heart failure (2), delirium (1), gastrointestinal upset (1), headache (1), syncope (1) and arrhythmia (1). Frailty scores showed an overall decline in function between admission and discharge, with mean (± SD) scores rising significantly, indicating increasing frailty, from 4.9 (± 1.6) to 6.7 (±2.1, p < 0.001).
Discussion
In this audit of older general medical inpatients with hospital-acquired sepsis, at the time of initial clinical deterioration, sepsis was not considered as a possible cause in almost three quarters of cases. Even when it was, adherence to each non-antibiotic component of the sepsis care bundle was less than 33%. No antibiotic was given within recommended timeframes in just over a third of cases, due to delays in patient review or nurse administration. In those in whom serum lactate was measured, such measurements were taken only once. While current guidelines do not recommend repeat measurements,3 recent studies suggest serum lactate clearance, which requires serial measurements, is a prognostic indicator in severe sepsis.18 SIRS criteria also proved more sensitive in identifying septic patients than qSOFA criteria, as has been reported by others,2,7 although the former rely on a definition of ‘severe sepsis’ which limits their sensitivity.
The under-recognition and under-treatment of sepsis in hospitalised patients is well recognised internationally,19 and likely accentuated in older, frail, multi-morbid general medical patients whose baseline observations are usually closer to the boundaries of normalcy4 and which obscures early sepsis recognition. Other contributing factors may include staffing of general medicine units by inexperienced junior medical clinicians, lack of awareness of the various components of the sepsis care bundle, variability in sepsis identification strategies, work pressures and competing priorities for nursing staff, and concerns about excessive antimicrobial use.10
Australian studies suggest sepsis pathways implemented across multiple hospitals can significantly (p < 0.05) improve care and outcomes. Safer Care Victoria’s “Think sepsis. Act fast” collaboration reduced inpatient sepsis-related mortality by 50%, from 11.4% to 5.8%.20 The New South Wales Clinical Excellence Commission’s SEPSIS KILLS! Program increased administration of intravenous antimicrobials within 60 minutes of triage from 29.3% in 2009–2011 to 52.2% in 2013.21 The Clinical Excellence Queensland’s Queensland Sepsis Program – Could this be sepsis? increased antibiotic administration within 1-hour for septic shock cases (85% vs 74%), improved 3-hour bundle compliance (63% vs 48%), and reduced ICU admissions (17.5% vs 26.5%).17 A nurse-initiated sepsis pathway in a tertiary oncology unit increased serum lactate measurements (75.0% vs 17.2%), halved the time to antibiotics (55 vs 110 min), reduced ICU admissions (17.1% vs 35.5%) and decreased sepsis-related mortality (5.0% vs 16.2%).22 These studies complement others performed internationally which support sepsis pathways in improving sepsis care compliance and patient outcomes.23 Importantly, these studies have not reported an increase in inappropriate antibiotic use, despite limitations in the definition and duration of time frames for antibiotic administration.24
We have no reason to believe that such initiatives cannot improve sepsis recognition and management in older, multi-morbid patients, and our findings underscore the need for more sepsis-specific education campaigns and implementation of sepsis pathways which target this vulnerable population. Automated, EMR-embedded machine-learning algorithms that utilise continuous monitoring of vital signs, blood parameters and other patient data have been shown to accurately predict sepsis onset many hours prior to clinical deterioration and may afford a longer time window for earlier, more effective intervention.25 Similarly, point of care biosensors may hold promise for earlier diagnosis of sepsis.26 Senior clinician champions capable of influencing practice norms, nurses empowered to implement sepsis screening strategies and generate alerts, and regular benchmarked feedback on care compliance and patient outcomes can also improve performance.10 Finally, the high readmission rate and our novel finding of sepsis-induced accentuation of frailty suggests the need for closer post-discharge follow-up of older patients and, where appropriate, referral to rehabilitation services.27 Following a septic episode, older patients develop an average of one or two new functional limitations, including cognitive impairment,28 and have a higher risk of rehospitalisation and death compared to matched controls.29
Our study has several limitations. The use of ICD-10-AM sepsis codes to identify the study sample may have missed cases that were not coded as sepsis, and the exclusion of 34 cases incorrectly coded as having sepsis as the discharge diagnosis, 33 cases for incorrect assignment of the treating specialty, and 43 cases for incomplete medical records underscores the inaccuracy of current coding practices and inadequacy of medical documentation, both recognised challenges for retrospective, record-based research in sepsis.30 The resulting small sample taken from only seven hospitals may not represent practice involving older medical inpatients in all Queensland public hospitals. Verification of probable or confirmed sepsis could not be performed using Sepsis-3 criteria as the reference standard, and instead relied on more limited criteria which may have resulted in some missed cases of sepsis. We did not collect data on the existence of formal resuscitation plans but excluded palliative care patients and noted all patients, or their substitute decision makers, indicated a desire for intravenous therapy, irrespective of preferences for cardiopulmonary resuscitation or invasive life-prolonging therapies. However, in an older adult living with advanced frailty, there may be good reasons why the clinical team, in consultation with the patient, decides not to rigidly follow a sepsis care bundle. Study strengths included measurement of risk factors, care processes and key clinical outcomes, including changes in frailty status.
In conclusion, there would appear to be room for improvement in every step of recognising and managing hospital-acquired sepsis among older general medical inpatients in Queensland public hospitals.
Data Sharing Statement
All data in the REDcap database are the property of Queensland Health and are unavailable for public release.
Funding
There was no funding for this study.
Disclosure
All authors have no conflicts of interest in this work.
References
1. Fleischmann C, Mellhammar L, Rose N, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1552–1562. doi:10.1007/s00134-020-06151-x
2. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
3. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi:10.1007/s00134-017-4683-6
4. Yang Y, Yang KS, Hsann YM, Lim V, Ong BC. The effect of comorbidity and age on hospital mortality and length of stay in patients with sepsis. J Crit Care. 2010;25(3):398–405. doi:10.1016/j.jcrc.2009.09.001
5. Fernando SM, McIsaac DI, Perry JJ, et al. Frailty and associated outcomes and resource utilization among older ICU patients with suspected infection. Crit Care Med. 2019;47(8):e669–e76. doi:10.1097/CCM.0000000000003831
6. Abugroun A, Nayyar A, Abdel-Rahman M, Patel P. Impact of malnutrition on hospitalization outcomes for older adults admitted for sepsis. Am J Med. 2021;134(2):221–226.e1. doi:10.1016/j.amjmed.2020.06.044
7. Fernando SM, Tran A, Taljaard M, et al. Prognostic accuracy of the quick sequential organ failure assessment for mortality in patients with suspected infection: a systematic review and meta-analysis. Ann Intern Med. 2018;168(4):266–275. doi:10.7326/M17-2820
8. Alberto L, Marshall AP, Walker R, Aitken LM. Screening for sepsis in general hospitalized patients: a systematic review. J Hosp Infect. 2017;96(4):305–315. doi:10.1016/j.jhin.2017.05.005
9. Ljungstrom L, Pernestig AK, Jacobsson G, Andersson R, Usener B, Tilevik D. Diagnostic accuracy of procalcitonin, neutrophil-lymphocyte count ratio, C-reactive protein, and lactate in patients with suspected bacterial sepsis. PLoS One. 2017;12(7):e0181704. doi:10.1371/journal.pone.0181704
10. Schorr C, Odden A, Evans L, et al. Implementation of a multicenter performance improvement program for early detection and treatment of severe sepsis in general medical-surgical wards. J Hosp Med. 2016;11(Suppl 1):S32–S9. doi:10.1002/jhm.2656
11. Hughes C, Pain C, Braithwaite J, et al. Between the flags: implementing a rapid response system at scale. BMJ Qual Saf. 2014;23(9):714–717. doi:10.1136/bmjqs-2014-002845
12. Campbell V, Conway R, Carey K, et al. Predicting clinical deterioration with Q-ADDS compared to NEWS, between the flags, and eCART track and trigger tools. Resuscitation. 2020;153:28–34. doi:10.1016/j.resuscitation.2020.05.027
13. Davis JS, Cheng AC, McMillan M, Humphrey AB, Stephens DP, Anstey NM. Sepsis in the tropical top end of Australia’s Northern Territory: disease burden and impact on indigenous Australians. Med J Aust. 2011;194(10):519–524. doi:10.5694/j.1326-5377.2011.tb03088.x
14. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi:10.1503/cmaj.050051
15. Queensland Department of Health. Emergency department non-pregnant adult sepsis pathway; 2021.
16. von Elm E, Altman DG, Egger M, et al. The strengthening of the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi:10.1136/bmj.39335.541782.AD
17. Venkatesh B, Schlapbach L, Mason D, et al. Impact of 1-hour and 3-hour sepsis time bundles on patient outcomes and antimicrobial use: a before and after cohort study. Lancet Glob Health. 2022;18:100305. doi:10.1016/j.lanwpc.2021.100305
18. Lee SG, Song J, Park DW, et al. Prognostic value of lactate levels and lactate clearance in sepsis and septic shock with initial hyperlactatemia: a retrospective cohort study according to the Sepsis-3 definitions. Medicine. 2021;100(7):e24835. doi:10.1097/MD.0000000000024835
19. Rhodes A, Phillips G, Beale R, et al. The surviving sepsis campaign bundles and outcome: results from the international multicentre prevalence study on sepsis (the IMPreSS study). Intensive Care Med. 2015;41(9):1620–1628. doi:10.1007/s00134-015-3906-y
20. Sykes K, Thursky K, Travis D, et al. Program evaluation 2017–18 ‘Think sepsis. Act fast.’ scaling collaboration. Melbourne: SaferCare Victoria; 2019. Available from: www.safercare.vic.gov.au.
21. Burrell AR, McLaws ML, Fullick M, Sullivan RB, Sindhusake D. SEPSIS KILLS: early intervention saves lives. Med J Aust. 2016;204(2):73. doi:10.5694/mja15.00657
22. Thursky K, Lingaratnam S, Jayarajan J, et al. Implementation of a whole of hospital sepsis clinical pathway in a cancer hospital: impact on sepsis management, outcomes and costs. BMJ Open Qual. 2018;7(3):e000355. doi:10.1136/bmjoq-2018-000355
23. Damiani E, Donati A, Serafini G, et al. Effect of performance improvement programs on compliance with sepsis bundles and mortality: a systematic review and meta-analysis of observational studies. PLoS One. 2015;10(5):e0125827. doi:10.1371/journal.pone.0125827
24. Weinberger J, Rhee C, Klompas M. A critical analysis of the literature on time-to-antibiotics in suspected sepsis. J Infect Dis. 2020;222(Suppl 2):S110–8. doi:10.1093/infdis/jiaa146
25. Shimabukuro DW, Barton CW, Feldman MD, Mataraso SJ, Das R. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
26. Bonini A, Carota AG, Poma N, et al. Emerging biosensing technologies towards early sepsis diagnosis and management. Biosensors. 2022;12:894. doi:10.3390/bios12100894
27. Lee HY, Lee J, Jung YS, et al. Korean Sepsis Alliance (KSA) Investigators: preexisting clinical frailty is associated with worse clinical outcomes in patients with sepsis. Crit Care Med. 2022;50(5):780–790. doi:10.1097/CCM.0000000000005360
28. Prescott HC, Iwashyna TJ, Blackwood B, et al. Understanding and enhancing sepsis survivorship. priorities for research and practice. Am J Respir Crit Care Med. 2019;200(8):972–981. doi:10.1164/rccm.201812-2383CP
29. Farrah K, McIntyre L, Talarico R, Coyle D, Thavorn K. Health outcomes and health system costs associated with sepsis: a population-based, retrospective cohort study. Value Health. 2019;22:S663. doi:10.1016/j.jval.2019.09.1393
30. Rhee C, Murphy MV, Li L, et al. Comparison of trends in sepsis incidence and coding using administrative claims versus objective clinical data. Clin Infect Dis. 2015;60:88–95. doi:10.1093/cid/ciu750
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
