Back to Journals » Cancer Management and Research » Volume 15
Real-World Treatment and Outcomes of ALK-Positive Metastatic Non–Small Cell Lung Cancer in a Southeast Asian Country
Authors Poh ME , How SH, Ho GF , Pang YK , Hasbullah HH, Tho LM, Muhamad Nor I, Lim BC , Ho KF, Thiagarajan M, Samsudin A, Omar A, Ong CK, Soon SY, Tan JYK, Zainal Abidin MA
Received 18 October 2022
Accepted for publication 28 December 2022
Published 13 January 2023 Volume 2023:15 Pages 31—41
DOI https://doi.org/10.2147/CMAR.S393729
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Mau Ern Poh,1 Soon Hin How,2,3 Gwo Fuang Ho,4 Yong Kek Pang,1 Harissa H Hasbullah,5,6 Lye Mun Tho,7 Ibtisam Muhamad Nor,6 Bee Chiu Lim,3 Kean Fatt Ho,8 Muthukkumaran Thiagarajan,6 Azlina Samsudin,9 Azza Omar,10 Choo Khoon Ong,11 Sing Yang Soon,12 Justin Yu Kuan Tan,9 Muhammad Adil Zainal Abidin2
1Department of Medicine, Faculty of Medicine, Universiti Malaya, Kuala Lumpur, Malaysia; 2Kulliyyah of Medicine, International Islamic University Malaysia, Kuantan, Pahang, Malaysia; 3Hospital Tengku Ampuan Afzan, Kuantan, Pahang, Malaysia; 4Clinical Oncology Unit, Faculty of Medicine, Universiti Malaya, Kuala Lumpur, Malaysia; 5Faculty of Medicine, Universiti Teknologi Mara, Sungai Buloh, Selangor, Malaysia; 6Oncology and Radiotherapy Department, General Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; 7Department of Clinical Oncology, Beacon Hospital, Petaling Jaya, Selangor, Malaysia; 8Mount Miriam Cancer Hospital, Tanjong Bungah, Penang, Malaysia; 9Hospital Sultanah Nur Zahirah, Kuala Terengganu, Terengganu, Malaysia; 10Respiratory Unit, Medical Department, Hospital Raja Perempuan Zainab II, Kota Bharu, Kelantan, Malaysia; 11Gleneagles Penang, George Town, Penang, Malaysia; 12Sarawak Heart Centre, Kuching, Sarawak, Malaysia
Correspondence: Mau Ern Poh, Department of Medicine, Faculty of Medicine, Universiti Malaya, Kuala Lumpur, 50603, Malaysia, Tel +60-3-7841-4000, Email [email protected]
Purpose: Anaplastic lymphoma kinase (ALK) inhibitors are associated with good overall survival (OS) for ALK-positive metastatic non–small cell lung cancer (NSCLC). However, these treatments can be unavailable or limited by financial constraints in developing countries. Using data from a nationwide lung cancer registry, the present study aimed to identify treatment patterns and clinical outcomes of ALK-positive NSCLC in Malaysia.
Methods: This retrospective study examined data of patients with ALK-positive NSCLC from 18 major hospitals (public, private, or university teaching hospitals) throughout Malaysia between January 1, 2015 and December 31, 2020 from the National Cardiovascular and Thoracic Surgical Database (NCTSD). Data on baseline characteristics, treatments, radiological findings, and pathological findings were collected. Overall survival (OS) and time on treatment (TOT) were calculated using the Kaplan–Meier method.
Results: There were 1581 NSCLC patients in the NCTSD. Based on ALK gene-rearrangement test results, only 65 patients (4.1%) had ALK-positive advanced NSCLC. Of these 65 patients, 59 received standard-of-care treatment and were included in the analysis. Crizotinib was the most commonly prescribed ALK inhibitor, followed by alectinib and ceritinib. Patients on ALK inhibitors had better median OS (62 months for first-generation inhibitors, not reached at time of analysis for second-generation inhibitors) compared to chemotherapy (27 months), but this was not statistically significant (P=0.835) due to sample-size limitations. Patients who received ALK inhibitors as first-line therapy had significantly longer TOT (median of 11 months for first-generation inhibitors, not reached for second-generation inhibitors at the time of analysis) compared to chemotherapy (median of 2 months; P< 0.01).
Conclusion: Patients on ALK inhibitors had longer median OS and significantly longer TOT compared to chemotherapy, suggesting long-term benefit.
Keywords: ALK inhibitors, chemotherapy, ALK-positive, NSCLC
Introduction
ALK gene translocation is a common genetic aberration in patients with lung cancer.1 Studies in both Western and Asian populations have revealed that about 4%–5% of non–small cell lung cancer (NSCLC) patients harbor ALK translocations.1–7 In one Asian study, the prevalence of ALK translocations in NSCLC patients was up to 6.7% of NSCLC patients.1,2 Patients who harbor ALK translocations are likely to be young (<50 years old), female, nonsmokers, or have adenocarcinoma.8
ALK genetic aberrations are diagnosed with tumor-cell testing via immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), reverse-transcription polymerase chain reaction, or fusion transcription (RNA next-generation sequencing).6,9–11 While FISH is the gold-standard test for detecting ALK rearrangements, IHC is cost-effective and easier to incorporate into the routine laboratory workflow.12 A local study showed that IHC is a reliable and practical test for ALK expression.13 The average turnaround time for FISH is 2–5 days, while the turnaround time for IHC is 1–2 days.14
Patients with ALK translocations are increasingly being treated with targeted therapies (ALK inhibitors). Crizotinib, a first-generation ALK inhibitor, was approved by the US Food and Drug Administration (FDA) in 2011, by the European Medicines Agency (EMA) in 2012, and in Malaysia in 2012. Ceritinib, a second-generation ALK inhibitor, was approved by the FDA in 2014, by the EMA in 2015, and in Malaysia in 2016. The FDA approved alectinib, another second-generation ALK inhibitor in 2015; while in Malaysia, alectinib was approved in 2018. These ALK inhibitors are associated with significantly better progression-free survival (PFS) than chemotherapy, with second-generation ALK inhibitors having significantly better PFS than first-generation inhibitors.15 However, ALK inhibitors can be unavailable or limited by cost constraints in developing countries. A budget analysis from a neighboring country, Thailand, revealed that only affluent patients could afford an ALK inhibitor.16 Even in the USA, the use of these treatments is associated with high economic burden.17 The objectives of this retrospective study were to assess clinical outcomes and treatment patterns among advanced NSCLC patients harboring ALK translocations in Malaysia based on data from a nationwide lung cancer registry.
Methods
Study Design and Data Sources
All procedures were carried out in accordance with relevant guidelines and regulations. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was reviewed and approved by the Universiti Malaya Medical Centre Medical Research Ethics Committee (MECID 20201115–9217). The committee waived the need for informed consent, as patient confidentiality was preserved using identification code numbers. The study was also performed in accordance with the principles stated in the Declaration of Helsinki.
This retrospective study used the National Cardiovascular and Thoracic Surgical Database (NCTSD), a nationwide hospital database that captures detailed information on the diagnosis and management of adult lung cancer patients (age ≥18 years) in Malaysia with a diagnosis of primary lung cancer (confirmed cytologically or histologically). Eighteen major hospitals throughout Malaysia, included public, university, and private hospitals, contribute data to the registry via an electronic case report form (eCRF). The eCRF was developed by the participating investigators, who consisted of oncologists, surgeons and respiratory physicians. Each source-data provider accessed its own data, and only authorized users (investigator or other members of the study team) were allowed to enter data remotely at their site via a web application. All data were de-identified to protect patients’ privacy.
For this study, patients from the registry with advanced stage IIIB, IIIC, or IV NSCLC harboring ALK translocations were included. Data on diagnosis date, age, sex, ethnicity, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status, staging, histology, radiology findings, pathological findings, and treatment records (including type of drugs, duration of treatment, and line of therapy) were analyzed.
Study Population
In this study, eligible patients were ≥18 years at the time of diagnosis, had advanced stage IIIB, IIIC, or IV NSCLC with positive ALK -rearrangement test results on FISH or IHC, and had been included in the registry database between January 1, 2015 and December 31, 2020. Patients had at least one measurable lesion as per Response Evaluation Criteria in Solid Tumors version 1.1 and ECOG performance-status score of 0–4 with adequate organ function. Staging was done with contrast-enhanced CT of the thorax and whole abdomen with/without bone scan (when indicated) or whole-body FDG-PET/CT. Symptomatic patients were diagnosed using MRI of the central nervous system or according to the practice of the participating hospital. Patients with stage IIIA NSCLC were excluded, as surgery is indicated in this group of patients. The study population consisted of patients in both the public (including the university teaching hospitals) and private institutions.
Genomic Analysis
ALK gene arrangements detected by FISH used the break-apart probe on the ALK gene according to the manufacturer’s instructions and analyzed using a fluorescence microscope equipped with orange, green, and 49,6-diamidino-2-phenylindole filters. If 15% of the tumor cells had a split red and green signal and/or a single red signal, the specimen was considered ALK FISH-positive. ALK gene arrangements were detected by IHC using an IHC detection kit according to the manufacturer’s recommendations for the visualization of the bound primary antibody and analyzed using light microscopy. If the tumor cells had strong granular cytoplasmic brown staining, the specimen was considered ALK-positive. Specific numbers of patients were not captured, as there is a high level of concordance in ALK-positivity detection between IHC and FISH.18
Treatment
All patients in the study received standard-of-care treatment following international guidelines. Patients received at least one line of systemic therapy (chemotherapy or ALK inhibitor).
Outcome Variables
The demographic and clinical characteristics of patients were gathered. Comorbidities included hypertension, diabetes mellitus, and cardiovascular or chronic kidney disease. Type of treatment (chemotherapy or targeted therapy), time on treatment (TOT), disease progression, and overall survival (OS) were assessed. The primary end point was OS, defined as the time from initiation of therapy until death from any cause. The secondary end point was TOT, which was defined as the duration between the first day of therapy to the last day of therapy.
Statistical Analysis
Data were retrieved from the registry and screened for missing values. Any missing data were cross-examined by the site investigator. Data analysis was performed using SPSS version 23. Descriptive data are presented as percentages. Continuous data (age and TOT) are presented as medians. The duration of time is described in months. All P values reported are two-sided and considered significant at the 0.05 threshold. The Kaplan–Meier method was used to estimate the OS and TOT, and the log-rank test was used to test the survival differences between groups. Multivariate analysis was performed using Cox proportional hazard regression to determine the independent prognostic factors affecting survival. P<0.05 was considered statistically significant. Univariate factors that were statistically significant were selected for the final model. HRs are given with 95% CIs.
Results
Baseline Demographic and Clinical Characteristics
There were 1581 NSCLC patients in the NCTSD. Based on ALK gene rearrangement test results (either FISH or IHC), 65 patients (4.1%) had advanced NSCLC with ALK translocations. Of the 65 ALK-positive patients, six were excluded because they died before ALK results were available (n=3), refused treatment (n=2), or the staging was not clear (n=1). A total of 59 patients received standard-of-care treatment and were included in the analysis. The median age of the patients was 55 (24–76) years. A majority (76.7%) were nonsmokers, 59.3% had no comorbidities, and 73.3% had an ECOG score of 0 or 1 (Table 1). Most patients had adenocarcinoma (93.2%) and stage IV disease (88.3%; Table 1).
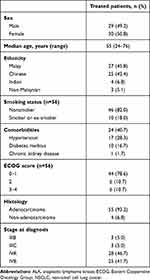 |
Table 1 Baseline demographic and clinical characteristics (n=59) |
Overall Survival
Patients on ALK inhibitors had better median OS (62 months for first-generation inhibitor, not reached for second-generation inhibitors) compared to chemotherapy (27 months; Table 2 and Figures 1 and 2), but this was not statistically significant (P=0.835, Figure 2). On subgroup analysis, patients with ALK-positive adenocarcinoma had significantly longer median OS than those with ALK-positive non-adenocarcinoma (62 months versus 8 months, P<0.05, Table 2). Patients on more than one ALK inhibitor had higher mean OS (57 months) than those who were only on one (26 months; Figure 3). However, this difference was not significant (P=0.079). The OS data for the second-generation ALK inibitors were not mature at the time of primary cutoff, and the median had not been reached.
 |
Table 2 Associations between patient demographic and clinical characteristics and overall survival |
 |
Figure 3 Kaplan–Meier OS curves by number of ALK-inhibitor lines. Abbreviations: ALK, anaplastic lymphoma kinase; OS, overall survival. Notes: Patients were treated with one or more ALK inhibitors. |
Treatment Patterns
Of the 59 treated patients, eleven received first-line chemotherapy (cisplatin and pemetrexed) (Table 3). The remaining 48 patients were started on ALK inhibitors. Crizotinib was the most commonly prescribed ALK inhibitor (39.6%) followed by alectinib (33.3%) and ceritinib (27.1%). At the time of analysis, all eleven patients on first-line chemotherapy had progressed compared to only 19 (39.5%) patients on first-line ALK inhibitors (Table 3). All eleven patients who progressed on first-line chemotherapy went on to receive second-line treatment (Table 3). Approximately half (45.5%) of these patients received chemotherapy again as second-line treatment, while another half received ALK inhibitors.
 |
Table 3 Overall response rate and time on treatment (n=59) |
Majority of patients on first-line crizotinib (94.7%) received a second-generation ALK inhibitor as second-line treatment upon disease progression. At the time of analysis, two patients from this group went on to receive crizotinib as third-line treatment, and one patient received crizotinib as fourth-line treatment. No rebiopsies were performed when disease progressed on an ALK inhibitor.
Patients who received ALK inhibitors as first-line therapy had significantly longer TOT (11 months for first-generation inhibitors and median not reached at the time of analysis for second-generation inhibitors) compared to chemotherapy (2 months; P<0.01; Table 4 and Figure 4). The median TOT for ceritinib and alectinib as first-line therapy was not reached as most of the patients were still on first-line targeted treatment at the time of analysis. Majority of the patients who received alectinib were started on the drug at the end of 2019, when the drug was made available commercially.
 |
Table 4 Associations between patient demographic and clinical characteristics and time on treatment |
Discussion
As there is a scarcity of clinical trial data on the use of ALK inhibitors in countries with limited resources,19 our retrospective data analysis provides real-world insights on treatment and outcomes of NSCLC patients with ALK translocations in a developing country — Malaysia. The median OS was longer for the first-generation ALK inhibitor crizotinib (62 months) compared to chemotherapy (27 months). The median OS for the second-generation ALK inhibitors (ceritinib and alectinib) had not been reached at the end of the study. However, there was no significant difference in the OS curves between the treatment arms (P=0.835). Likewise, in the PROFILE studies, those on the crizotinib arm showed significant improvements in their PFS compared to those on chemotherapy, but there was no difference in OS, which was postulated to have been caused by the crossover of participants between the two study arms at disease progression.15 In our study, 54.5% of patients who failed first-line chemotherapy went on to receive an ALK inhibitor.
In contrast, most patients who progressed on an ALK inhibitor in the first-line setting received another type of ALK inhibitor in the second-line setting instead of chemotherapy. These patients had longer OS than those who had received only one ALK inhibitor. Similarly, another real-world study showed that the use of more than one ALK inhibitor line significantly improved OS.20
Patients on ALK inhibitors in our study had significantly longer TOT than those on chemotherapy, suggesting long-term benefit. In real-world NSCLC studies, TOT or time to treatment discontinuation can be used as a surrogate end point to assess the effectiveness of treatment, as it correlates with PFS and OS (as determined through analysis of randomized controlled trials).21–23 In another real-world study of NSCLC patients with ALK translocations, the median time to treatment failure (due to progression, death, or other reasons) for crizotinib was 10 months.24 TOT for the first-generation ALK inhibitor (crizotinib) was slightly longer, 11 months, as some patients continued treatment with first-line ALK inhibitors despite disease progression. This is partly because a lot of patients prefer oral therapy over chemotherapy and second-generation ALK inhibitors were more expensive than crizotinib at that time. This phenomenon was also observed in the PROFILE 1014 study, where 73% of patients continued to receive crizotinib beyond disease progression for a median of 3.1 months.25 In contrast, a real-world study done in the USA showed a TOT of 7 months for first-line ALK inhibitors and 9 months for second-line ALK inhibitors, which is much shorter than the PFS in clinical trials.26 This is perhaps due to the patients in the USA study being older (median 61 years) compared to the patients in our study (median 53 years), with a trend towards starting second-generation ALK inhibitors earlier upon progression, as these were more freely available in the USA.
Nevertheless, randomized controlled trials of NSCLC patients with ALK translocations have demonstrated the superior efficacy of ALK inhibitors. Compared to chemotherapy, crizotinib significantly increased PFS in treatment-naïve (PROFILE 1014 study) and treatment-experienced (PROFILE 1007 study) patients.15 Furthermore, head-to-head trials of first- versus second-generation ALK inhibitors in the first-line setting (ALEX and J-ALEX) showed significantly better PFS with alectinib than crizotinib.15
About 20% of patients in our study had an ECOG score of 2–4, but median TOT and OS were not reached, suggesting that ALK inhibitors can be given even in patients with poor ECOG performance status. This is consistent with other studies, where ALK inhibitors have also been reported to be efficacious despite patients having poor ECOG scores (>2).27–30 In addition, treatment-related serious adverse events with ALK inhibitors are uncommon and at similar to or lower rates than chemotherapy, highlighting their safety,15,31 making them a good choice for frail patients with poor ECOG scores.
It is well known that a delayed presentation of disease followed by delayed testing can cause patients to miss the window of opportunity for efficacious treatment. In this study, a few patients with ALK translocations did not receive treatment because they died before test results were available. In Malaysia, sequential testing of EGFR mutations followed by ALK testing is a common practice in government hospitals due to laboratory-testing budget restrictions. This sequential testing approach is time-consuming and delays diagnosis. The move to adopt next-generation sequencing, which tests multiple genetic mutations simultaneously, should be encouraged, as it can be cost-effective and time-efficient with its ability to produce test results faster.32
Rebiopsies were not performed for any patients who relapsed after an ALK inhibitor. This is probably because studies of second-line or third-line ALK inhibitors do not require rebiopsies to initiate new treatment and next-generation sequencing were expensive during the study period (US$2500–5000 per test). However, performing a rebiopsy at relapse can provide important prognostic information and help physicians to determine the next course of treatment. In a study by Haratake et al, 2019,33 patients who failed an ALK inhibitor and were given a subsequent ALK inhibitor based on rebiopsy results had a higher objective response rate.
Of the 1581 NSCLC patients included in the lung registry during the period of our study, 4.1% had ALK translocations. This figure is in keeping with international studies (4%–5%),1–8 but lower than another Asian study that had a prevalence of ALK translocation in NSCLC patients of 6.7%.1,2 The lower prevalence can probably be attributed to the low rate of ALK-translocation testing in patients with adenocarcinoma in Malaysia. This is despite ALK translocation being common among adenocarcinoma patients in Malaysia (13% based on earlier data in selected patients with negative EGFR mutations)34 and ALK tests being freely available in public hospitals.34 Testing for ALK translocation is not a priority among physicians, mainly because of the expense of ALK inhibitors, especially among government-supported patients.35 The lack of access to treatment options for ALK mutations will affect testing rates.36 Despite the small sample, the heterogeneous clinical characteristics of patients in our study and the use of different generations of ALK inhibitors reflect the conditions of actual clinical practice, similar to a real-world Canadian study of ALK-positive patients receiving ALK inhibitors.30
Conclusion
In this retrospective multi-institutional cohort of ALK-positive metastatic NSCLC patients, ALK inhibitors led to longer OS and significantly longer TOT than chemotherapy. Similar to data from clinical trials and other real-world studies, our results showed that regardless of the type or line of therapy, ALK inhibitors provided better clinical outcomes than chemotherapy. Therefore, clinicians should advocate for better access to state-of-the-art treatments, ie, ALK inhibitors, for these patients.
Acknowledgments
The authors wish to thank the Malaysian Association for Thoracic and Cardiovascular Surgery for maintaining the lung cancer database, study coordinators at each of the participating centers, and Anne John Michael for help in preparing the manuscript.
Funding
This work was supported by the Malaysian Thoracic Society.
Disclosure
Professor Dr Gwo Fuang Ho reports grants and/or nonfinancial support from Eli Lily, Regeneron, MSD, AB Science, Astellas, Tessa Therapeutics, and Arcus Bioscience, grants and personal fees from Roche, Boehringer Ingelheim, and Janssen Pharmaceuticals, grants, personal fees, and nonfinancial support from AstraZeneca, Pfizer, and Ipsen, personal fees from Bristol Myers Squibb, and nonfinancial support from Taiho outside the submitted work. Dr Harissa H Hasbullah reports personal fees from MSD and AstraZeneca and grants from Novartis and Roche outside the submitted work. Dr Lye Mun Tho reports personal fees from Novartis, Pfizer, Roche, AstraZeneca, and Bristol Myers Squibb outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Lee VHF, Mok TSK, Goto Y., et al. Differences between the east and the west in managing advanced-stage non-small cell lung cancer. Clin Oncol. 2020;32(1):e1–e9. doi:10.1016/j.clon.2019.07.014
2. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi:10.1038/nature05945
3. Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275–4283. doi:10.1158/1078-0432.CCR-08-0168
4. Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8(1):11–23. doi:10.1038/nrc2291
5. Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol. 2013;31(8):1105–1111. doi:10.1200/JCO.2012.44.5353
6. Chia PL, Mitchell P, Dobrovic A, John T. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin Epidemiol. 2014;6:423–432. doi:10.2147/CLEP.S69718
7. Desai A, Mohammed T, Rakshit S, Krull J. The landscape of ALK alterations in non-small cell lung cancer. J Thorac Oncol. 2021;16(suppl_4):S704–S710. doi:10.1016/S1556-0864(21)01863-3
8. Chang GC, Yang TY, Chen KC, et al. ALK variants, PD-L1 expression, and their association with outcomes in ALK-positive NSCLC patients. Sci Rep. 2020;10(1):21063. doi:10.1038/s41598-020-78152-1
9. Kozuma Y, Toyokawa G, Seto T. ALK testing methods: is there a winner or loser? Expert Rev Anticancer Ther. 2019;19(3):237–244. doi:10.1080/14737140.2019.1562343
10. Friedlaender A, Banna G, Patel S, Addeo A. Diagnosis and treatment of ALK aberrations in metastatic NSCLC. Curr Treat Options Oncol. 2019;20(10):79. doi:10.1007/s11864-019-0675-9
11. McLeer-Florin A, Duruisseaux M, Pinsolle J, et al. ALK fusion variants detection by targeted RNA-next generation sequencing and clinical responses to crizotinib in ALK-positive non-small cell lung cancer. Lung Cancer. 2018;116:15–24. doi:10.1016/j.lungcan.2017.12.004
12. Selinger CI, Rogers TM, Russell PA, et al. Testing for ALK rearrangement in lung adenocarcinoma: a multicenter comparison of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol. 2013;26(12):1545–1553. doi:10.1038/modpathol.2013.87
13. Mohamad N, Jayalakshmi P, Rhodes A, et al. Anaplastic lymphoma kinase (ALK) mutations in patients with adenocarcinoma of the lung. Br J Biomed Sci. 2017;74(4):176–180. doi:10.1080/09674845.2017.1331520
14. Doshi S, Ray D, Stein K, et al. Economic analysis of alternative strategies for detection of ALK rearrangements in non small cell lung cancer. Diagnostics. 2016;6(1):4. doi:10.3390/diagnostics6010004
15. Elliott J, Bai Z, Hsieh SC, et al. ALK inhibitors for non-small cell lung cancer: a systematic review and network meta-analysis. PLoS One. 2020;15(2):e0229179. doi:10.1371/journal.pone.0229179
16. Thongprasert S, Permsuwan U. Crizotinib treatment for advanced non-small-cell lung cancer patients: a budget impact analysis based in Thailand. Curr Med Res Opin. 2017;33(5):955–961. doi:10.1080/03007995.2017.1297929
17. Lin HM, Pan X, Hou P, et al. Economic burden in patients with ALK+ non-small cell lung cancer, with or without brain metastases, receiving second-line anaplastic lymphoma kinase (ALK) inhibitors. J Med Econ. 2022;23(8):894–901. doi:10.1080/13696998.2020.1762620
18. Wynes MW, Sholl LM, Dietel M, et al. An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. J Thorac Oncol. 2014;9(5):631–638. doi:10.1097/JTO.0000000000000115
19. Patel A, Batra U, Prasad KT, et al. Real world experience of treatment and outcome in ALK-rearranged metastatic nonsmall cell lung cancer: a multicenter study from India. Curr Probl Cancer. 2020;44(3):100571. doi:10.1016/j.currproblcancer.2020.100571
20. Britschgi C, Addeo A, Rechsteiner M, et al. Real-World Treatment Patterns and Survival Outcome in Advanced Anaplastic Lymphoma Kinase (ALK) Rearranged Non-Small-Cell Lung Cancer Patients. Front Oncol. 2020;10:1299. doi:10.3389/fonc.2020.01299
21. Blumenthall GM, Gong Y, Kehl K, et al. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small cell lung cancer. Ann Oncol. 2019;30(5):830–838. doi:10.1093/annonc/mdz060
22. Liu SV, Hu X, Li Y, Burke T, Piperdi B. 108P - Real-world time on treatment (rwToT) analysis for first-line pembrolizumab combination therapy in advanced nonsquamous NSCLC. J Thorac Oncol. 2021;16(suppl_4):S748–S7802. doi:10.1016/S1556-0864(21)01950-X
23. Velcheti V, Hu X, Piperdi B, Burke T. Real-world outcomes of first-line pembrolizumab plus pemetrexed-carboplatin for metastatic nonsquamous NSCLC at US oncology practices. Sci Rep. 2021;11:9222. doi:10.1038/s41598-021-88453-8
24. Reynolds C, Masters ET, Black-Shinn J, et al. Real-world use and outcomes of ALK-positive crizotinib-treated metastatic NSCLC in US community oncology practices: a retrospective observational study. J Clin Med. 2018;7(6):129. doi:10.3390/jcm7060129
25. Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi:10.1056/NEJMoa1408440
26. Jahanzeb M, Lin HM, Pan X, et al. Real-world treatment patterns and progression-free survival associated with anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor therapies for ALK+ non-small cell lung cancer. Oncologist. 2020;25(10):867–877. doi:10.1634/theoncologist.2020-0011
27. Noronha V, Ramaswamy A, Patil VM, et al. ALK positive lung cancer: clinical profile, practice and outcomes in a developing country [published correction appears in PLoS One. 2016 Dec 9; 11(12): e0168221]. PLoS One. 2016;11(9):e0160752. doi:10.1371/journal.pone.0160752
28. Malapelle U, Rossi A, Bria E. Relationship between performance status or younger age and osimertinib therapy in T790M-positive NSCLC: are the available data convincing? J Thorac Dis. 2019;11(Suppl 15):S1837–S1840. doi:10.21037/jtd.2019.08.99
29. Gounant V, Duruisseaux M, Soussi G, et al. Does very poor performance status systematically preclude single agent anti-PD-1 immunotherapy? A multicenter study of 35 consecutive patients. Cancers. 2021;13(5):1040. doi:10.3390/cancers13051040
30. Gibson AJW, Box A, Dean ML, et al. Retrospective real-world outcomes for patients with ALK-rearranged lung cancer receiving ALK receptor tyrosine kinase inhibitors. JTO Clin Res Rep. 2021;2:100157. doi:10.1016/j.jtocrr.2021.100157
31. Wang L, Wang W. Safety and efficacy of anaplastic lymphoma kinase tyrosine kinase inhibitors in non‑small cell lung cancer (Review). Oncol Rep. 2021;45(1):13–28. doi:10.3892/or.2020.7851
32. Pennell NA, Mutebi A, Zhou ZY, et al. Economic impact of next-generation sequencing versus single-gene testing to detect genomic alterations in metastatic non-small-cell lung cancer using a decision analytic model. JCO Precis Oncol. 2019;3:1–9. doi:10.1200/PO.18.00356
33. Haratake N, Seto T, Takamori S, et al. Short progression-free survival of ALK inhibitors sensitive to secondary mutations in ALK-positive NSCLC patients. Thorac Cancer. 2019;10:1779–1787. doi:10.1111/1759-7714.13143
34. Rajadurai P, How SH, Liam CK, Sachithanandan A, Soon SY, Tho LM. Lung cancer in Malaysia. J Thorac Oncol. 2020;15(3):317–323. doi:10.1016/j.jtho.2019.10.021
35. Lin HM, Wu Y, Yin Y, et al. Real-world ALK Testing Trends in Patients With Advanced Non-Small-Cell Lung Cancer in the United States. Clin Lung Cancer. 2022;22:213.
36. How SH, Tho LM, Liam CK, et al. Programmed death-ligand 1 expression and use of immune checkpoint inhibitors among patients with advanced non-small-cell lung cancer in a resource-limited country. Thorac Cancer. 2022;13(11):1676–1683. doi:10.1111/1759-7714.14442
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



